Abstract
Mercury is a ubiquitous contaminant in aquatic ecosystems, posing a significant health risk to humans and wildlife that eat fish. Mercury accumulates in aquatic food webs as methylmercury (MeHg), a particularly toxic and persistent organic mercury compound. While mercury in the environment originates largely from anthropogenic activities, MeHg accumulation in freshwater aquatic food webs is not a simple function of local or regional mercury pollution inputs. Studies show that even sites with similar mercury inputs can produce fish with mercury concentrations ranging over an order of magnitude. While much of the foundational work to identify the drivers of variation in mercury accumulation has focused on freshwater lakes, mercury contamination in stream ecosystems is emerging as an important research area. Here, we review recent research on mercury accumulation in stream-dwelling organisms. Taking a hierarchical approach, we identify a suite of characteristics of individual consumers, food webs, streams, watersheds, and regions that are consistently associated with elevated MeHg concentrations in stream fish. We delineate a conceptual, mechanistic basis for explaining the ecological processes that underlie this vulnerability to MeHg. Key factors, including suppressed individual growth of consumers, low rates of primary and secondary production, hydrologic connection to methylation sites (e.g. wetlands), heavily forested catchments, and acidification are frequently associated with increased MeHg concentrations in fish across both streams and lakes. Hence, we propose that these interacting factors define a syndrome of characteristics that drive high MeHg production and bioaccumulation rates across these freshwater aquatic ecosystems. Finally, based on an understanding of the ecological drivers of MeHg accumulation, we identify situations when anthropogenic effects and management practices could significantly exacerbate or ameliorate MeHg accumulation in stream fish.
Keywords: biomagnification, methylmercury, stream fish, trace elements, trophic transfer
INTRODUCTION
Methylmercury (MeHg) is among the most widespread and potentially harmful contaminants in aquatic ecosystems. Worldwide, MeHg is the most frequent target of advisories to limit fish consumption. In the US, fish from thousands of inland streams and lakes have been declared unsafe for unrestricted human consumption due to elevated MeHg levels1. In all, >35% of US fresh waters are currently subject to some limit to fish consumption due to concern over elevated MeHg levels 1. This concern is warranted because MeHg is a potent neurotoxin, it is particularly harmful to developing children and fetuses, and it may cause cardiovascular damage in adults 2,3. Human exposure to MeHg is directly linked to fish consumption 4–6, with approximately 8% of U.S. women of child-bearing age thought to have blood mercury concentrations exceeding the US EPA safe level 7. While MeHg in the human diet comes partly from commercially-harvested marine fish, freshwater fish can contain similarly hazardous MeHg concentrations 8. Concern over MeHg contamination in fish and associated broad-scale consumption advisories may reduce the consumption of all fish products, even those that have clear health benefits 9,10. Such concern also appears likely to limit recreational use of freshwater fisheries, even those not particularly contaminated, resulting in negative effects on local communities 11.
Vulnerability to MeHg toxicity depends on the amount of contaminated fish in the diet, which is manageable for human populations. However, many wildlife species are strict fish-eaters and so are extremely vulnerable to MeHg. Studies have linked elevated MeHg concentrations in wild piscivorous birds and mammals with reduced reproductive success, behavioral and hormonal changes, and motor skill impairment 12,13. Best-documented for loons Gavia immer, these deleterious effects of MeHg can yield strong demographic effects on piscivorous wildlife populations 14 and may lead to population declines where MeHg concentrations are elevated 15. Laboratory studies also show that environmentally realistic levels of MeHg contamination can suppress reproductive success in some fish species 16, adding an additional conservation concern for MeHg pollution.
Given the hazards of MeHg contamination for humans and wildlife, research aimed at identifying factors that exacerbate susceptibility to MeHg accumulation and developing appropriate remediation strategies is essential to inform public policy. This is challenging because there is tremendous variation in MeHg concentrations in fish across sites and species, driven by variation in anthropogenic inputs as well as MeHg accumulation rates which respond to complex and dynamic ecological processes 17–19. In this review, we identify a suite of factors that are consistently associated with elevated susceptibility to MeHg accumulation in freshwater food webs and outline a conceptual, mechanistic basis for these relationships. Our focus here is on temperate small-stream ecosystems. Much of the foundational work on MeHg in food webs was conducted in freshwater lakes, while mercury accumulation in stream food webs is recently receiving increased research attention 20 but has not previously been reviewed. We review recent empirical studies from streams to identify the key factors driving variation in MeHg concentrations and assess the generality of these drivers between temperate zone streams and lakes.
Our limited understanding of MeHg accumulation in small-stream food webs is an important knowledge gap. Increasing evidence shows that MeHg concentrations in stream-dwelling fish can reach levels dangerous to consumers even at sites with no point source Hg inputs 20–25, yet much of the work on Hg contamination in stream biota focuses on sites affected by point source Hg inputs 26–29 yielding little information about ecological drivers of variation. Relative to lentic systems, some streams may actually be disproportionately susceptible to MeHg accumulation, with higher concentrations in stream-dwelling organisms than for the same taxa in lakes and other habitats 30–32. Stream fisheries are often intensively harvested by humans 33,34 and stream fish and other biota are an important food source for numerous wildlife species 35,36. Thus, MeHg accumulation in stream food webs represents a potentially important route of MeHg exposure for humans and wildlife 37.
Mercury in the environment
Originating largely from industrial sources (e.g. coal burning, incinerators), inorganic mercury (Hg) is transported long distances in the atmosphere, deposited across the landscape, and transported hydrologically through catchments 38–40. In recipient wetlands and waterbodies, Hg is transformed to MeHg (methylated), largely by sulfate-reducing bacteria in anoxic sediments. Relative to inorganic Hg, MeHg is more toxic, more prone to bioaccumulation, and more persistent in tissues, so the rate of production of MeHg from inorganic Hg is a key control of Hg accumulation 41,42. Entering the food web, MeHg is bioconcentrated from water in suspended algal cells or benthic periphyton, yielding concentrations ca. 104 to 106 fold higher than ambient water 41,43. Higher in the food web, accumulation of MeHg is almost entirely from consumption of contaminated food rather than water 44,45. MeHg in food is efficiently assimilated by consumers and is highly persistent in tissues. Thus, MeHg concentrations biomagnify through the food web, often increasing 2–5 fold across trophic levels and yielding concentrations in piscivorous fish and wildlife that are potentially dangerous to consumers 41.
High MeHg concentrations in fish and aquatic food webs are sometimes associated with elevated inorganic Hg inputs from spatial variation in atmospheric deposition and regional industrial sources 19,46 or local point sources from mining or industry 29,47. Yet, even across sites where Hg inputs are similar, concentrations in fish can differ up to 10-fold 18,24,48,49. Previous studies in lakes have identified characteristics of lakes and their watersheds that are consistently associated with increased susceptibility to MeHg accumulation 48. Here, we briefly review some of the major factors identified in studies of lakes below and then evaluate their relevance to stream ecosystems.
Drivers of increased methylmercury in lake fish
Recent reviews by Evers et al. and Driscoll et al. aimed to identify the drivers of increased MeHg concentrations in lake fish in the northeastern United States 19,50. These reviews identify two categories of factors that can lead to local “hotspots” of elevated MeHg in fish: high inputs of Hg, largely from regional industrial pollution; and high “landscape sensitivity,” which defines a suite of factors that determine the propensity for Hg accumulation in the food web. Our goal is to evaluate the factors identified in these studies, as well as other potentially important drivers of MeHg accumulation, to define individual, food web, and landscape sensitivity to MeHg for small-stream ecosystems.
Evers et al. 19 associate high landscape sensitivity to MeHg accumulation in lakes in the northeastern United States with factors that increase the local deposition of Hg, transport to waterbodies, methylation, and accumulation in the food web. The primary landscape driver of local Hg deposition they identified was forest cover. Heavy forest cover leads to increased local Hg deposition, as trees effectively scavenge Hg from the atmosphere that is then deposited with throughfall or leaf litter 51–53. Transport of Hg through watersheds is mediated by dissolved organic carbon (DOC), an important carrier of Hg through watersheds. Therefore, landscape characteristics that enhance DOC flux also enhance transport of Hg to lakes 54. Methylation rate is enhanced by increased availability of suitable methylation sites in the lake and its watershed (e.g. presence of wetlands 48) and low pH, as acidic conditions and sulfate inputs enhance Hg bioavailability and methylation activity 55. Finally, low productivity exacerbates bioaccumulation through the food web by preventing the dilution of MeHg in increased biomass (“bloom dilution” 42,56–58). These key characteristics that define landscape sensitivity to MeHg accumulation for lakes 19 are consistently associated with increased MeHg concentrations in lake fish across large-scale studies of hundreds of lakes 48,59,60 and have proven useful at predicting lakes that are susceptible to MeHg accumulation 61 and for developing strategies to reduce MeHg concentrations in lake-dwelling fish 62,63.
Unlike the extensive work in lakes, there is relatively little information available about the ecological drivers of variation in MeHg concentrations in stream-dwelling fish. Factors linked to increased inputs and bioavailability of MeHg in lakes may operate similarly to promote MeHg accumulation in streams. Yet, there are important functional differences between lakes and streams and their food webs that may alter these relationships. In lakes, benthic organic matter in sediments is a large storage pool for Hg and often an important site of methylation, providing a direct source of MeHg to the water column and internal food web 41. Streams, though, are defined by unidirectional movement of water, solutes, and suspended particulates often with stepwise uptake, processing and loss through organisms 64,65. While organic sediments in streams may be a storage pool and methylation site for Hg 20,66, dissolved MeHg that enters the water column in streams is primarily available for uptake downstream. Stream Hg dynamics may thus be governed more by sources and methylation of Hg upstream and in the watershed than by local stream characteristics. In fact, streams have garnered considerable attention as conduits for Hg transport to downstream waterbodies 67–69, but the relationship of Hg transport through streams to bioaccumulation of MeHg within the stream food web is not known. Also unlike most lake ecosystems, secondary production in small streams may rely as much on allochthonous inputs from terrestrial detritus and insects as on in-stream autochthonous production 35,70,71. These allochthonous inputs may be an important source of Hg to stream food webs 53, yet these inputs could obscure relationships between MeHg accumulation in stream fish and characteristics of the stream and its food web.
HIERARCHICAL CONTOLS OF METHYLMERCURY ACCUMULATION IN STREAM FISH
We take a hierarchical approach to identify the key factors that drive variation in MeHg accumulation in stream organisms in order to examine the interactions among factors acting on individuals, food webs, streams, watersheds, and regions. With this approach, we identify factors that drive increased MeHg accumulation by synergistically affecting processes at different scales. Accounting for the hierarchical structure of ecological systems when developing a conceptual understanding can yield unique insights into important mechanisms by identifying emergent properties and inconsistencies across scales 72,73.
Throughout this review, we present empirical examples from a case study of Hg accumulation in stream-dwelling Atlantic salmon Salmo salar in small streams in New Hampshire and Massachusetts from 2005 to 2008. The case study follows methods in Ward et al. 24, which contains the data from 2005 and 2006. Briefly, juvenile Atlantic salmon from a single source were stocked at 15–20 study sites per year, then collected after one growing season to measure Hg concentrations. At each site, concentrations of Hg in salmon prey invertebrates and physical and chemical characteristics of the site were measured. Given the uniform initial conditions and age at capture, the stocked salmon at these sites represent a unique standardized measure of Hg accumulation. Thus, the case study serves to illustrate the tremendous variability in Hg accumulation (5–13 fold range in site mean Hg concentrations across sites over the four years), the potential to explain this variation based on hypothesized drivers, as well as the interactions and confounding among potential drivers at different scales.
Individual
Fish accumulate MeHg almost entirely from the consumption of contaminated food 44,45. As MeHg is not strongly regulated physiologically 74, accumulation in individual fish can be described by a simple linear mass-balance model where the MeHg concentration at steady state (MeHgss, ng•g−1) is defined as MeHgss=(AE•I•Cf)•(Ke+G)−1; where AE is assimilation efficiency, I is specific ingestion rate (g•g−1•d−1), Cf is the MeHg concentration in food (ng•g−1), Ke is Hg efflux (g•g−1•d−1), and G is specific growth (g•g−1•d−1) 56,75,76. For fish, variation in MeHg assimilation efficiency and elimination rate is generally small relative to other factors 75,77. Hence, the primary factors that drive variation in MeHg concentration among individuals are the MeHg concentration in the prey, consumption rate, and growth rate (or growth efficiency, the ratio of consumption rate to growth rate; see below). Larger-scale patterns of variation in MeHg concentration in fish are likely mediated largely by changes in these proximate factors affecting individuals (Figure 1).
Figure 1.
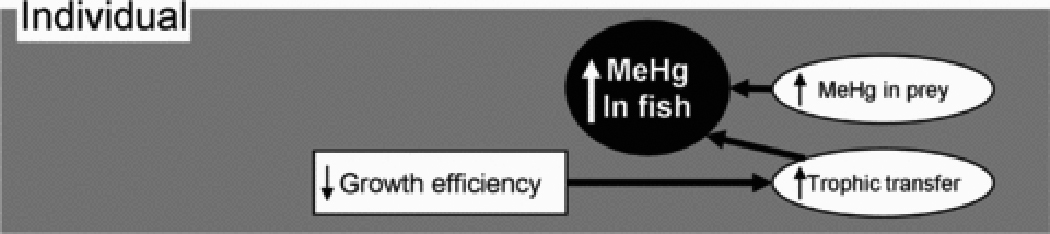
Conceptual map of factors that affect methylmercury (MeHg) in individual fish. Environmental and ecological factors are in boxes, components of the mercury cycle are in ovals. Arrows between boxes indicate causal relationships. Arrows within boxes indicate the direction of change.
Given the clear evidence for transmission of MeHg through food webs 44, many studies attribute variation in fish MeHg concentrations to factors affecting MeHg concentrations lower in the food web, taking the relationship between fish MeHg concentrations and prey MeHg concentrations as a given. While numerous studies in other systems 48,57 and controlled feeding experiments 44,45 show that increased MeHg in prey is associated with increased MeHg in fish, this relationship is rarely actually measured in situ in streams. Some studies even report elevated MeHg in fish from streams that do not have elevated MeHg in invertebrate prey 78. Nonetheless, increased MeHg in prey is clearly a dominant risk factor for increased MeHg in stream fish 24.
Fish growth and consumption rates also can control MeHg concentrations, but their overall effects remain less clear than effects of increased MeHg concentrations in prey. Rapid growth can reduce MeHg concentrations in fast-growing fish relative to slow growing fish via somatic growth dilution. This occurs when fast growers assimilate biomass from prey more efficiently than slow growers (i.e. higher growth efficiency) 24,56,75. Fast-growing fish with high growth efficiency add more tissue per unit MeHg consumed in prey, diluting the MeHg in a larger biomass. Conversely, if fast growth is due simply to fast-growing fish eating more prey, and thus taking in more MeHg, then MeHg will not be effectively diluted 24,75. Individual growth is one of the most variable traits across all fish populations, and recent evidence suggests that much variation in growth is associated with differences in growth efficiency 79–81. Thus, growth efficiency and somatic growth dilution likely play a sizeable role in mediating trophic transfer of MeHg.
Observed MeHg concentrations in fish integrate MeHg concentrations in prey and fish growth efficiency. MeHg in prey sets a baseline contamination level and growth efficiency determines the rate of trophic transfer, or deviations from the linear relationship between fish MeHg and prey MeHg 82. The relative importance of MeHg in prey and growth efficiency is difficult to assess in situ, as growth efficiency is difficult to measure in the field 83. Yet, assessing variation in growth rate alone can yield useful information. In the juvenile salmon case study, Hg concentrations in prey and mean individual growth rate together explained 70–95% of the variation in mean Hg concentrations in salmon across sites over the four years (analysis on log-transformed data). Variance partitioning shows that 48–53% of the variation is explained by independent effects of prey Hg on fish Hg and 23–45% is explained by independent effects of growth (analyzing years separately) 24,84. In all years, fast growth led to reduced Hg concentrations in salmon, suggesting that variation in growth efficiency led to somatic growth dilution (Figure 2).
Figure 2.
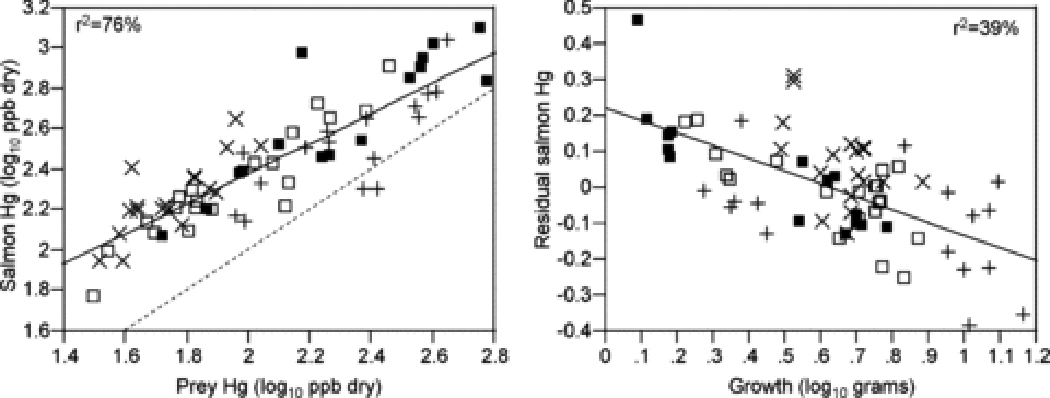
Relationship between mean mercury (Hg) concentrations in salmon and mercury concentrations in their prey (left panel) and relationship between the residuals from the salmon-prey regression, an index of trophic transfer, and salmon growth as mean final mass (right panel). Each point is a study site and different symbols indicate different years (■: 2005; +: 2006; ×:2007; □: 2008). Solid lines are simple linear fits to pooled data. The dashed line is the 1:1 relationship between salmon and prey Hg concentrations. Based on a subset of samples analyzed for Hg speciation, 96% of Hg in salmon and 85% of mercury in prey is methylmercury.
Individual differences in growth efficiency and prey MeHg concentrations directly drive the tremendous variation fish MeHg concentrations. The example above involved comparisons of individuals within a species. Yet, these individual differences are also relevant to comparisons across fish species. Fish species can differ widely in prey MeHg and growth efficiency due to species-specific prey preferences and physiology. These differences could lead to large changes in the MeHg concentrations of the fish community as a whole with a shift in fish community composition, independent of changes in Hg inputs or MeHg production. Such community-level effects on MeHg accumulation are addressed further below in the context of the larger food web.
Finally, assessing the relationship between individual size of a fish and its MeHg concentration does not equate to accounting for growth effects unless age is also factored into the analysis. The commonly reported positive relationship between fish size and MeHg concentration is largely due to exposure time (when larger fish are older) and to increased MeHg in prey for larger fish, which feed at a higher trophic level, rather than to variation in growth rate or growth efficiency (but see ref. 75). Comparisons of parameters (slope, intercept) of the individual size-MeHg concentration relationship across sites can yield information about the ecological factors that drive variation in MeHg accumulation, but further information (fish age, prey MeHg concentrations) is required to isolate the underlying mechanisms 85. Further, from a human health perspective, relationships between fish size and MeHg concentration are very useful for establishing consumption restrictions for specific fish that are a particular risk for consumers.
Food web
The best-studied relationship of MeHg concentrations to food web characteristics is an increase in MeHg concentration in consumers with an increase in trophic level 86,87. This biomagnification of MeHg occurs across trophic levels in streams when comparing consumers to their resources 21,22,25,88, including in the salmon case study (Figure 2). While MeHg concentrations increase with trophic level within a stream, variation in food chain length does not seem to be an important driver of variation in MeHg concentration in fish across streams 22. This is likely because variation in food chain length is very small relative to variation in MeHg concentrations at the base of the food web. Yet, changes in community structure that cause a focal fish species to feed at higher trophic level could lead to increased MeHg concentrations. This has been observed in lakes 47,89 but not yet reported in streams.
Changes in the productivity and community structure at the base of stream food webs can also directly influence MeHg concentrations at low trophic levels (Figure 3). Bloom dilution, where increased primary production dilutes MeHg in producers with increased biomass, occurs in benthic periphyton in streams 90–92 just as in suspended phytoplankton in lakes 42. Hill and Larsen 90 showed experimentally that stream periphyton growing in high light treatments gain more biomass, yielding 3-fold lower MeHg concentrations than periphyton in shaded treatments. Models suggest that high biomass and low MeHg concentrations in producers at highly productive sites can propagate directly up the food chain to yield high biomass and low MeHg concentrations in primary consumers 93, called density dilution in studies of zooplankton MeHg in lakes 57,94. While no studies have empirically linked spatial variation in MeHg concentrations in periphyton to consumers in streams, the pattern in the salmon case study is consistent with these dilution processes. Sites with heavy shading from riparian canopy trees had lower invertebrate biomass and higher Hg concentrations, but the proportion of variation explained by these relationships is low (Figure 4). Conversely, Tsui et al. 23 report higher MeHg concentrations in invertebrates at highly productive sites where the periphyton community shifts to dominance by abundant filamentous algae rather than a benthic biofilm, potentially because mats of filamentous algae harbor methylating bacteria and increase MeHg production. Thus, a shift in community composition that favors increased methylation at highly productive sites could potentially override the effects of bloom dilution.
Figure 3.
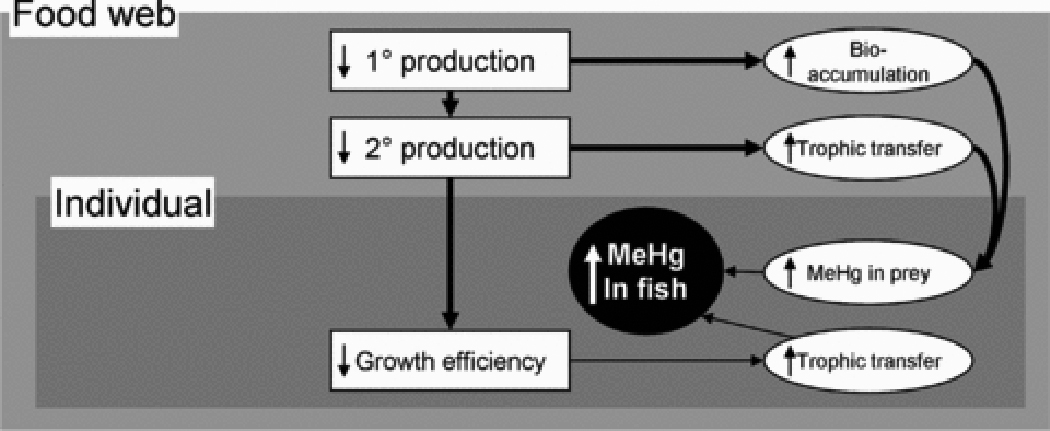
Expanded conceptual map showing factors at the food web level, with relationships at this level highlighted with heavy arrows. Symbols as in Figure 1.
Figure 4.
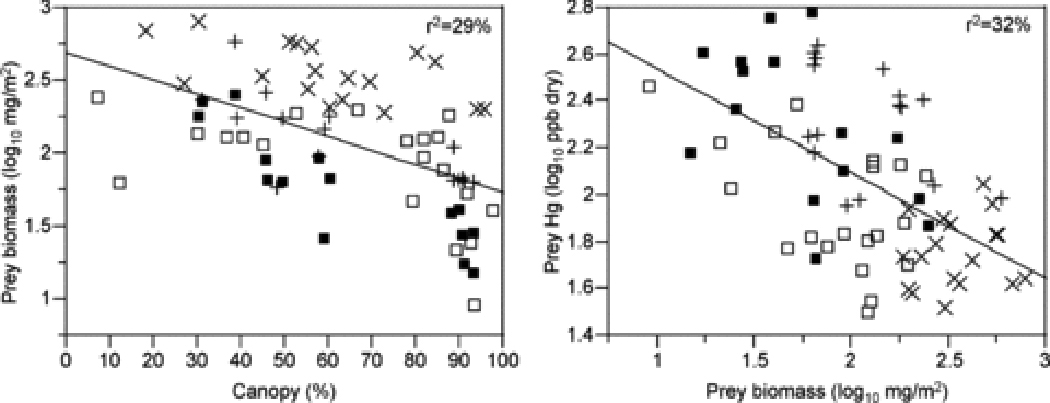
Relationship between prey biomass and canopy cover (left panel) and prey mercury (Hg) concentration and prey biomass (right panel). Symbols as in Figure 2.
Changes in primary and secondary production and biomass could affect trophic transfer of MeHg in addition to affecting MeHg levels in resources (Figure 3). Eating high quality autochthonous algae, versus allochthonous detritus, can enhance growth efficiency of generalist primary consumers in streams 95. Such variation in resource quality and growth efficiency reduced MeHg trophic transfer to zooplankton primary consumers in laboratory experiments 56. At higher trophic levels, when prey biomass increases, fish can reduce energy spent foraging without slowing growth 96. This suggests there will be greater growth efficiency and lower trophic transfer of MeHg to fish at sites with high prey biomass.
Food web effects on MeHg accumulation in streams can be mediated by changes in autochthonous primary production. However, forested small-stream food webs are noted for reliance on inputs of allochthonous detritus35,70,71. We hypothesized that low-quality allochthonous detritus could exacerbate trophic transfer to generalist primary consumers. Yet, the effects of allochthonous input on MeHg concentrations in invertebrates that are prey for predatory fish depend on the relative concentrations of MeHg in detritus and periphyton, trophic transfer to both generalist and specialist primary consumers, and the feeding preferences of fish. There is little empirical evidence to resolve the relative importance of autochthonous and allochthonous materials as paths of MeHg transfer to fish. We suggest that the autochthonous food web is a more likely path, as studies that characterize invertebrate feeding relationships have found that MeHg concentrations in shredders that eat allochthonous detritus are generally low 25, with elevated concentrations in algae-grazing scrapers 78,97 or generalist collectors and filterers 23,98. Further work is needed to determine whether algae-grazing invertebrate functional groups represent keystone conduits (sensu Pickhardt et al.99) for the trophic transfer of MeHg to stream fish.
Stream
At the stream scale, biogeochemical factors that determine MeHg production (via methylation) and delivery can have strong effects on MeHg concentrations in fish, mediated by elevated accumulation of MeHg at the base of the food web (Figure 5). As in lakes, most of the Hg in upper-trophic-level stream organisms is MeHg while most of the ambient Hg in water and sediments is inorganic 25. This suggests that high local MeHg production rate can drive elevated MeHg in fish without elevated external Hg loading. This relationship is well established in lakes 19,50, where methylated Hg is available to local organisms. In streams, dissolved MeHg concentrations in stream water do predict MeHg concentrations in fish in large-scale surveys 20,22, yet the source of this MeHg is not clear. Most studies attribute MeHg in stream water to methylation in upstream wetlands, but availability of wetland methylation sites is not necessary for elevated MeHg in stream organisms (see Watershed below). Transport of MeHg to streams from wetlands is dominated by episodic flood events 100, so bioavailability to stream organisms may be low. In-stream methylation, which peaks during warm low-flow periods 66,100, could be an important continuous source of MeHg to stream organisms during the growing season.
Figure 5.
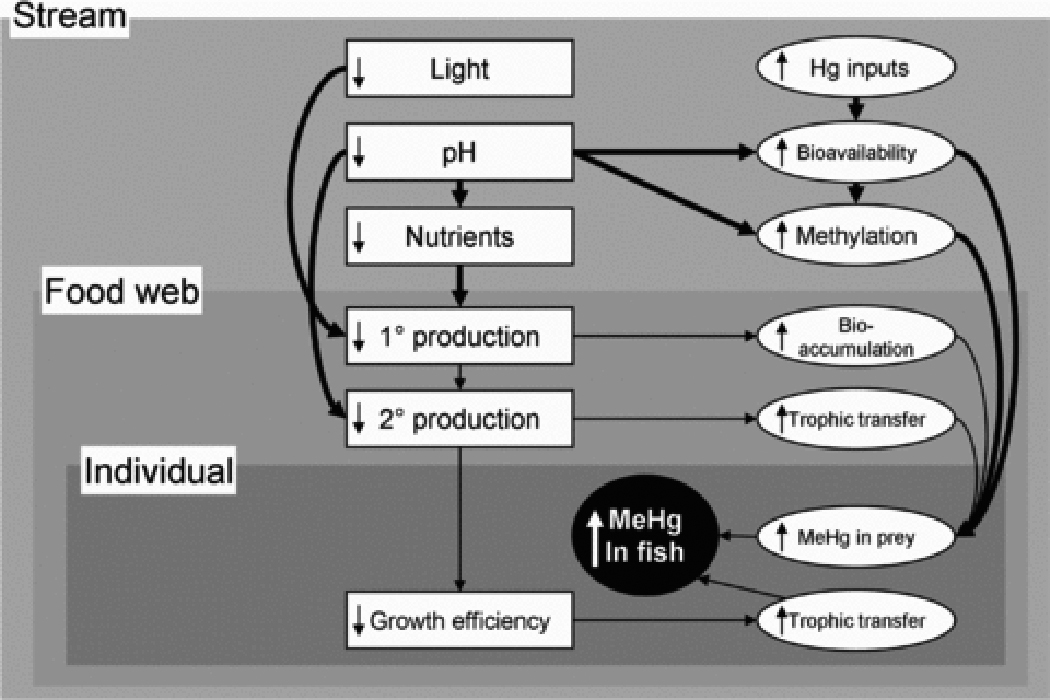
Expanded conceptual map showing factors at the stream level, with relationships at this level highlighted with heavy arrows. Symbols as in Figure 1.
While the role of methylation in the stream itself as a source of MeHg to stream fish remains unclear, it is clear that organic sediments in streams can hold high concentrations of Hg and that microbial Hg methylation does occur in stream sediments 66,100. However, dissolved MeHg and Hg concentrations in stream water do not necessarily reflect local sediment Hg concentration or methylation rate 20,66. Other potential methylation sites in streams include dense mats of filmentous algae 23 and the deep hyporheic zones 101 and periphyton biofilms 102 associated with coarse mineral substrates. Although these latter sites with low organic matter concentrations will not hold large pools of Hg, they may be hotspots of Hg methylation and delivery to stream biota due to high bioavailability, frequent exchange with surface water 101, and the direct role of periphyton methylation sites as a food source for macroinvertebrates 102.
Water-quality factors associated with increased MeHg in fish include reduced pH and elevated dissolved organic carbon (DOC) 20,24,25,78,103 (but see ref. 21). Stream water pH alone is an excellent predictor of Hg concentrations in fish and their prey in the salmon case study (Figure 6). The similar effects of pH on MeHg concentrations in lake fish are generally attributed to sulfate additions in acidified lakes stimulating methylation activity by sulfate-reducing bacteria in lake sediments 18. Thus, low pH in streams may be an indicator of high sulfate loads and increased sulfate-reducing bacteria activity in upstream methylation sites 104 or in the stream itself. Low-pH induced increases in MeHg in fish mediated by increased methylation may be reinforced by increased trophic transfer of MeHg in acidified streams. Even moderately suppressed pH in streams is associated with reduced biomass of susceptible invertebrate species, particularly mayflies (Ephemeroptera) 105,106. Mayflies are preferred prey of stream-dwelling trout and salmon, and growth of these taxa can be suppressed where mayfly biomass is low 107, preventing growth dilution of MeHg. Further, acid-sensitive fish species suffer energetic stress at low pH due to the costs of maintaining ion balance, further suppressing growth efficiency 108 and potentially exacerbating trophic transfer of MeHg.
Figure 6.
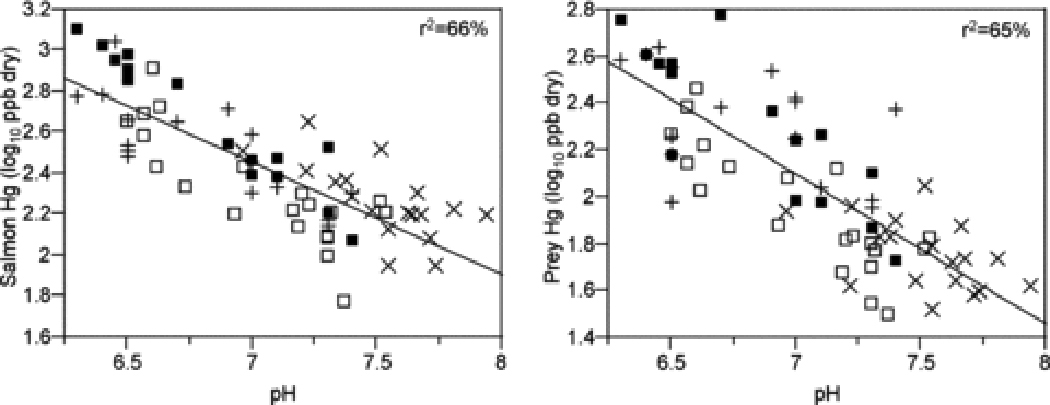
Relationship between salmon salmon Hg concentration and pH (left panel) and prey Hg concentration and pH (right panel). Symbols as in Figure 2.
High concentrations of dissolved organic carbon (DOC) in stream water are also frequently associated with increased MeHg concentrations in stream organisms 20,22,103. In solution, DOC effectively binds and transports MeHg and Hg, so the correlation of DOC concentrations with MeHg in organisms may be a simple function of increased loading and inputs to the base of the food web. However, studies with lake phytoplankton suggest that DOC-bound MeHg is not readily available for uptake in producers 109, and the relationship of stream water DOC to MeHg concentrations in stream periphyton has not been measured. As an alternative pathway for DOC-bound MeHg into the food web, some stream invertebrates (e.g. Simuliidae) directly consume suspended organic carbon that is nominally in the dissolved fraction (e.g. < 0.45 µm), suggesting that DOC-bound MeHg may be available directly to some stream consumers 103.
Watershed
Watershed geology and land cover can affect MeHg concentrations in stream fish by affecting local Hg deposition, transport to streams, and availability of upstream methylation sites or by mediating the in-stream processes described above. Each factor likely acts on processes at multiple scales. For example, extensive forest cover can enhance Hg local deposition, as trees, particularly conifers 110, effectively capture Hg from the atmosphere 51–53. In addition to increased Hg deposition, forest cover can mediate Hg transport, methylation, and stream food web structure (Figure 7). Forests mediate Hg transport to streams via effects on particulate organic carbon (POC) and DOC export, particurly when forests are disturbed 111,112. In the northeastern US, forest harvest and regrowth contribute to soil base cation depletion, increasing acid sensitivity 113 and potentially promoting methylation at low pH. Finally, young forests effectively intercept nutrients and light 114, suppressing in-stream productivity and potentially exacerbating MeHg bioaccumulation and trophic transfer. All of these factors could contribute to increased MeHg in fish in heavily forested watersheds, as observed in nation-wide surveys 20 and the salmon case study, although the proportion of variation in fish MeHg explained by forest cover alone is low (Figure 8).
Figure 7.
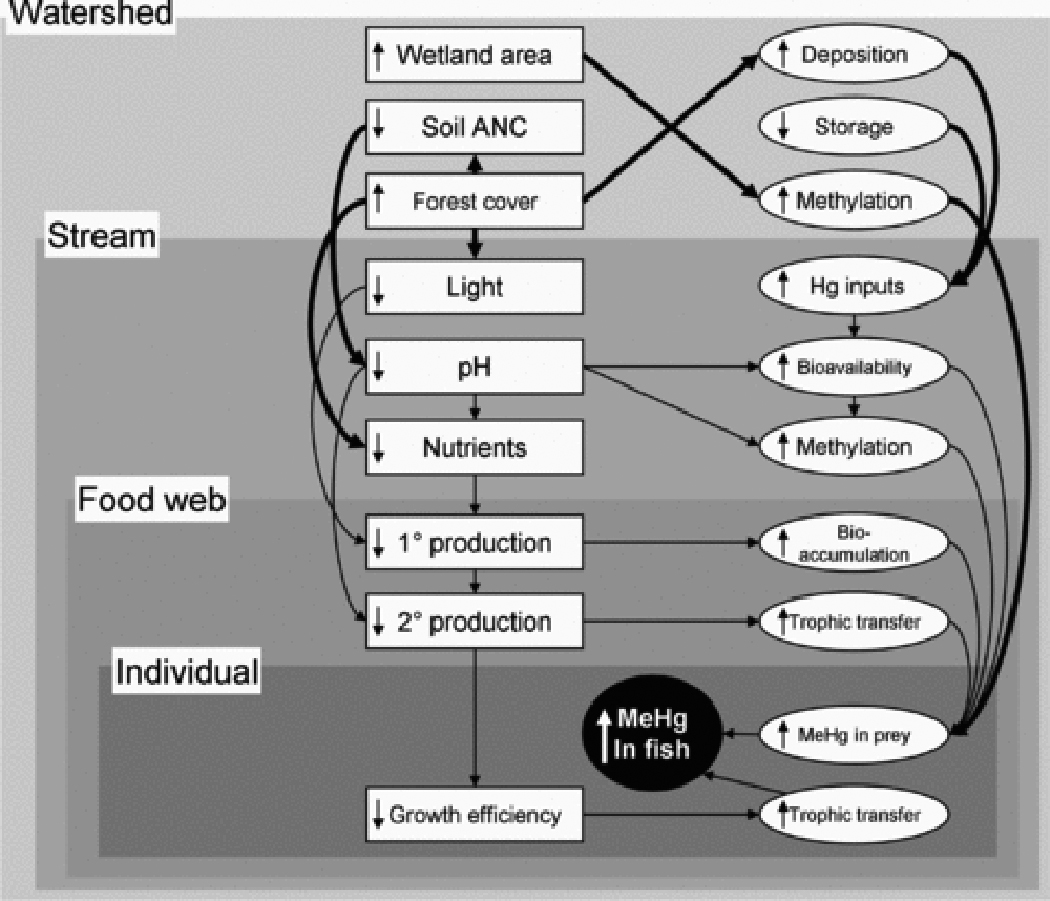
Expanded conceptual map showing factors at the watershed level, with relationships at this level highlighted with heavy arrows. Symbols as in Figure 1.
Figure 8.
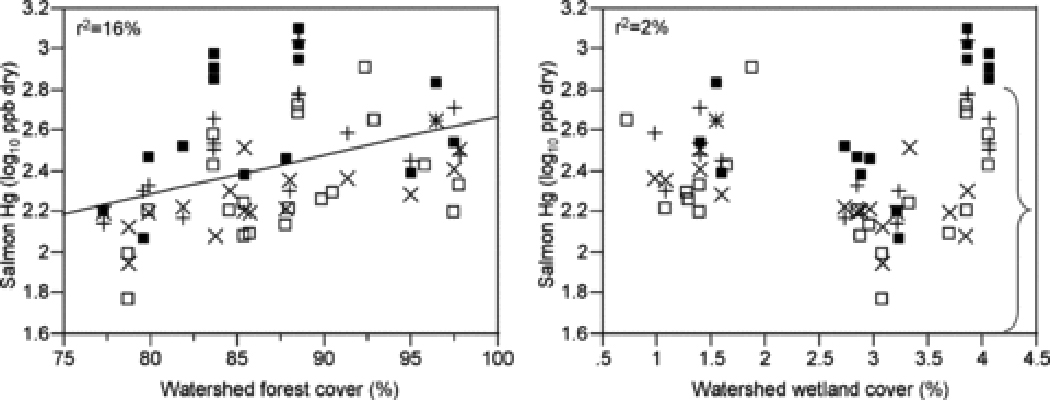
Relationships between salmon mercury (Hg) concentration and the percent forest cover (left panel) and wetland cover (right panel) in the watershed. Symbols as in Figure 2. The bracket indicates the range in small-fish Hg concentrations observed by Chasar et al. 22.
Of all watershed land-cover characteristics, a high proportion of wetland area is most often implicated as a risk factor for increased MeHg in fish 22,78. Numerous observational and experimental studies show that wetlands are MeHg sources, related to high methylation potential in saturated organic soils 54,115. Further, many wetlands are DOC sources 116, and exported DOC could enhance Hg and MeHg transport to downstream food webs. These patterns are reinforced by the very high Hg concentrations in fish from high-DOC rivers draining wetlands in the southeastern US, matching or exceeding concentrations at sites impacted by Hg mining 20. However, high wetland area is sufficient, but not necessary, for elevated MeHg in fish. While Chasar et al. 22 observed a strong, linear increase in fish Hg concentrations across a gradient of watershed wetland area ranging up to 35%, concentrations in juvenile salmon in the case study span the entire range of small-fish concentrations observed by Chasar et al. over a range in watershed wetland area up to only 5% (Figure 8). Furthermore, Tsui et al. 23 observed similar highly variable MeHg concentrations in stream invertebrates across multiple sites with no watershed wetlands.
Watershed-scale Hg budgets show that most Hg deposited from the atmosphere is retained in terrestrial soil 117,118. As noted above, Hg transport to streams and wetlands is mediated by hydrologic transport of DOC and POC, which effectively bind and carry Hg 54. Absent disturbance that affects soil carbon storage and export, most soils remain an Hg sink. However, the long-term dynamics of the large pool of anthropogenic Hg stored in terrestrial soils remains uncertain. Recent increases in DOC export from forest soils, associated with reduced acid deposition, suggest that Hg export from terrestrial soils may increase 119–121. In any case, the large pool of anthropogenic Hg remaining in watersheds suggests that MeHg contamination of sensitive freshwater ecosystems could continue even when anthropogenic Hg emissions decline.
Finally, at the watershed scale, gold and Hg mining and industrial point sources can lead to elevated MeHg in stream biota 20,29. Despite the potential for increased local anthropogenic Hg inputs with anthropogenic disturbance, there is a surprising negative relationship between developed area or human population density in the watershed and MeHg concentrations in fish in multiple studies in streams and lakes (correlation with percent developed area in the salmon case study r=−0.35) 20,48. Reduced MeHg in fish from developed streams may reflect reduced connectivity between streams and methylation sites in floodplain wetlands and soils 122–124 or increased nutrient inputs that promote productivity 125. That stream fish MeHg concentrations can decline with increased human population density despite increased Hg concentrations in stream sediments 20 suggests that some human impacts reduce bioaccumulation and trophic transfer of MeHg through food webs, emphasizing the importance of understanding these ecological drivers of MeHg accumulation.
Region
At larger scales, the primary drivers of variation in MeHg in stream biota are climatic factors and the location of emissions sources that drive spatial variation in Hg and acid deposition over multiple watersheds (Figure 9). Across the US, states that receive high wet Hg deposition produce fish with higher mean Hg concentrations 46. But high Hg deposition and high MeHg in fish are not always linked. In the southeastern US, Rypel et al. 124 found no relationship between atmospheric Hg deposition and riverine fish Hg concentrations for the southeastern coastal plain; most of the variation in fish concentrations was explained by characteristics of the local ecosystem. Very low Hg deposition rates can likely prevent MeHg contamination in fish, and recent work suggests that reduced Hg deposition can produce a rapid decline in MeHg in fish 126. Yet is is also clear that even moderate deposition can drive high MeHg concentrations in sensitive streams. Reducing Hg emissions world-wide is the long-solution to MeHg contamination in fish. Continued work to identify what makes a stream sensitive to Hg inputs will be essential to mitigating MeHg in impacted systems as emissions decline.
Figure 9.
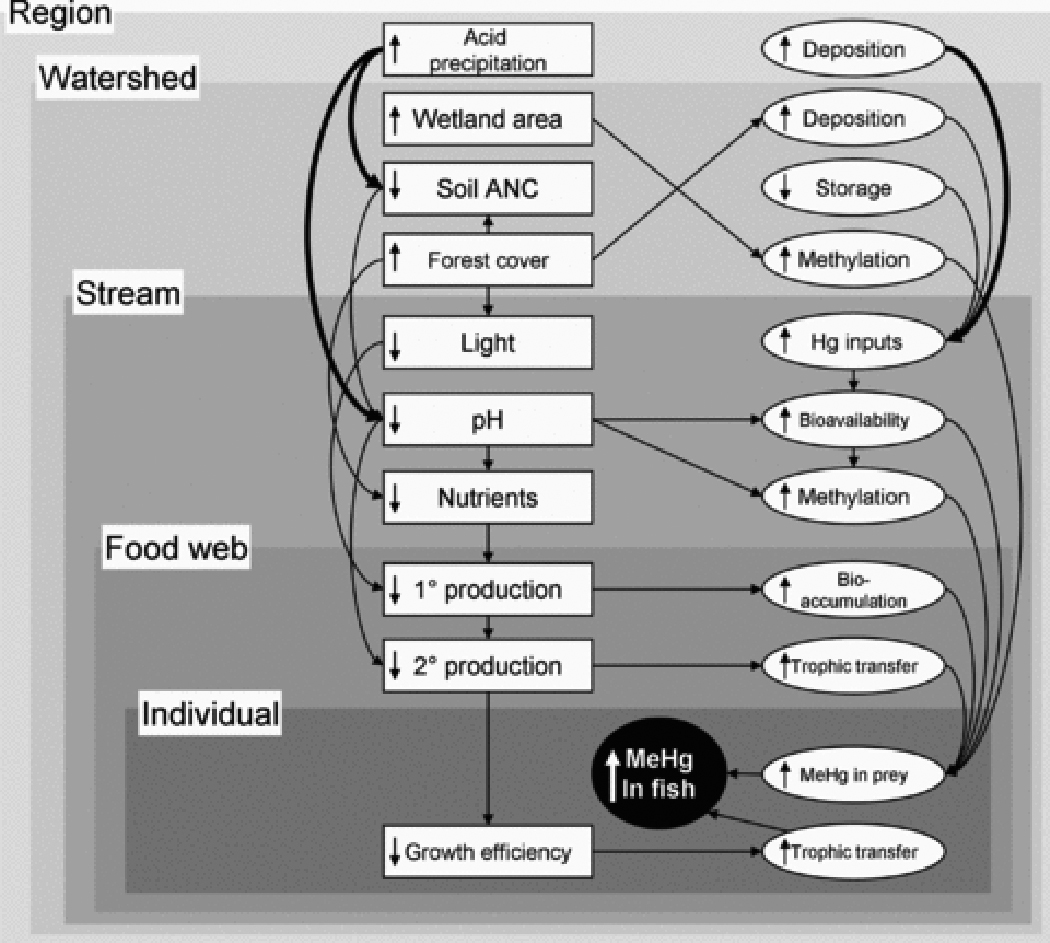
Expanded conceptual map showing factors at the region level, with relationships at this level highlighted with heavy arrows. Symbols as in Figure 1.
A BIOACCUMULATION SYNDROME
From the review above, we note a suite of factors that interact through multiple mechanisms and across spatial scales to yield increased MeHg concentrations in stream-dwelling fish. For individual fish, high MeHg concentrations in prey and low growth efficiency lead to high MeHg accumulation. Suppressed primary and secondary productivity potentially act via both prey and growth to increase MeHg in fish, increasing MeHg in prey by preventing bloom dilution and suppressing growth efficiency through energetic costs of increased foraging effort. At the stream and watershed scale, both low pH and high forest cover are associated with reduced primary or secondary productivity, with these effects exacerbated by increased inputs or methylation of Hg. Wetlands are methylation sites and some wetland types export acid-sensitive, high DOC waters that further enhance Hg transport and methylation 116,127. No one of these factors alone can predict MeHg in stream fish across regions, but each is a risk factor for increased concentrations.
The risk factors we identified for increased sensitivity to MeHg accumulation in stream food webs are similar to those described for freshwater lakes 19,48. Therefore, these factors may indicate a syndrome of environmental characteristics that drive high MeHg production and bioaccumulation rates that is applicable across freshwater ecosystems. This similarity in syndrome factors could indicate that Hg dynamics in both small-stream and lake ecosystems are largely determined by factors that affect Hg inputs, transport, and transformation at the watershed scale and not bioaccumulation and trophic transfer within streams or lakes. However, there is compelling evidence that changes in productivity, food web structure, and internal Hg cycling can affect MeHg accumulation in individual streams and lakes without changes at the watershed scale 42,90,128,129, so we suggest that the common syndrome factors are due to similar mechanisms at all scales of the hierarchy.
Mechanistically, syndrome factors are those that either increase MeHg availability via increased inputs and methylation or increase trophic transfer by preventing somatic growth dilution and bloom dilution. Availability and trophic transfer are the fundamental drivers of MeHg accumulation in all food webs, including freshwater streams and lakes. Yet, there are key differences in ecosystem structure between streams and lakes that will determine the relative importance of each factor and the spatial and temporal scale of effects on MeHg in fish. For example, due to unidirectional movement of water and high shoreline to area ratio, streams are more a product of their landscapes than lakes and management interventions may require a larger spatial scale. Further, streams are subject to a unique array of anthropogenic impacts that are not relevant to lakes (see Anthropogenic impacts and management below). Thus, despite common syndrome factors between streams and lakes, stream ecosystems will likely require unique MeHg management and mitigation strategies.
The factors we identify as a bioaccumulation syndrome include some that are frequent targets of river restoration and management efforts. For example, replanting riparian forest, restoring and enhancing connectivity to floodplain wetlands, and reducing productivity by eliminating nutrient pollution are important components of stream ecosystem management efforts to protect fish populations and improve water quality 130,131. That these factors appear to be associated with increased MeHg accumulation in stream fish suggests that, at current levels of Hg emissions, river restoration efforts may not produce healthy freshwater fisheries for humans and wildlife. Considering the response of syndrome factors in assessment of stream restoration and other anthropogenic impacts and management activities may aid in predicting the effects on MeHg concentrations in fish.
ANTHROPOGENIC IMPACTS AND MANAGEMENT
Throughout the world, many streams are dammed, diverted, channelized, and used as drains for waste disposal 132. In many areas, stream fish communities are replaced with exotic species 133, stocked with hatchery-produced fish 134, and heavily harvested 34. These widespread impacts can completely reshape stream food webs, disrupt nutrient cycles, and alter material transport 132. Given these impacts, humans may have as much influence on Hg dynamics in streams through indirect effects on cycling, uptake and trophic transfer as by direct inputs of Hg pollution. Here, we briefly review some of the major categories of anthropogenic impacts on streams and their potential effects on MeHg accumulation in stream-dwelling fish.
Dams and hydrologic alteration
With nearly 1 million dams on streams and rivers worldwide and more than 50% of the world’s freshwater runoff appropriated for human use, anthropogenic impacts on hydrologic cycles are vast in scale 135. Impacts of dams on Hg cycling have received considerable attention because of elevated MeHg concentrations in fish and other organisms in impoundments 62. Newly flooded organic soils harbor Hg and provide ideal methylation sites, yielding a spike in MeHg concentrations in reservoir water and organisms and substantial MeHg export downstream. Concentrations of MeHg in reservoir organisms can remain elevated for decades, yielding increased MeHg in riverine fish feeding on organisms lost downstream 136. However, long-term export dynamics from reservoirs to downstream riverine ecosystems are less clear. Many old reservoirs accumulate organic sediments and nutrients, suggesting in the long term there may be a sink of Hg in reservoir sediments that would become a concern on dam removal 137–139.
In addition to Hg transformation and export from reservoirs, regulated rivers downstream from dams have low flow variability that reduces exchange between the river and flood plain wetlands and soils 140. Channelization and urban development in general further constrain streams and increase the disconnect between rivers and floodplains 125. This impact of river regulation may serve to reduce Hg loading from terrestrial soils and reduce MeHg inputs from methylation sites in floodplain wetlands 122. Consistent with this hypothesis, Rypel et al. 124 observed a consistent reduction in Hg concentrations in fish from regulated rivers throughout the Southeast. However, it is unclear whether this pattern would hold in regions with less floodplain extent and duration.
Forestry and forest disturbance
Worldwide, the large majority of forested watersheds have been subject to timber extraction or conversion to other land uses 141. These forestry practices can impact Hg dynamics at multiple levels. In the short term, soil disturbance associated with clearcutting and soil preparation for regeneration can increase DOC and POC export to streams, increasing Hg inputs to streams 142 and MeHg concentrations in biota 143. The longer-term effects are much less clear. In some cases, reduced shading and increased nutrient loading in streams draining logged catchments can increase primary and secondary production 144–146. As noted above for the salmon case study, this can reduce Hg concentrations in fish via bloom dilution and growth dilution. Yet, this effect of forest harvest may be context-dependent. In regions where forest harvest increases fine sediment yields to streams, reduced interstitial shelter space can reduce fish growth rates 147 and potentially exacerbate MeHg accumulation. In addition to these effects, selective forestry practices can change tree species composition in ways that affect Hg dynamics. In particular, shifting forest composition from deciduous to coniferous species can increase Hg loading and stream chemistry, potentially increasing MeHg accumulation in fish 110,148.
A large body of research and practice has developed to mitigate negative impacts of forestry on aquatic ecosystems. What are the impacts of these mitigation efforts for MeHg accumulation in stream fish? The most common mitigation technique is the use of riparian buffers, strips of relatively undisturbed vegetation along streams that ameliorate effects of land use in the rest of the watershed. 149. Buffers prevent sediment and POC associated with disturbance from entering streams 150. It is possible that they prevent short-term pulses of Hg following timber harvest, but there are no studies evaluating Hg dynamics post harvest in streams with and without buffers. Provision of shade and interception of nutrients also are important goals for riparian buffer management 150. Yet this could reduce primary and secondary productivity and offset advantages of reduced Hg inputs by increasing trophic transfer. Thus, buffers appear unlikely to ameliorate Hg accumulation due to forest harvest.
In addition to forestry practices, ecosystem scientists are increasingly aware that disturbance processes are fundamental to the structure and function of forests and their watersheds. Fire is a major natural and anthropogenic disturbance in many forests 151, with potentially great consequences for Hg dynamics in surface waters. Fire can release large amounts of Hg that is redeposited locally 152, promote DOC and Hg export from soil, and stimulate soil Hg methylation 153. Consistent with these increased inputs, some studies of lakes in burned catchments find increased MeHg concentrations in organisms 143,154. Bank et al. 155 however, found that Hg concentrations were lower in stream-dwelling salamanders from a burned watershed in Maine. Such effects may be mediated by changes in productivity of streams. Fire can result in substantial increases in light and nutrients in receiving streams, stimulating increased primary and secondary productivity 156,157. Allen et al. 158 found that elevated lake productivity associated with nutrient inputs after fire led to reduced MeHg concentrations in invertebrates and fish.
Given the major changes in fire regime associated with fire suppression, human development, and climate change, a better understanding of the links between fire and Hg accumulation is vital. For example, prescribed fire is increasingly used as a management technique to provide fire-dependent habitats and ecosystem processes 159, and to reduce fuel loads to lessen the magnitude and severity of large fires 160. Wickman et al. 161 found that prescribed fire increased inputs of MeHg to wetlands in Upper Great Lakes region, yet it is unclear how these effects translate into MeHg concentrations in biota.
Fisheries management
Stocking of piscivorous game fish can essentially add a trophic level to freshwater food webs 134, increasing the scope for biomagnification of MeHg. Many fisheries management activities also alter individual consumption and growth rates of the fish community, the very factors that determine MeHg intake and trophic transfer 24. Prey consumption and growth rates are often density-dependent in stream fish populations 107,162. Fish stocking increases fish population density and can suppress fish growth 163, potentially increasing MeHg trophic transfer. There are millions of fish introduced annually through stocking programs worldwide 11. Given the diverse effects of stocking on ecosystems134, their potential to affect MeHg concentrations in stocked and native fish is enormous.
Fish stocking and other fisheries management tools can also be applied to intentionally affect MeHg in fish 164. Multiple studies in lakes have shown that increasing fish growth rates by intensive fishing to reduce population density 129,165,166 or lake fertilization 128 can reduce fish MeHg concentration. Such actions may have unpredictable effects due to shifts in community composition or foraging behavior. For example, Lepak et al. 89 found that fish in an Adirondack lake grew faster after removal of competitors, but shifted to more contaminated prey at a higher trophic level, preventing growth dilution from reducing MeHg in fish. Nonetheless, fisheries management approaches to MeHg control in stream fish merit further consideration as they offer a potential rapid, local-scale solution where Hg inputs cannot be controlled.
Acidification and Liming of Surface Waters
Acid precipitation has been recognized as a threat to aquatic ecosystems since the 1970s. Since the passage of legislation limiting nitrogen and sulfur emissions in North America and northern Europe, some recovery in stream chemistry has occurred 167. However, due to the long-term depletion of base cations in forest soils, many surface waters remain either chronically or episodically acidified, with long time scales of recovery even with emissions reductions 168. As a result, the management application of buffering minerals (generally limestone-based, hence the generic term ‘liming’) has become well established in vulnerable areas of eastern North America and northern Europe 169. Given the strong relationships between pH and Hg methylation, and between acid-neutralizing capacity and stream productivity, liming acidified surface waters is expected to decrease MeHg in fish tissue. This effect has been observed in Scandinavian lakes 170,171, where major declines in MeHg (up to 80% in a ten-year period) are reported in perch (Perca fluviatilis), particularly in lakes that were moderately acidified (pH 5.4 – 5.8). Similar studies have not been conducted in flowing-water ecosystems. Given the wide range of liming techniques used in surface waters, an explicit analysis of the effects of stream and watershed liming on Hg accumulation would be valuable in evaluating the costs and benefits of these management actions. Further, there is a particular knowledge gap concerning the effects of liming on Hg methylation and accumulation in systems subject to episodic, as opposed to chronic acidification.
CONCLUSIONS
A suite of factors, including suppressed individual growth of consumers, low primary and secondary production, hydrologic connection to methylation sites (e.g. wetlands), heavily forested catchments, and acidification, drive sensitivity to MeHg accumulation in fish across both streams and lakes. These factors interact across a hierarchy of levels, from individual fish to regional landscapes, to increase MeHg concentrations in fish prey and increase trophic transfer of MeHg. Given the similarity of the driving factors of MeHg accumulation in streams to those identified as important determinants of MeHg accumulation in lakes, we suggest that these factors represent a syndrome of increased bioaccumulation risk for freshwaters. The bioaccumulation syndrome includes factors that are frequent targets of stream restoration efforts, suggesting that these efforts alone will not produce healthy fisheries.
Other anthropogenic impacts and management actions have diverse effects and may increase or decrease MeHg in stream fish, yet these effects may be predictable by assessing interactions with syndrome factors. For two human interventions, fisheries management and acid mitigation, effects on syndrome factors yield clear predictions for activities that can reduce MeHg accumulation in fish. These techniques have already been applied to lake ecosystems and merit further attention for reducing MeHg in stream fish. In contrast, hydrologic alteration and forest disturbance have complex and diverse effects on syndrome factors. As a result it is not currently possible to make clear, general prediction of their effects on MeHg concentrations in fish. Continued progress in elucidating the mechanisms underlying these influences should increase our ability to make predictions under specified ranges of environmental conditions. For example, a critical aspect of both hydrologic alteration and forest disturbance effects on fish MeHg is the relative importance of altered MeHg inputs (mobilization and methylation) compared to altered trophic transfer (stream productivity and fish growth rate). Evaluating these effects across a range of conditions will allow us to generate testable predictions on when they will predominate in determining MeHg accumulation in fish.
Looming in the background, and providing a context for all of the effects we have discussed, climate change represents an enormous potential driving force for MeHg in fish. Effects of warming are a particular concern because processes from methylation 102,172 to fish growth efficiency 173 respond to increased temperatures in ways that can increase MeHg accumulation in fish. In essence, the strong temperature dependence of respiration at the level of the ecosystem (which governs carbon dynamics) and at the level of individual physiology (which governs growth efficiency), may set the stage for long-term and widespread changes in both overall bioaccumulation, and in the way that syndrome factors interact to produce variation in Hg dynamics across the landscape.
ACKNOWLEDGEMENTS
Our work was funded by NIEHS-SBRP grant ES07373 and the USFS Northern Research Station. We thank Celia Chen, the staff of the Dartmouth Trace Element Analysis lab, and the Dartmouth Toxic Metals Research Program for their feedback and assistance with our mercury research. William Schlesinger and an anonymous reviewer provided helpful reviews on this manuscript.
REFERENCES
- 1.US EPA. 2008 Biennial national listing of fish advisories. Washington, DC: U.S. EPA; 2009 Technical report EPA-823-F-09-007.
- 2.Mergler D, et al. Methylmercury exposure and health effects in humans: A worldwide concern. Ambio. 2007;36:3–11. doi: 10.1579/0044-7447(2007)36[3:meahei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA-J. Am. Med. Assoc. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 4.Rees JR, Sturup S, Chen C, Folt C, Karagas MR. Toenail mercury and dietary fish consumption. J. Expo. Sci. Env. Epid. 2007;17:25–30. doi: 10.1038/sj.jes.7500516. [DOI] [PubMed] [Google Scholar]
- 5.Oken E, et al. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am. J. Epidemiol. 2008;167:1171–1181. doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oken E, et al. Maternal fish consumption, hair mercury, and infant cognition in a US cohort. Environ. Health Perspect. 2005;113:1376–1380. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schober SE, et al. Blood mercury levels in US children and women of childbearing age, 1999–2000. JAMA-J. Am. Med. Assoc. 2003;289:1667–1674. doi: 10.1001/jama.289.13.1667. [DOI] [PubMed] [Google Scholar]
- 8.Abdelouahab N, et al. Ecosystem matters: Fish consumption, mercury intake and exposure among fluvial lake fish-eaters. Sci. Total Environ. 2008;407:154–164. doi: 10.1016/j.scitotenv.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Hibbeln JR, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. Lancet. 2007;369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 10.Swain EB, et al. Socioeconomic consequences of mercury use and pollution. Ambio. 2007;36:45–61. doi: 10.1579/0044-7447(2007)36[45:scomua]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Caudill J. The economic effects of rainbow trout stocking by Fish and Wildlife Service hatcheries in FY 2004. Arlington, VA: U.S. Fish and Wildlife Service, Division of Economics; 2005. [Google Scholar]
- 12.Scheulhammer AM, Meyer MW, Sandheinrich MB, Murray MW. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio. 2007;36:12–18. doi: 10.1579/0044-7447(2007)36[12:eoemot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe MF, Schwarzbach S, Sulaiman RA. Effects of mercury on wildlife: A comprehensive review. Environ. Toxicol. Chem. 1998;17:146–160. [Google Scholar]
- 14.Meyer MW, Evers DC, Hartigan JJ, Rasmussen PS. Patterns of common loon (Gavia immer) mercury exposure, reproduction, and survival in Wisconsin, USA. Environ. Toxicol. Chem. 1998;17:184–190. [Google Scholar]
- 15.Evers DC, et al. Adverse effects from environmental mercury loads on breeding common loons. Ecotoxicology. 2008;17:69–81. doi: 10.1007/s10646-007-0168-7. [DOI] [PubMed] [Google Scholar]
- 16.Hammerschmidt CR, Sandheinrich MB, Wiener JG, Rada RG. Effects of dietary methylmercury on reproduction of fathead minnows. Environ. Sci. Technol. 2002;36:877–883. doi: 10.1021/es011120p. [DOI] [PubMed] [Google Scholar]
- 17.Folt CL, Chen CY, Pickhardt PC. Using plankton food web variables as indicators for the accumulation of toxic metals in fish. In: Wilson SH, Suk WA, editors. Biomarkers of environmentally associated disease: Technologies, concepts, and perspectives. Boca Raton: CRC Press/Lewis Publishers; 2002. pp. 287–306. [Google Scholar]
- 18.Wiener JG, et al. Mercury in soils, lakes, and fish in Voyageurs National Park (Minnesota): Importance of atmospheric deposition and ecosystem factors. Environ. Sci. Technol. 2006;40:6261–6268. doi: 10.1021/es060822h. [DOI] [PubMed] [Google Scholar]
- 19.Evers DC, et al. Biological mercury hotspots in the northeastern United States and southeastern Canada. Bioscience. 2007;57:29–43. [Google Scholar]
- 20.Scudder BC, et al. Mercury in fish, bed sediment, and water from streams across the United States, 1998–2005. U.S. Geological Survey Scientific Investigations Report 2009–5109. 2009 [Google Scholar]
- 21.Peterson SA, Van Sickle J, Herlihy AT, Hughes RM. Mercury concentration in fish from streams and rivers throughout the western united states. Environ. Sci. Technol. 2007;41:58–65. doi: 10.1021/es061070u. [DOI] [PubMed] [Google Scholar]
- 22.Chasar LC, Scudder BC, Stewart AR, Bell AH, Aiken GR. Mercury cycling in stream ecosystems. 3. Trophic dynamics and methylmercury bioaccumulation. Environ. Sci. Technol. 2009;43:2733–2739. doi: 10.1021/es8027567. [DOI] [PubMed] [Google Scholar]
- 23.Tsui MTK, Finlay JC, Nater EA. Mercury bioaccumulation in a stream network. Environ. Sci. Technol. 2009;43:7016–7022. doi: 10.1021/es901525w. [DOI] [PubMed] [Google Scholar]
- 24.Ward DM, Nislow KH, Chen CY, Folt CL. Rapid, efficient growth reduces mercury concentrations in stream-dwelling Atlantic salmon. Trans. Am. Fish. Soc. 2010;139:1–10. doi: 10.1577/T09-032.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason RP, Laporte JM, Andres S. Factors controlling the bioaccumulation of mercury, methylmercury, arsenic, selenium, and cadmium by freshwater invertebrates and fish. Arch. Environ. Contam. Toxicol. 2000;38:283–297. doi: 10.1007/s002449910038. [DOI] [PubMed] [Google Scholar]
- 26.Hill WR, Stewart AJ, Napolitano GE. Mercury speciation and bioaccumulation in lotic primary producers and primary consumers. Can. J. Fish. Aquat. Sci. 1996;53:812–819. [Google Scholar]
- 27.Jardine TD, et al. Water striders (family Gerridae): Mercury sentinels in small freshwater ecosystems. Environ. Pollut. 2005;134:165–171. doi: 10.1016/j.envpol.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Southworth GR, Turner RR, Peterson MJ, Bogle MA, Ryon MG. Response of mercury contamination in fish to decreased aqueous concentrations and loading of inorganic mercury in a small stream. Environ. Monit. Assess. 2000;63:481–494. [Google Scholar]
- 29.Burger J, et al. Use of Central stonerollers (Cyprinidae : Campostoma anomalum) from Tennessee as a bioindicator of metal contamination. Environ. Monit. Assess. 2005;110:171–184. doi: 10.1007/s10661-005-6689-8. [DOI] [PubMed] [Google Scholar]
- 30.Sackett DK, Aday DD, Rice JA, Cope WG. A statewide assessment of mercury dynamics in North Carolina water bodies and fish. Trans. Am. Fish. Soc. 2009;138:1328–1341. [Google Scholar]
- 31.Pennuto CM, Lane OP, Evers DC, Taylor RJ, Loukmas J. Mercury in the northern cray fish, Orconectes virilis (Hagen), in New England, USA. Ecotoxicology. 2005;14:149–162. doi: 10.1007/s10646-004-6266-x. [DOI] [PubMed] [Google Scholar]
- 32.Lake JL, Ryba SA, Serbst J, Brown CF, Gibson L. Mercury and stable isotopes of carbon and nitrogen in mink. Environ. Toxicol. Chem. 2007;26:2611–2619. doi: 10.1897/06-607.1. [DOI] [PubMed] [Google Scholar]
- 33.Allan JD, et al. Overfishing of inland waters. Bioscience. 2005;55:1041–1051. [Google Scholar]
- 34.Post JR, et al. Canada's recreational fisheries: The invisible collapse? Fisheries. 2002;27:6–17. [Google Scholar]
- 35.Baxter CV, Fausch KD, Saunders WC. Tangled webs: Reciprocal flows of invertebrate prey link streams and riparian zones. Freshwat. Biol. 2005;50:201–220. [Google Scholar]
- 36.Steinmetz J, Kohler SL, Soluk DA. Birds are overlooked top predators in aquatic food webs. Ecology. 2003;84:1324–1328. [Google Scholar]
- 37.Cristol DA, et al. The movement of aquatic mercury through terrestrial food webs. Science. 2008;320:335. doi: 10.1126/science.1154082. [DOI] [PubMed] [Google Scholar]
- 38.Lovett GM, et al. Year in Ecology and Conservation Biology 2009. Oxford: Blackwell Publishing; 2009. Effects of air pollution on ecosystems and biological diversity in the eastern United States; pp. 99–135. [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald WF, Engstrom DR, Mason RP, Nater EA. The case for atmospheric mercury contamination in remote areas. Environ. Sci. Technol. 1998;32:1–7. [Google Scholar]
- 40.Pacyna EG, Pacyna JM, Steenhuisen F, Wilson S. Global anthropogenic mercury emission inventory for 2000. Atmos. Environ. 2006;40:4048–4063. [Google Scholar]
- 41.Watras CJ, et al. Bioaccumulation of mercury in pelagic freshwater food webs. Sci. Total Environ. 1998;219:183–208. doi: 10.1016/s0048-9697(98)00228-9. [DOI] [PubMed] [Google Scholar]
- 42.Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD. Algal blooms reduce the uptake of toxic methylmercury in freshwater food webs. Proc. Natl. Acad. Sci. USA. 2002;99:4419–4423. doi: 10.1073/pnas.072531099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pickhardt PC, Fisher NS. Accumulation of inorganic and methylmercury by freshwater phytoplankton in two contrasting water bodies. Environ. Sci. Technol. 2007;41:125–131. doi: 10.1021/es060966w. [DOI] [PubMed] [Google Scholar]
- 44.Hall BD, Bodaly RA, Fudge RJP, Rudd JWM, Rosenberg DM. Food as the dominant pathway of methylmercury uptake by fish. Water Air Soil Poll. 1997;100:13–24. [Google Scholar]
- 45.Pickhardt PC, Stepanova M, Fisher NS. Contrasting uptake routes and tissue distributions of inorganic and methylmercury in mosquitofish (Gambusia affinis) and redear sunfish (Lepomis microlophus) Environ. Toxicol. Chem. 2006;25:2132–2142. doi: 10.1897/05-595r.1. [DOI] [PubMed] [Google Scholar]
- 46.Hammerschmidt CR, Fitzgerald WF. Methylmercury in freshwater fish linked to atmospheric mercury deposition. Environ. Sci. Technol. 2006;40:7764–7770. doi: 10.1021/es061480i. [DOI] [PubMed] [Google Scholar]
- 47.Eagles-Smith CA, Suchanek TH, Colwell AE, Anderson NL, Moyle PB. Changes in fish diets and food web mercury bioaccumulation induced by an invasive planktivorous fish. Ecol. Appl. 2008;18:A213–A226. doi: 10.1890/06-1415.1. [DOI] [PubMed] [Google Scholar]
- 48.Chen CY, Stemberger RS, Kamman NC, Mayes BM, Folt CL. Patterns of Hg bioaccumulation and transfer in aquatic food webs across multi-lake studies in the northeast US. Ecotoxicology. 2005;14:135–147. doi: 10.1007/s10646-004-6265-y. [DOI] [PubMed] [Google Scholar]
- 49.Wiener JG, Martini RE, Sheffy TB, Glass GE. Factors influencing mercury concentrations in walleyes in northern Wisconsin lakes. Trans. Am. Fish. Soc. 1990;119:862–870. [Google Scholar]
- 50.Driscoll CT, et al. Mercury contamination in forest and freshwater ecosystems in the Northeastern United States. BioScience. 2007;57:17–28. [Google Scholar]
- 51.Miller EK, et al. Estimation and mapping of wet and dry mercury deposition across northeastern North America. Ecotoxicology. 2005;14:53–70. doi: 10.1007/s10646-004-6259-9. [DOI] [PubMed] [Google Scholar]
- 52.Rea AW, Keeler GJ, Scherbatskoy T. The deposition of mercury in throughfall and litterfall in the Lake Champlain watershed: A short-term study. Atmos. Environ. 1996;30:3257–3263. [Google Scholar]
- 53.St Louis VL, et al. Importance of the forest canopy to fluxes of methyl mercury and total mercury to boreal ecosystems. Environ. Sci. Technol. 2001;35:3089–3098. doi: 10.1021/es001924p. [DOI] [PubMed] [Google Scholar]
- 54.Grigal DF. Inputs and outputs of mercury from terrestrial watersheds: A review. Environ. Rev. 2002;10:1–39. [Google Scholar]
- 55.Gilmour CC, Henry EA. Mercury methylation in aquatic systems affected by acid deposition. Environ. Pollut. 1991;71:131–169. doi: 10.1016/0269-7491(91)90031-q. [DOI] [PubMed] [Google Scholar]
- 56.Karimi R, Chen CY, Pickhardt PC, Fisher NS, Folt CL. Stoichiometric controls of mercury dilution by growth. Proc. Natl. Acad. Sci. USA. 2007;104:7477–7482. doi: 10.1073/pnas.0611261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen CY, Folt CL. High plankton densities reduce mercury biomagnification. Environ. Sci. Technol. 2005;39:115–121. [PubMed] [Google Scholar]
- 58.Luengen AC, Flegal AR. Role of phytoplankton in mercury cycling in the San Francisco Bay estuary. Limnol. Oceanogr. 2009;54:23–40. [Google Scholar]
- 59.Chen CY, Pickhardt PC, Xu MQ, Folt CL. Mercury and arsenic bioaccumulation and eutrophication in Baiyangdian Lake, China. Water Air Soil Pollut. 2008;190:115–127. doi: 10.1007/s11270-007-9585-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hogan LS, Marschall E, Folt C, Stein RA. How non-native species in Lake Erie influence trophic transfer of mercury and lead to top predators. J. Great Lakes Res. 2007;33:46–61. [Google Scholar]
- 61.Roue-Legall A, Lucotte M, Carreau J, Canuel R, Garcia E. Development of an ecosystem sensitivity model regarding mercury levels in fish using a preference modeling methodology: Application to the Canadian boreal system. Environ. Sci. Technol. 2005;39:9412–9423. doi: 10.1021/es048220q. [DOI] [PubMed] [Google Scholar]
- 62.Mailman M, Stepnuk L, Cicek N, Bodaly RA. Strategies to lower methyl mercury concentrations in hydroelectric reservoirs and lakes: A review. Sci. Total Environ. 2006;368:224–235. doi: 10.1016/j.scitotenv.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 63.Munthe J, et al. Recovery of mercury-contaminated fisheries. Ambio. 2007;36:33–44. doi: 10.1579/0044-7447(2007)36[33:romf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 64.Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980;37:130–137. [Google Scholar]
- 65.Newbold JD, Elwood JW, Oneill RV, Vanwinkle W. Measuring nutrient spiraling in streams. Can. J. Fish. Aquat. Sci. 1981;38:860–863. [Google Scholar]
- 66.Marvin-DiPasquale M, et al. Mercury cycling in stream ecosystems. 2. Benthic methylmercury production and bed sediment-pore water partitioning. Environ. Sci. Technol. 2009;43:2726–2732. doi: 10.1021/es802698v. [DOI] [PubMed] [Google Scholar]
- 67.Scherbatskoy T, Shanley JB, Keeler GJ. Factors controlling mercury transport in an upland forested catchment. Water Air Soil Poll. 1998;105:427–438. [Google Scholar]
- 68.Aastrup M, Johnson J, Bringmark E, Bringmark L, Iverfeldt A. Occurrence and transport of mercury within a small catchment area. Water Air Soil Poll. 1991;56:155–167. [Google Scholar]
- 69.Brigham ME, Wentz DA, Aiken GR, Krabbenhoft DP. Mercury cycling in stream ecosystems. 1. Water column chemistry and transport. Environ. Sci. Technol. 2009;43:2720–2725. doi: 10.1021/es802694n. [DOI] [PubMed] [Google Scholar]
- 70.Minshall GW. Role of allochthonous detritus in trophic structure of a woodland springbrook community. Ecology. 1967;48:139. [Google Scholar]
- 71.Finlay JC. Stable-carbon-isotope ratios of river biota: Implications for energy flow in lotic food webs. Ecology. 2001;82:1052–1064. [Google Scholar]
- 72.Lowe WH, Likens GE, Power ME. Linking scales in stream ecology. BioScience. 2006;56:591–597. [Google Scholar]
- 73.Folt CL, Nislow KH, Power ME. Implications of temporal and spatial scale for Atlantic salmon (Salmo salar) research. Can. J. Fish. Aquat. Sci. 1998;55:9–21. [Google Scholar]
- 74.Karimi R, Folt CL. Beyond macronutrients: Element variability and multielement stoichiometry in freshwater invertebrates. Ecol. Lett. 2006;9:1273–1283. doi: 10.1111/j.1461-0248.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 75.Trudel M, Rasmussen JB. Bioenergetics and mercury dynamics in fish: A modelling perspective. Can. J. Fish. Aquat. Sci. 2006;63:1890–1902. [Google Scholar]
- 76.Luoma SN, Rainbow PS. Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environ. Sci. Technol. 2005;39:1921–1931. doi: 10.1021/es048947e. [DOI] [PubMed] [Google Scholar]
- 77.Trudel M, Rasmussen JB. Modeling the elimination of mercury by fish. Environ. Sci.Technol. 1997;31:1716–1722. [Google Scholar]
- 78.Castro MS, Hilderbrand RH, Thompson J, Heft A, Rivers SE. Relationship between wetlands and mercury in brook trout. Arch. Environ. Contam. Toxicol. 2007;52:97–103. doi: 10.1007/s00244-006-0057-8. [DOI] [PubMed] [Google Scholar]
- 79.Trudel M, Rasmussen JB. Predicting mercury concentration in fish using mass balance models. Ecol. Appl. 2001;11:517–529. [Google Scholar]
- 80.Rennie MD, Collins NC, Shuter BJ, Rajotte JW, Couture P. A comparison of methods for estimating activity costs of wild fish populations: more active fish observed to grow slower. Can. J. Fish. Aquat. Sci. 2005;62:767–780. [Google Scholar]
- 81.Chipps SR, Wahl DH. Fish bioenergetics modeling in the 21st century: Reviewing new insights and revisiting old constraints. Trans. Am. Fish. Soc. 2008;137:298–313. [Google Scholar]
- 82.Mathews T, Fisher NS. Evaluating the trophic transfer of cadmium, polonium, and methylmercury in an estuarine food chain. Environ. Toxicol. Chem. 2008;27:1093–1101. doi: 10.1897/07-318.1. [DOI] [PubMed] [Google Scholar]
- 83.Kennedy BP, Nislow KH, Folt CL. Habitat-mediated foraging limitations drive survival bottlenecks for juvenile salmon. Ecology. 2008;89:2529–2541. doi: 10.1890/06-1353.1. [DOI] [PubMed] [Google Scholar]
- 84.Mac Nally R. Multiple regression and inference in ecology and conservation biology: Further comments on identifying important predictor variables. Biodivers. Conserv. 2002;11:1397–1401. [Google Scholar]
- 85.Simoneau M, Lucotte M, Garceau S, Laliberte D. Fish growth rates modulate mercury concentrations in walleye (Sander vitreus) from eastern Canadian lakes. Environ. Res. 2005;98:73–82. doi: 10.1016/j.envres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 86.Sabo JL, Finlay JC, Post DM. In Year in Ecology and Conservation Biology 2009. Oxford: Blackwell Publishing; 2009. Food chains in freshwaters; pp. 187–220. [DOI] [PubMed] [Google Scholar]
- 87.Cabana G, Rasmussen JB. Modeling food-chain structure and contaminant bioaccumulation using stable nitrogen isotopes. Nature. 1994;372:255–257. [Google Scholar]
- 88.Peterson SA, Herlihy AT, Hughes RM, Motter KL, Robbins JM. Level and extent of mercury contamination in Oregon, USA, lotic fish. Environ. Toxicol. Chem. 2002;21:2157–2164. [PubMed] [Google Scholar]
- 89.Lepak JM, Robinson JM, Kraft CE, Josephson DC. Changes in mercury bioaccumulation in an apex predator in response to removal of an introduced competitor. Ecotoxicology. 2009;18:488–498. doi: 10.1007/s10646-009-0306-5. [DOI] [PubMed] [Google Scholar]
- 90.Hill WR, Larsen IL. Growth dilution of metals in microalgal biofilms. Environ. Sci. Technol. 2005;39:1513–1518. doi: 10.1021/es049587y. [DOI] [PubMed] [Google Scholar]
- 91.Bell AH, Scudder BC. Mercury accumulation in periphyton of eight river ecosystems. J. Am. Water Resour. Ass. 2007;43:957–968. [Google Scholar]
- 92.Desrosiers M, Planas D, Mucci A. Total mercury and methylmercury accumulation in periphyton of Boreal Shield Lakes: Influence of watershed physiographic characteristics. Sci. Total Environ. 2006;355:247–258. doi: 10.1016/j.scitotenv.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 93.Herendeen RA, Hill WR. Growth dilution in multilevel food chains. Ecol. Model. 2004;178:349–356. [Google Scholar]
- 94.Chen CY, Folt CL. High plankton abundance reduces trophic transfer of mercury. SETAC Globe 2005. 2005 [Google Scholar]
- 95.Torres-Ruiz M, Wehr JD, Perrone AA. Trophic relations in a stream food web: Importance of fatty acids for macroinvertebrate consumers. J. N. Am. Benthol. Soc. 2007;26:509–522. [Google Scholar]
- 96.Orpwood JE, Griffiths SW, Armstrong JD. Effects of food availability on temporal activity patterns and growth of Atlantic salmon. J. Anim. Ecol. 2006;75:677–685. doi: 10.1111/j.1365-2656.2006.01088.x. [DOI] [PubMed] [Google Scholar]
- 97.Cremona F, Hamelin S, Planas D, Lucotte M. Sources of organic matter and methylmercury in littoral macroinvertebrates: A stable isotope approach. Biogeochemistry. 2009;94:81–94. [Google Scholar]
- 98.Snyder CD, Hendricks AC. Effect of seasonally changing feeding habits on whole animal mercury concentrations in Hydropsyche morosa (Trichoptera, Hydropsychidae) Hydrobiologia. 1995;299:115–123. [Google Scholar]
- 99.Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD. Impacts of zooplankton composition and algal enrichment on the accumulation of mercury in an experimental freshwater food web. Sci. Total Environ. 2005;339:89–101. doi: 10.1016/j.scitotenv.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 100.Schuster PF, et al. Mercury and organic carbon dynamics during runoff episodes from a northeastern USA watershed. Water, Air, Soil, Pollut. 2008;187:89–108. [Google Scholar]
- 101.Stoor RW, Hurley JP, Babiarz CL, Armstrong DE. Subsurface sources of methyl mercury to Lake Superior from a wetland-forested watershed. Sci. Total Environ. 2006;368:99–110. doi: 10.1016/j.scitotenv.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 102.Desrosiers M, Planas D, Mucci A. Mercury methylation in the epilithon of boreal shield aquatic ecosystems. Environ. Sci. Technol. 2006;40:1540–1546. doi: 10.1021/es0508828. [DOI] [PubMed] [Google Scholar]
- 103.Harding KM, Gowland JA, Dillon PJ. Mercury concentration in black flies Simulium spp. (Diptera, Simuliidae) from soft-water streams in Ontario, Canada. Environ. Pollut. 2006;143:529–535. doi: 10.1016/j.envpol.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 104.Jeremiason JD, et al. Sulfate addition increases methylmercury production in an experimental wetland. Environ. Sci. Technol. 2006;40:3800–3806. doi: 10.1021/es0524144. [DOI] [PubMed] [Google Scholar]
- 105.Rosemond AD, Reice SR, Elwood JW, Mulholland PJ. The effects of stream acidity on benthic invertebrate communities in the south-eastern United States. Freshwat. Biol. 1992;27:193–209. [Google Scholar]
- 106.Courtney LA, Clements WH. Effects of acidic pH on benthic macroinvertebrate communities in stream microcosms. Hydrobiologia. 1998;379:135–145. [Google Scholar]
- 107.Ward DM, Nislow KH, Folt CL. Increased population density and suppressed prey biomass: Relative impacts on juvenile Atlantic salmon growth. Trans. Am. Fish. Soc. 2009;138:135–143. [Google Scholar]
- 108.Magee JA, Obedzinski M, McCormick SD, Kocik JF. Effects of episodic acidification on Atlantic salmon (Salmo salar) smolts. Can. J. Fish. Aquat. Sci. 2003;60:214–221. [Google Scholar]
- 109.Gorski PR, Armstrong DE, Hurley JP, Krabbenhoft DP. Influence of natural dissolved organic carbon on the bioavailability of mercury to a freshwater alga. Environ. Pollut. 2008;154:116–123. doi: 10.1016/j.envpol.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 110.Kolka RK, Nater EA, Grigal DF, Verry ES. Atmospheric inputs of mercury and organic carbon into a forested upland bog watershed. Water Air and Soil Pollution. 1999;113:273–294. [Google Scholar]
- 111.Goodale CL, Aber JD, McDowell WH. The long-term effects of disturbance on organic and inorganic nitrogen export in the White Mountains, New Hampshire. Ecosystems. 2000;3:433–450. [Google Scholar]
- 112.Kreutzweiser DP, Hazlett PW, Gunn JM. Logging impacts on the biogeochemistry of boreal forest soils and nutrient export to aquatic systems: A review. Environ. Rev. 2008;16:157–179. [Google Scholar]
- 113.Likens GE, et al. The biogeochemistry of calcium at Hubbard Brook. Biogeochemistry. 1998;41:89–173. [Google Scholar]
- 114.Nislow KH. Forest change and stream fish habitat: Lessons from 'Olde' and New England. J. Fish Biol. 2005;67 Supplement B:186–204. [Google Scholar]
- 115.St Louis VL, et al. Importance of wetlands as sources of methyl mercury to boreal forest ecosystems. Can. J. Fish. Aquat. Sci. 1994;51:1065–1076. [Google Scholar]
- 116.Johnston CA, et al. Wetland types and wetland maps differ in ability to predict dissolved organic carbon concentrations in streams. Sci. Total Environ. 2008;404:326–334. doi: 10.1016/j.scitotenv.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 117.Grigal DF. Mercury sequestration in forests and peatlands: A review. J. Environ. Qual. 2003;32:393–405. doi: 10.2134/jeq2003.3930. [DOI] [PubMed] [Google Scholar]
- 118.Shanley JB, et al. Comparison of total mercury and methylmercury cycling at five sites using the small watershed approach. Environ. Pollut. 2008;154:143–154. doi: 10.1016/j.envpol.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 119.De Wit HA, Mulder J, Hindar A, Hole L. Long-term increase in dissolved organic carbon in streamwaters in Norway is response to reduced acid deposition. Environ. Sci. Technol. 2007;41:7706–7713. doi: 10.1021/es070557f. [DOI] [PubMed] [Google Scholar]
- 120.Monteith DT, et al. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature. 2007;450:537-U9. doi: 10.1038/nature06316. [DOI] [PubMed] [Google Scholar]
- 121.Evans C, et al. Does elevated nitrogen deposition or ecosystem recovery from acidification drive increased dissolved organic carbon loss from upland soil? A review of evidence from field nitrogen addition experiments. Biogeochemistry. 2008;91:13–35. [Google Scholar]
- 122.Selvendiran P, Driscoll CT, Montesdeoca MR, Bushey JT. Inputs, storage, and transport of total and methyl mercury in two temperate forest wetlands. J. Geophys. Res.-Biogeo. 2008;113 [Google Scholar]
- 123.Craig LS, et al. Stream restoration strategies for reducing river nitrogen loads. Front. Ecol. Environ. 2008;6:529–538. [Google Scholar]
- 124.Rypel AL, Arrington DA, Findlay RH. Mercury in southeastern US riverine fish populations linked to water body type. Environ. Sci. Technol. 2008;42:5118–5124. doi: 10.1021/es8001772. [DOI] [PubMed] [Google Scholar]
- 125.Bernhardt ES, Band LE, Walsh CJ, Berke PE. Year in Ecology and Conservation Biology 2008. Oxford: Blackwell Publishing; 2008. Understanding, managing, and minimizing urban impacts on surface water nitrogen loading; pp. 61–96. [DOI] [PubMed] [Google Scholar]
- 126.Harris RC, et al. Whole-ecosystem study shows rapid fish-mercury response to changes in mercury deposition. P Natl Acad Sci USA. 2007;104:16586–16591. doi: 10.1073/pnas.0704186104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gorham E, et al. The chemistry of streams in southwestern and central Nova Scotia, with particular reference to catchment vegetation and the influence of dissolved organic carbon primarily from wetlands. Wetlands. 1998;18:115–132. [Google Scholar]
- 128.Essington TE, Houser JN. The effect of whole-lake nutrient enrichment on mercury concentration in age-1 yellow perch. Trans. Am. Fish. Soc. 2003;132:57–68. [Google Scholar]
- 129.Surette C, Lucotte M, Doire J, Tremblay A. Mercury bioaccumulation in fish: Effects of intensive fishing in three natural lakes of Northern Quebec, Canada. J. Phys. IV. 2003;107:1443–1443. [Google Scholar]
- 130.Bernhardt ES, Palmer MA. Restoring streams in an urbanizing world. Freshwat. Biol. 2007;52:738–751. [Google Scholar]
- 131.Bernhardt ES, et al. Synthesizing US river restoration efforts. Science. 2005;308:636–637. doi: 10.1126/science.1109769. [DOI] [PubMed] [Google Scholar]
- 132.Malmqvist B, Rundle S. Threats to the running water ecosystems of the world. Environ. Conserv. 2002;29:134–153. [Google Scholar]
- 133.Rahel FJ. Homogenization of fish faunas across the United States. Science. 2000;288:854–856. doi: 10.1126/science.288.5467.854. [DOI] [PubMed] [Google Scholar]
- 134.Eby LA, Roach WJ, Crowder LB, Stanford JA. Effects of stocking-up freshwater food webs. Trends Ecol. Evol. 2006;21:576–584. doi: 10.1016/j.tree.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 135.Rosenberg DM, McCully P, Pringle CM. Global-scale environmental effects of hydrological alterations: Introduction. Bioscience. 2000;50:746–751. [Google Scholar]
- 136.Schetagne R, Doyon JF, Fournier JJ. Export of mercury downstream from reservoirs. Sci. Total Environ. 2000;260:135–145. doi: 10.1016/s0048-9697(00)00557-x. [DOI] [PubMed] [Google Scholar]
- 137.Harrison JA, et al. The regional and global significance of nitrogen removal in lakes and reservoirs. Biogeochemistry. 2009;93:143–157. [Google Scholar]
- 138.Hart DD, et al. Dam removal: Challenges and opportunities for ecological research and river restoration. Bioscience. 2002;52:669–681. [Google Scholar]
- 139.Stanley EH, Doyle MW. Trading off: The ecological removal effects of dam removal. Front. Ecol. Environ. 2003;1:15–22. [Google Scholar]
- 140.Magilligan FJ, Nislow KH, Graber BE. Scale-independent assessment of discharge reduction and riparian disconnectivity following flow regulation by dams. Geology. 2003;31:569–572. [Google Scholar]
- 141.Houghton RA. The worldwide extent of land use change. BioScience. 1994;44:305–313. [Google Scholar]
- 142.Porvari P, Verta M, Munthe J, Haapanen M. Forestry practices increase mercury and methyl mercury output from boreal forest catchments. Environ. Sci. Technol. 2003;37:2389–2393. doi: 10.1021/es0340174. [DOI] [PubMed] [Google Scholar]
- 143.Garcia E, Carignan R. Mercury concentrations in fish from forest harvesting and fire-impacted Canadian boreal lakes compared using stable isotopes of nitrogen. Environ. Toxicol. Chem. 2005;24:685–693. doi: 10.1897/04-065r.1. [DOI] [PubMed] [Google Scholar]
- 144.Gurtz ME, Wallace JB. Substrate-mediated response of stream invertebrates to disturbance. Ecology. 1984;65:1556–1569. [Google Scholar]
- 145.Kiffney PM, Richardson JS, Bull JP. Responses of periphyton and insects to experimental manipulation of riparian buffer width along forest streams. J. Appl. Ecol. 2003;40:1060–1076. [Google Scholar]
- 146.Nislow KH, Lowe WH. Influences of logging history and riparian forest characteristics on macroinvertebrates and brook trout (Salvelinus fontinalis) in headwater streams (New Hampshire, USA) Freshwat. Biol. 2006;51:388–397. [Google Scholar]
- 147.Suttle KB, Power ME, Levine JM, McNeely C. How fine sediment in riverbeds impairs growth and survival of juvenile salmonids. Ecol. Appl. 2004;14:969–974. [Google Scholar]
- 148.Witt EL, Kolka RK, Nater EA, Wickman TR. Influence of the forest canopy on total and methyl mercury deposition in the boreal forest. Water Air Soil Poll. 2009;199:3–11. [Google Scholar]
- 149.Lowrance R. Riparian forest ecosystems as filters. In: Pace ML, Groffman P, editors. Successes, limitations and frontiers in ecosystem science. New York: Springer; 1998. pp. 113–164. [Google Scholar]
- 150.Barling RD, Moore ID. Role of buffer strips in management of waterway pollution: A review. Environ. Manage. 1994;18:543–558. [Google Scholar]
- 151.Attiwill PM. The disturbance of forest ecosystems: the ecological basis for conservative management. For. Ecol. Manage. 1994;63:247–300. [Google Scholar]
- 152.Witt EL, Kolka RK, Nater EA, Wickman TR. Forest fire effects on mercury deposition in the boreal forest. Environ. Sci. Technol. 2009;43:1776–1782. doi: 10.1021/es802634y. [DOI] [PubMed] [Google Scholar]
- 153.Amirbahman A, Ruck PL, Fernandez IJ, Haines TA, Kahl JS. The effect of fire on mercury cycling in the soils of forested watersheds: Acadia National Park, Maine, USA. Water Air Soil Poll. 2004;152:313–331. [Google Scholar]
- 154.Kelly EN, Schindler DW, St. Louis VL, Donald DB, Vladicka KE. Forest fire increases mercury accumulation by fishes via food web restructuring and increased mercury inputs. P. Natl. Acad. Sci. USA. 2006;103:19380–19385. doi: 10.1073/pnas.0609798104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bank MS, Loftin CS, Jung RE. Mercury bioaccumulation in northern two-lined salamanders from streams in the northeastern United States. Ecotoxicology. 2005;14:181–191. doi: 10.1007/s10646-004-6268-8. [DOI] [PubMed] [Google Scholar]
- 156.Minshall GW. Responses of stream benthic macroinvertebrates to fire. For. Ecol. Manage. 2003;178:155–161. [Google Scholar]
- 157.Mellon CD, Wipfli MS, Li JL. Effects of forest fire on headwater stream macroinvertebrate communities in eastern Washington, USA. Freshwat. Biol. 2008;53:2331–2343. [Google Scholar]
- 158.Allen EW, Prepas EE, Gabos S, Strachan WMJ, Zhang WP. Methyl mercury concentrations in macroinvertebrates and fish from burned and undisturbed lakes on the boreal plain. Can. J. Fish. Aquat. Sci. 2005;62:1963–1977. [Google Scholar]
- 159.Tiedemann AR, Klemmedson JO, Bull EL. Solution of forest health problems with prescribed fire: Are forest productivity and wildlife at risk? For. Ecol. Manage. 2000;127:1–18. [Google Scholar]
- 160.Fernandes PM, Botelho HS. A review of prescribed burning effectiveness in fire hazard reduction. Int. J. Wildland Fire. 2003;12:117–128. [Google Scholar]
- 161.Wickman T, et al. Influence of prescribed fire on the re-mobilization of mercury in the Boundary Waters Canoe Area Wilderness: Update. 2007 International Lake of the Woods Water Quality Forum, International Falls, MN. 2007 [Google Scholar]
- 162.Grant JWA, Imre I. Patterns of density-dependent growth in juvenile stream-dwelling salmonids. J. Fish Biol. 2005;67 Supplement B:100–110. [Google Scholar]
- 163.Bohlin T, Sundstrom LF, Johnsson JI, Hojesjo J, Pettersson J. Density-dependent growth in brown trout: Effects of introducing wild and hatchery fish. J. Anim. Ecol. 2002;71:683–692. [Google Scholar]
- 164.Stow CA, Carpenter SR, Madenjian CP, Eby LA, Jackson LJ. Fisheries management to reduce contaminant consumption. BioScience. 1995;45:752–758. [Google Scholar]
- 165.Verta M. Changes in fish mercury concentrations in an intensively fished lake. Can. J. Fish. Aquat. Sci. 1990;47:1888–1897. [Google Scholar]
- 166.Gothberg A. Intensive fishing: a way to reduce the mercury level in fish. Ambio. 1983;12:259–261. [Google Scholar]
- 167.Driscoll CT, Driscoll KM, Roy KM, Mitchell MJ. Chemical response of lakes in the Adirondack Region of New York to declines in acidic deposition. Environ. Sci. Technol. 2003;37:2036–2042. doi: 10.1021/es020924h. [DOI] [PubMed] [Google Scholar]
- 168.Clair TA, Dennis IF, Cosby BJ. Probable changes in lake chemistry in Canada's Atlantic Provinces under proposed North American emission reductions. Hydrol. Earth Syst. Sci. 2003;7:574–582. [Google Scholar]
- 169.Clair TA, Hindar A. Liming for the mitigation of acid rain effects in freshwaters: A review of recent results. Environ. Rev. 2005;13:91–128. [Google Scholar]
- 170.Lindqvist O, et al. Mercury in the Swedish environment: Recent research on causes, consequences and corrective methods. Water Air Soil Poll. 1991;55 R11-&. [Google Scholar]
- 171.Andersson P, Borg H, Karrhage P. Mercury in fish muscle in acidified and limed lakes. Water Air Soil Poll. 1995;80:889–892. [Google Scholar]
- 172.Benoit JM, Gilmour CC, Heyes A, Mason RP, Miller CL. Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. Biogeochemistry of Environmentally Important Trace Elements. 2003:262–297. [Google Scholar]
- 173.Elliott JM. Energetics of feeding, metabolism and growth of brown trout (Salmo trutta L.) in relation to body weight, water temperature and ration size. J. Anim. Ecol. 1976;45:923–948. [Google Scholar]


