Abstract
Several reports indicate that autism spectrum disorder (ASD) is associated with increased rate of head growth in early childhood. Increased rate of growth may index aberrant processes during early development, may precede the onset of symptoms, and may predict severity of the disease course. We examined rate of change in occipitofrontal circumference measurements (abstracted from medical records) in 28 boys with ASD and in 8 boys with developmental delay without autism from birth to age 36 months. Only children who had more than 3 occipitofrontal circumference measurements available during this age period were included. All data were converted to z scores based on the Centers for Disease Control and Prevention norms. Rate of growth from birth to age 36 months was statistically significantly higher for the ASD group than the developmental delay group, with children with ASD showing a statistically significant increase in occipitofrontal circumference relative to norms between 7 and 10 months; this group difference in rate of growth was more robust when height was used as a covariate. Rate of growth was not found to be different for children with ASD whose parents reported a history of loss of skills (regression) vs those whose parents reported early onset of autism symptoms. Findings from this study suggest that the aberrant growth is present in the first year of life and precedes the onset of diagnosis in children with ASD with and without a history of autistic regression.
Keywords: autism, head circumference, growth
Autism spectrum disorder (ASD) is a category of neurodevelopmental disorders characterized by impairments in social relationships and communication and by the presence of repetitive interests and stereotyped behaviors. Basic impairments, such as lack of attention to others and failure to orient to name, often appear within the first year of life.1 Using retrospective investigations, other subtle impairments in the domains of motor, sensory perception, attention, and social behaviors are present in the first year.1,2 In a study of high-risk infants, Zwaigenbaum et al3 found that by 12 months of age infants who later were diagnosed as having ASD differed from nonrisk infants in terms of their eye contact, failure to orient to name, impoverished social interest and affect, atypical visual attention and temperament, and delayed language development. By preschool age, behavioral symptoms of ASD include impairments in social orienting, eye contact, joint attention, imitation, responses to the emotional displays of others, and face recognition.4–8 Although the social behavior of individuals with ASD has been well characterized, the basic neurobiological mechanisms underlying these impairments are poorly understood.
Neuroimaging studies9,10 of children with ASD revealed abnormalities in the cerebral cortex, medial temporal lobe, and cerebellum. These abnormalities may have their origins in early prenatal life.11 In a study of children with ASD who were 3 to 4 years of age vs children with developmental delay and typical development, Sparks et al12 found increased total cerebral volume and proportionally large amygdala volume in the children with ASD. Findings suggest increased volume early in development but slowed growth of the frontal (white and gray matter), temporal (white and gray matter), and parietal (white matter) regions in children with ASD compared with control subjects.13
This atypical pattern of early enlargement in brain size can also be found using occipitofrontal circumference measurements.14–16 During early life, occipitofrontal circumference measures correlate with gray matter volumes observed in magnetic resonance imaging investigations at 2 to 3 years of age and may be a good early proxy for brain volume.17,18 At older ages, occipitofrontal circumference is no longer as strongly correlated with brain volume.19,20 At birth, children who are later diagnosed as having ASD have been shown to have normal to small occipitofrontal circumference. In a sample of 42 children with ASD, the mean ± SD occipitofrontal circumference percentile at birth was 48% ± 29%.14 In a meta-analysis by Redcay and Courschesne,20 birth occipitofrontal circumference was 13% smaller in children with ASD than in controls. The meta-analysis included birth samples from 3 studies, each of which suggested variability in head size at birth. For example, Gillberg and de Souza21 found that of 10 of 13 children with Asperger syndrome who were macrocephalic after 16 months of age had been macrocephalic at birth; 4 of 11 children with autism had been macrocephalic at birth. In contrast, Courschesne et al15 reported that the mean occipitofrontal circumference z score for children with ASD in their sample was between −0.66 (15 children with birth and longitudinal data) and −0.41 (33 children with a measurement at birth). Finally, Lainhart et al16 found that the mean birth occipitofrontal circumference of 8 girls with ASD was greater than the comparison mean, while the mean birth occipitofrontal circumference of 37 boys did not differ from the mean. Only 2 of 9 children who were later identified as macrocephalic had been macrocephalic at birth.16
Torrey and colleagues22 found that 15 children with ASD identified through records from the National Collaborative Perinatal Project had slightly but not statistically significantly larger occipitofrontal circumferences at birth and no difference at age 4 months compared with approximately 40 000 children who did not have ASD. In this sample, body weight and length were larger in the group with ASD.17,23–25 Using a population screen of all Swedish children born between 1974 and 1993 (Swedish Inpatient Register), Hultman et al26 found that ASD was associated with pregnancy, delivery, and maternal variables but not with atypical occipitofrontal circumference at birth. However, this sample only reflects children who received inpatient care.
Studies14,16,21,23,27–33 that evaluated individuals with ASD across a large age span report that 10% to 30% of individuals with ASD are macroencephalic. There is some suggestion that the rate of macrocephaly in ASD is elevated or is similar to that of other developmental disorders such as attention-deficit/hyperactivity disorder,30 tuberous sclerosis, seizure disorder,28 and other developmental disabilities.34 Of note, the rate of macrocephaly in first-degree relatives of individuals with ASD is also elevated.28,31
More recently, researchers have examined age-related differences in occipitofrontal circumference in ASD. In a study by Lainhart et al16 among a subsample of 11 participants with ASD with multiple data points between birth and age 3 years, 6 had typical rates of growth, 4 had slower rates of growth, and 1 had an increased rate of growth. In another sample of individuals with ASD, occipitofrontal circumference z scores were found to increase from −0.66 at birth to 0.18 at age 3 to 5 months and to 1.01 at age 6 to 14 months.15 This represented a z score increase of 1.67 between birth and age 6 to 14 months. Furthermore, Dementieva et al14 found in a subsample of 17 children who had measurements at birth that 11 had a 25-percentage point or greater increase in occipitofrontal circumference between 2 consecutive time points. In 15 children who had 4 measurements across the first year of life, 11 children had an increase of 25 percentage points or more between time point 1 (birth to age 1 month) and time point 2 (age 1 month to age 2 months).
Results are unclear with respect to the extent to which accelerated head growth is correlated with variations in symptom severity. Dementieva et al14 found accelerated head growth associated with higher levels of adaptive functioning on the Vineland Adaptive Behavioral Scales. In another study,16 increased occipitofrontal circumference was associated with less severe core features of ASD in participants who had large occipitofrontal circumference for age and sex. In contrast, Courchesne et al15 found that increased occipitofrontal circumference was associated more severe stereotyped and repetitive behavior, later onset of first word, and higher likelihood of an autism diagnosis, compared with the milder diagnosis of pervasive developmental disorder-not otherwise specified. Occipitofrontal circumference was also found to be correlated with discrepancies in nonverbal IQ and verbal IQ. In a study27 of children with ASD aged 4 to 14 years, larger occipitofrontal circumference was associated with higher nonverbal IQ scores than verbal IQ scores.
As part of a larger National Institutes of Health-funded longitudinal study of children with ASD and children with idiopathic developmental delay (without autism), we examined the rate of change in occipitofrontal circumference in boys from birth to age 36 months. To be included in the analysis, children had to have 3 or more occipitofrontal circumference measurements in their medical records between birth and 36 months of age. In addition to occipitofrontal circumference, we collected concurrent height measurements from medical records to take into account overall body growth. Furthermore, for the group with ASD, we collected information from parents regarding the presence of early loss of skills (autistic regression). We also examined whether the pattern of occipitofrontal circumference was associated with levels of symptom severity as assessed by diagnostic status at 3 years of age. This study focused on a restricted age range (birth to 3-year developmental period), narrow age bins, and boys with ASD to reduce the heterogeneity in the sample. We included an aged-matched sample of boys with early developmental delay to examine the specificity of accelerated rate of growth in children with ASD.
Methods
Participants
Children were diagnosed at 3 to 4 years of age by the diagnostic team of the University of Washington Autism Center’s research program, which includes clinical psychologists and advanced graduate students in clinical psychology. Children with identifiable genetic syndromes (eg, Down syndrome, phenylketonuria, Williams syndrome, and fragile X syndrome), premature birth, cerebral palsy, sensory impairment, and known neurological disease or head trauma were excluded. Clinicians administered the Autism Diagnostic Interview-Revised35 and the Autism Diagnostic Observation Schedule-Generic.36 Both instruments assess the symptoms of autistic disorder listed in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV).37 In addition, clinicians made a separate clinical judgment of diagnosis based on the presence or absence of autism symptoms as defined in the DSM-IV. Diagnosis of autism disorder (autism) was defined as meeting criteria for autistic disorder on the Autism Diagnostic Observation Schedule-Generic and on the Autism Diagnostic Interview-Revised (within 2 points) and meeting DSM-IV criteria for autistic disorder based on clinical judgment. Diagnosis of pervasive developmental disorder-not otherwise specified was defined as meeting criteria for ASD on the Autism Diagnostic Observation Schedule-Generic, meeting criteria for autistic disorder on the Autism Diagnostic Interview-Revised (within 2 points), or at least meeting DSM-IV criteria for pervasive developmental disorder-not otherwise specified based on clinical judgment. For most analyses, the pervasive developmental disorder-not otherwise specified and autism groups were combined to form the ASD group.
Children with developmental delay had a diagnosis of idiopathic developmental delay based on a multidisciplinary diagnostic evaluation. To be included in the developmental delay group, the child demonstrated delays in 3 of the following 4 areas: verbal abilities, nonverbal problem solving, motor milestones, and adaptive behavior. Additional exclusionary criteria included the characteristics already listed for the autism group, as well as neurological disorder associated with autism (eg, Norrie syndrome, neurofibromatosis, and tuberous sclerosis), major physical abnormality (eg, cranial or facial deformity), or a sibling with ASD. Furthermore, the child did not meet the criteria for ASD as already defined.
As part of the participation in a larger longitudinal study, medical records were solicited. Twenty-eight boys with ASD (18 with autism and 10 with pervasive developmental disorder-not otherwise specified) and 8 boys with developmental delay were included.
Regression Status
Developmental regression was assessed via parent report of a history of loss of skills (regression) on the Autism Diagnostic Interview-Revised. Children classified as regressed were those who had received a score of 2 (definite) on at least 1 of the following 3 Autism Diagnostic Interview-Revised items: loss of spontaneous meaningful communicative speech at some level, loss of words used spontaneously but without clear communicative intent, and loss of skills in areas other than language development before age 5 years. Of 28 boys with ASD, 11 were classified as regressed (8 were diagnosed as having autism and 3 were diagnosed as having pervasive developmental disorder-not otherwise specified), and 17 had early-onset ASD.
Occipitofrontal Cortex Head Circumference and Body Length
Occipitofrontal circumference and body length were abstracted from medical records for each child. We attempted to reduce potential errors by dropping measurements that were clearly inaccurate or were grossly inconsistent with previous measurements; only 2 of 197 measurements were dropped. To compare the occipitofrontal circumference growth rates for children measured at varying ages from birth to 3 years of age, occipitofrontal circumference and height were transformed to z scores using the 2000 Centers for Disease Control and Prevention growth charts normative data for sex- and age-matched children,38 developed by the National Center for Health Statistics.
Occipitofrontal Circumference and Height
To determine whether occipitofrontal circumference was correlated with the overall body growth of the child, the relation between height and occipitofrontal circumference was examined. To adequately compare the ASD group and the developmental delay group, we pooled measurements in increments of age, resulting in the following 7 bins from birth to age 3 years: bin 1 (birth to age 0.99 months), bin 2 (age 1–3.99 months), bin 3 (age 4–6.99 months), bin 4 (age 7–9.99 months), bin 5 (age 10–12.99 months), bin 6 (age 13–21.99 months), and bin 7 (age 22–36 months). In the event that a child had 2 or more observations in the bin for a given period, the measurements were averaged such that each child contributed no more than 1 value per bin in time.
Macrocephalus and Microcephalus
Macrocephalus was diagnosed when the occipitofrontal circumference z score was greater than 1.88. Microcephalus was considered to be present in any child whose occipitofrontal circumference after age 16 months was more than 2 SDs below the Centers for Disease Control and Prevention normative values.
Comparison From Birth to Median Age of 10 Months
Participants for whom birth occipitofrontal circumference records (within the age range of 0–0.99 months) were valid and who had a valid occipitofrontal circumference measurement between age 6 to 14 months were selected for an analysis in which we replicated methods used by Courchesne et al.15 One value for each participant was chosen from each of the 2 bins, the value closest to birth and the value closest to age 10 months (median, 6–14 months age bin).
Results
All data are presented as occipitofrontal circumference z scores.
Occipitofrontal Circumference and Height
For the ASD group, there were 195 occipitofrontal circumference data points for 28 boys. The mean ± SD occipitofrontal circumference z score across all samples was 0.52 ± 1.10, and the mean ± SD height was 0.53 ± 1.20. For the developmental delay group, there were 61 occipitofrontal circumference data points for 8 boys. The mean ± SD occipitofrontal circumference was −0.75 ± 1.10, and the mean ± SD height was −0.65 ± 1.60.
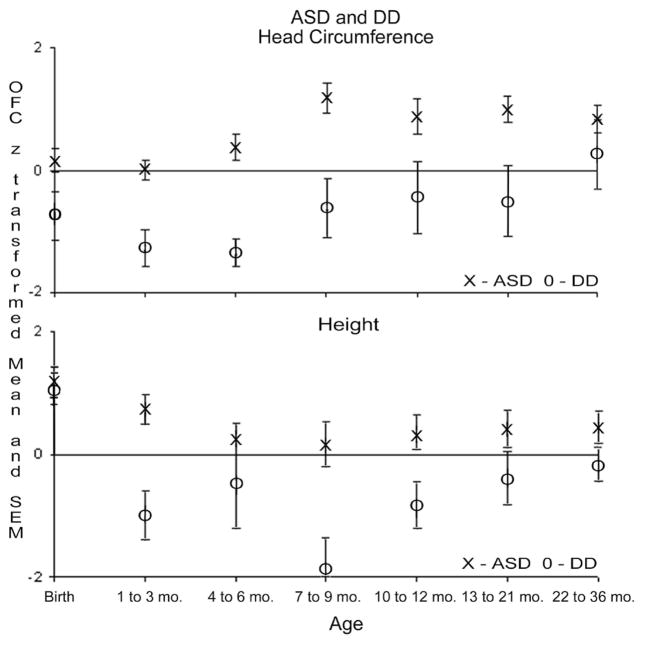
Seven bins from birth to age 3 years were used to compare the ASD and developmental delay groups with the normative Centers for Disease Control and Prevention values. To control for multiple comparisons, Bonferroni adjustments were applied to each set of 7 age-based occipitofrontal circumference scores. Therefore, the threshold for statistical significance became P = .007. Occipitofrontal circumference z scores in the ASD group did not differ from the normative value of zero in 3 bins from birth to age 6.99 months (t < 1.84, statistically nonsignificant). However, by age 7 to 9.99 months, the ASD group had a mean occipitofrontal circumference that was statistically significantly larger than the norms (t = 4.80, P < .001) and remained larger from age 13 through 36 months (t > 3.74, P δ .007). Because of somewhat larger variance, the scores from age 10 to 12.99 months did not achieve statistical significance but were of similar magnitude to the other elevated age bins. As summarized in Table 1, boys with ASD demonstrated a mean z-score increase in occipitofrontal circumference of 0.81 between age 4 to 6.99 months and age 7 to 9.99 months. The differences in the mean occipitofrontal circumference as a function of age are shown in Figure 1.
Table 1.
z Scores for Occipitofrontal Circumference (OFC) and Height for Children With Autism Spectrum Disorder (ASD) vs Developmental Delaya
| Age, mo | ASD |
DD |
ASD vs Norms t Value | DD vs Norms t Value | ASD vs DD z score | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Mean | SEM | No. | Mean | SEM | ||||
| OFC | |||||||||
| 0–0.99 | 22 | 0.16 | 0.19 | 5 | −0.75 | 0.40 | 0.83 | −1.86 | 2.00 |
| 1–3.99 | 24 | 0.00 | 0.17 | 7 | −1.29 | 0.30 | 0.02 | −4.24a | 2.98a |
| 4–6.99 | 25 | 0.38 | 0.21 | 7 | −1.36 | 0.23 | 1.84 | −5.91a | 3.49a |
| 7–9.99 | 15 | 1.19 | 0.25 | 4 | −0.62 | 0.50 | 4.80a | −1.25 | 2.50 |
| 10–12.99 | 17 | 0.87 | 0.30 | 6 | −0.45 | 0.59 | 2.91 | −0.76 | 1.86 |
| 13–21.99 | 19 | 0.99 | 0.22 | 6 | −0.52 | 0.58 | 4.54a | −0.89 | 2.23 |
| 22–36 | 17 | 0.84 | 0.23 | 5 | 0.25 | 0.56 | 3.74a | 0.45 | 0.90 |
| Height | |||||||||
| 0–0.99 | 20 | 1.15 | 0.25 | 6 | 1.05 | 0.27 | 4.67a | 3.88 | 0.52 |
| 1–3.99 | 21 | 0.72 | 0.25 | 7 | −1.01 | 0.39 | 2.92 | −2.55 | 2.79a |
| 4–6.99 | 23 | 0.25 | 0.23 | 7 | −0.47 | 0.72 | 1.11 | −0.66 | 1.69 |
| 7–9.99 | 11 | 0.16 | 0.34 | 5 | −1.88 | 0.48 | 0.45 | −3.92 | 2.50 |
| 10–12.99 | 16 | 0.33 | 0.28 | 6 | −0.84 | 0.36 | 1.18 | −2.35 | 1.96 |
| 13–21.99 | 13 | 0.41 | 0.29 | 6 | −0.41 | 0.44 | 1.40 | −0.91 | 1.40 |
| 22–36 | 14 | 0.43 | 0.24 | 5 | −0.19 | 0.28 | 1.78 | −0.67 | 1.76 |
Abbreviation: SEM, standard error of the mean.
Statistically significant after Bonferroni adjustment for multiple comparisons across the 7 age bins (P < .007).
Figure 1.
z-Score means and standard errors of the means for occipitofrontal circumference and height for children with ASD vs developmental delay.
Compared with normal values for height, the ASD group, on average, was statistically significantly longer or taller than the norms only during the first month of life (bin from birth to age 0.99 months, t = 4.67, P < .007) but not from age 1 to 36 months (t < 2.92, not statistically significant). During the window of greatest increase in occipitofrontal circumference in the ASD group (from age 4–6.99 months to age 7–9.99 months), the mean z score for height decreased by −0.10.
Compared with the normal values, occipitofrontal circumference for the developmental delay group was statistically significantly smaller at age 1 to 6.99 months (2 bins, t < –4.24, P < .005) but not at birth to age 0.99 months or from age 7 to 36 months (t range, −1.86 to 0.45; not statistically significant). The developmental delay group showed an increase in occipitofrontal circumference z score during the period from age 4 to 6.99 months to age 7 to 9.99 months of 0.74 and a height change of −1.41 (Figure 1).
Nonparametric (2-tailed Mann-Whitney) tests were used to compare the ASD group vs the developmental delay group and the autism group vs the pervasive developmental disorder-not otherwise specified group. The ASD group had statistically significantly greater occipitofrontal circumference values than the developmental delay group at 2 bins from age 1 to 6.99 months (z score, >2.98) and statistically significantly greater height at age 1 to 3.99 months (z score, 2.79). There were no statistically significant differences between the autism and pervasive developmental disorder-not otherwise specified groups at any of the age bins (Figure 2).
Figure 2.
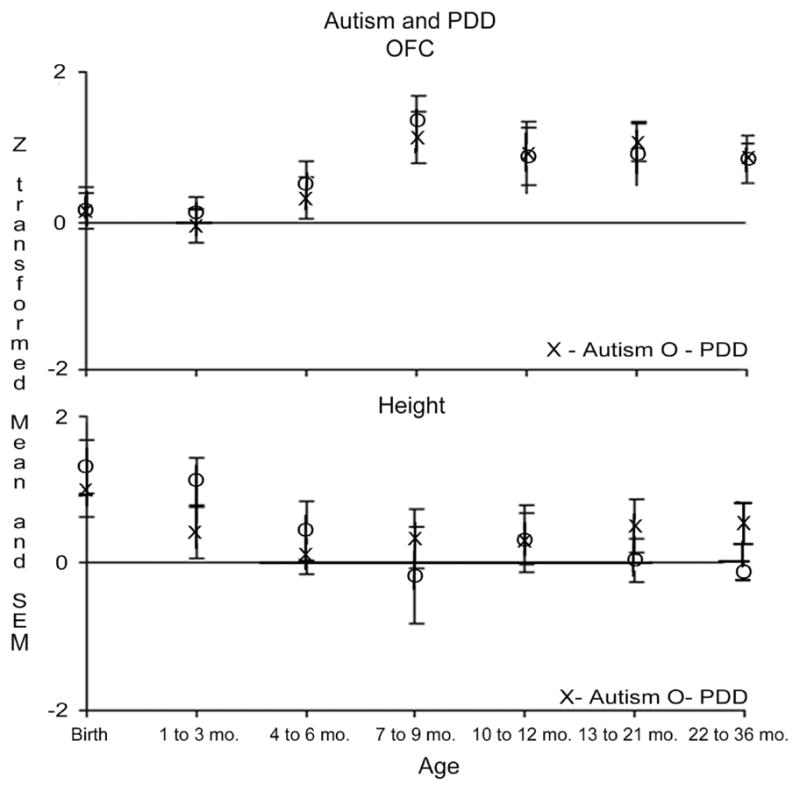
z-Score means and standard errors of the means for occipitofrontal circumference and height for children with autism vs pervasive developmental disorder-not otherwise specified.
Macrocephalus and Microcephalus
In this study, 6 of 28 boys with ASD (21.4%) met the criteria for macrocephalus (z score, >1.88) sometime between birth and age 36 months. Of these 6 children, 5 had early onset of ASD symptoms, and 4 met criteria for autism strictly defined. Although a greater proportion of children with macrocephalus were classified as having early onset of ASD symptoms (5 of 6 [83.3%]) compared with those with early onset without macrocephalus (12 of 22 [54.5%]), this difference was not statistically significant (P = .36, Fisher exact test).
Microcephalus was considered to be present in any child whose occipitofrontal circumference after age 16 months was less than 2 SDs below the Centers for Disease Control and Prevention normative values. In this sample, 2 children met the criteria for microcephalus at 16 months of age, both with a diagnosis of developmental delay.
Group Comparisons From Birth to Median Age of 10 Months
Twenty-four boys had a valid occipitofrontal circumference measurement between birth and age 0.99 months and a valid measurement between 6 and 14 months of age. The values closest to birth and closest to 10 months of age were retained for this comparison, such that each participant contributed only 1 value for each of the 2 age bins. Among 24 boys in this analysis, 4 were diagnosed as having developmental delay, 10 were later diagnosed as having pervasive developmental disorder-not otherwise specified, and 10 were diagnosed as having autism.
Table 2 gives the individual values in occipitofrontal circumference for the participants with autism and with pervasive developmental disorder-not otherwise specified and the change in occipitofrontal circumference values from birth to approximately age 10 months. The mean ± SE occipitofrontal circumference at birth was 0.052 ± 0.360 for the autism group, 0.02 ± 0.26 for the pervasive developmental disorder-not otherwise specified group, and −0.813 ± 0.480 for the developmental delay group. Therefore, children diagnosed as having autism or pervasive developmental disorder-not otherwise specified did not differ from the Centers for Disease Control and Prevention norms at birth. However, at 10 months of age, children later diagnosed as having ASD had statistically significantly larger occipitofrontal circumference values than the Centers for Disease Control and Prevention norms and were statistically significantly larger than the children with developmental delay in occipitofrontal circumference, height, and weight (P <.01 for all). The mean ± SE occipitofrontal circumference values were 0.80 ± 0.40 for autism, 0.92 ± 0.36 for pervasive developmental disorder-not otherwise specified, and −1.58 ± 0.34 for developmental delay. The autism and pervasive developmental disorder-not otherwise specified groups did not differ from each other on the 3 growth measures of head circumference, height, or weight at birth or at 10 months of age.
Table 2.
Occipitofrontal Circumference z Scores at Birth and at a Median Age of 10 Months for 10 Boys With Autism and 10 Boys With Pervasive Developmental Disorder-Not Otherwise Specified (PDD)
| Birth | Approximately Age 10 m | Change in z Score | Diagnosis at Age 3 y |
|---|---|---|---|
| −1.81 | −1.12 | 0.68a | PDD |
| −1.56 | 1.15 | 2.71a | Autism |
| −1.31 | −0.11 | 1.20a | Autism |
| −0.89 | 1.39 | 2.28a | Autism |
| −0.66 | −0.79 | −0.12 | PDD |
| −0.46 | −2.12 | −1.66a | Autism |
| −0.27 | 0.19 | 0.45 | PDD |
| −0.17 | 0.11 | 0.28 | Autism |
| −0.11 | 0.88 | 0.99a | PDD |
| 0.21 | 0.68 | 0.47 | Autism |
| 0.21 | 1.46 | 1.25a | PDD |
| 0.21 | 2.06 | 1.85a | PDD |
| 0.38 | 1.17 | 0.79a | PDD |
| 0.38 | 1.49 | 1.11a | PDD |
| 0.57 | 1.17 | 0.59 | Autism |
| 0.57 | 2.31 | 1.74a | PDD |
| 1.16 | 2.13 | 0.97a | Autism |
| 1.17 | 1.63 | 0.46 | Autism |
| 1.30 | 1.55 | 0.26 | PDD |
| 1.98 | 1.93 | −0.05 | Autism |
Change of ±0.68, representing 1 SD.
Regression vs Early Onset of ASD
Regression was assessed via parent report of a history of loss of skills (regression) on the Autism Diagnostic Interview-Revised. Of 28 boys with ASD, 11 were classified as regressed (of whom 8 were diagnosed as having autism and 3 as having pervasive developmental disorder-not otherwise specified). No statistically significant differences were found at any of the 7 age bins between the boys with ASD who had early onset of symptoms and those who had regression. Children with early onset and those with developmental regression demonstrated an increase of approximately 0.80 in the mean occipitofrontal circumference between age 4 to 6.99 months and age 7 to 9.99 months.
Discussion
This study examined changes in head circumferences within a narrow time window (birth to age 36 months) in boys diagnosed as having ASD vs developmental delay. Furthermore, we examined whether early growth of head circumference was different for children with autism vs pervasive developmental disorder-not otherwise specified and for children with ASD with and without a history of autistic regression. Consistent with data previously reported by Courchesne and colleagues,15 we found that the period of early overgrowth in occipitofrontal circumference in children with ASD occurred within the second half of the first year of life. Specifically, our analyses narrow this period to between 6 and 9 months of age.
In contrast to results from some previous findings,15 the occipitofrontal circumference for children with ASD in the present study did not differ from the Centers for Disease Control and Prevention norms at birth. The birth occipitofrontal circumference in the children with ASD ranged from −1.81 to 1.98, suggesting that there was extensive variability in birth occipitofrontal circumference, despite mean differences that did not differ from the Centers for Disease Control and Prevention norms. As seen in Figure 1, children with ASD showed occipitofrontal circumference values that were statistically significantly greater than the Centers for Disease Control and Prevention norms after 6 months of age through age 36 months (also see Sparks et al12). When examining the growth patterns using a z score of ±0.68 to represent a change of ±1 SD, we found that 11 of 20 children with ASD (in Table 2) demonstrated accelerated growth between birth and age 10 months, whereas only 1 of 20 children showed decelerated growth.
The pattern of accelerated occipitofrontal circumference growth did not differ for children with autism compared with those with pervasive developmental disorder-not otherwise specified. Furthermore, there was no difference in pattern of occipitofrontal circumference for children with early onset of autism vs those with a reported history of autistic regression. Both groups show early acceleration of occipitofrontal circumference growth. Werner and Dawson39 found that children with ASD with a history of regression used complex babble and words statistically significantly more than infants with early onset of ASD at 12 months of age; by 24 months of age, both groups with ASD performed worse than typical children on word use, vocalizations, pointing, social gaze, and orienting to name. Although the study by Werner and Dawson supports parental reports of behavioral regression in ASD, acceleration in occipitofrontal circumference growth may represent an early neurobiological sign that precedes onset of autistic regression, similar to findings of early regulatory difficulties in such children.40 More detailed work examining function between 6 and 12 months of age will be needed to understand the relation between occipitofrontal circumference growth and the onset of symptom expression.
Compared with the boys with ASD, boys with developmental delay had significantly smaller occipitofrontal circumference than the ASD group (compared with Centers for Disease Control and Prevention norms) at birth but also showed accelerated growth (compared with norms) during the first year of life. Given the small sample size in the developmental delay group, it is difficult to interpret the statistically significant rise in occipitofrontal circumference from age 4 to 6.99 months to age 7 to 9.99 months, especially because the number of participants with developmental delay included at each age point was not the same. A possibility is that subtle differences in prenatal history, which are known to result in different growth trajectories during the first year of life, affected the sample. For example, infants of primiparous pregnancies are smaller, lighter, and shorter at birth but catch up to infants of second or third pregnancies by 12 months of age.41 Moreover, it is important to note that the small numbers of boys with developmental delay who had occipitofrontal circumference data at birth and between 10 and 14 months of age actually showed a relative decrease in the rate of occipitofrontal circumference growth.
Similar to other studies,14,16,21,23,27–33 approximately 21% of our sample demonstrated macrocephalus between birth and 36 months of age. Although the prevalence of macrocephalus during later childhood in ASD surpasses that in the typical population, findings from studies14,20 suggest that macrocephalus is not characteristic of the infant period in children who later go on to develop ASD. Similarly, in our study 11 of 20 children demonstrated an increase of 1 SD from birth to age 10 months; of those 11, only 4 had macrocephalus by age 6 to 14 months. Of note, 4 of 11 children with accelerated growth had an occipitofrontal circumference at birth that was 1 SD below the Centers for Disease Control and Prevention norms.
What are the implications of these findings? Courchesne and Pierce42 proposed that a period of early abnormally accelerated rate of brain growth is followed by an abnormally reduced rate of brain growth by adolescence and that systems with protracted development are the most affected by this growth abnormality (eg, frontal cortex). Additional work is needed to characterize this later deceleration in growth as reflecting an early onset of normative deceleration or an aberrant process that results in failure of continual development. Regardless, these 2 processes, overgrowth and deceleration, occur during periods of rapid brain growth (late infancy and adolescence) and are associated with the onset and potential worsening, respectively, of ASD symptoms.
The use of the rate of occipitofrontal circumference growth as a risk factor for ASD poses important questions for the field. How specific is early accelerated head growth to ASD? In the Fels Longitudinal Study sample, 6% of healthy infants show statistically significant occipitofrontal circumference increases during the first year (as cited by Courchesne et al15). Also, for Rett syndrome, a decreasing rate of head growth is used as an essential diagnostic symptom for the disorder. In Rett syndrome, children have normal head circumference at birth, followed by a slowing of the rate of growth; this period of slowing can begin between age 3 months and age 4 years. There are several other syndromes (eg, Weaver syndrome and Soto syndrome) that are marked by rapid excessive growth and macrocephalus. These cases are rare compared with the current prevalence rates for ASD and often are associated with additional malformations that help to provide differential diagnosis. Commonalities across disorders in head growth patterns may suggest specific or common genetic pathways. In addition, modeling of accelerated head growth in animals may help to further our understanding of the neurobiological and genetic underpinnings of the disorder.
This study further adds to the growing body of evidence that children with ASD do not have abnormal occipitofrontal circumference values at birth but exhibit a rapid early acceleration of occipitofrontal circumference growth. We find that this period begins during the second half of the first year of life, between 6 and 9 months of age, and does not seem to be different for children with autism vs pervasive developmental disorder-not otherwise specified or for those with or without an early history of autism regression. Future work with larger samples is needed to further define and understand the clinical significance of early growth in head circumference in ASD and other development disorders.
Acknowledgments
This study was supported by grant U19HD35465 from the National Institute of Child Health and Human Development as part of its Collaborative Program of Excellence in Autism (GD) and by center grant U54MH066399 from the National Institute of Mental Health as part of the National Institutes of Health STAART (Studies to Advance Autism Research and Treatment) Centers Program (GD).
We thank the children and parents who participated in this study.
Footnotes
This research was conducted at the University of Washington, Seattle.
References
- 1.Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24(3):247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- 2.Maestro S, Muratori F, Cavallaro MC, et al. Attentional skills during the first 6 months of age in autism. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1239–1245. doi: 10.1097/00004583-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Zwaigenbaum L, Bryson S, Rogers T, et al. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Dawson G, Carver L, Meltzoff AN, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson G, Meltzoff AN, Osterling J, et al. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord. 1998;28(6):479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- 6.Dawson G, Toth K, Abbott R, et al. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev Psychol. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- 7.Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of autism: the contribution of non-verbal communication measures. J Child Psychol Psychiatry. 1986;27(5):657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 8.Sigman MD, Kasari C, Kwon JH, Yirmiya N. Responses to the negative emotions of others by autistic, mentally retarded, and normal children. Child Dev. 1992;63(4):796–807. [PubMed] [Google Scholar]
- 9.Kemper TL, Bauman MJ. Neuropathology of infantile autism. Mol Psychiatry. 2002;7(suppl 2):S12–S13. doi: 10.1038/sj.mp.4001165. [DOI] [PubMed] [Google Scholar]
- 10.Rodier PM. Converging evidence for brain stem injury in autism. Dev Psychopathol. 2002;14(3):537–557. doi: 10.1017/s0954579402003085. [DOI] [PubMed] [Google Scholar]
- 11.Rodier PM, Ingram JL, Tisdale B, et al. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol. 1996;370(2):247–261. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Sparks BF, Friedman SD, Shaw DWW, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59(2):184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 13.Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16(4):1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- 14.Dementieva Y, Vance D, Donnelly S, et al. Accelerated head growth in early development of individuals with autism. Pediatr Neurol. 2005;32(2):102–108. doi: 10.1016/j.pediatrneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290(3):337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 16.Lainhart JE, Piven J, Wzorek M, et al. Macrocephaly in children and adults with autism. J Am Acad Child Adolesc Psychiatry. 1997;36(2):282–290. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Courchesne E, Karns CM, Davis HR, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57(2):1–10. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 18.Aylward EH, Minshew NJ, Field K, et al. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59(2):175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- 19.Bartholomeusz HH, Courchesne E, Karns C. Relationship between OFC and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics. 2002;22(5):239–241. doi: 10.1055/s-2002-36735. [DOI] [PubMed] [Google Scholar]
- 20.Redcay E, Courchesne E. When is the brain enlarged in autism? a meta-analysis of all brain size reports. Biol Psychiatry. 2005;58(1):1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Gillberg C, de Souza L. Head circumference in autism, Asperger syndrome, and ADHD: a comparative study. Dev Med Child Neurol. 2002;44(5):296–300. doi: 10.1017/s0012162201002110. [DOI] [PubMed] [Google Scholar]
- 22.Torrey EF, Dhavale D, Lawlor JP, Yolken RH. Autism and OFC in the first year of life. Biol Psychiatry. 2004;56(11):892–894. doi: 10.1016/j.biopsych.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Davidovitch M, Patterson B, Gartside P. Head circumference measurements in children with autism. J Virol. 1996;11(5):389–393. doi: 10.1177/088307389601100509. [DOI] [PubMed] [Google Scholar]
- 24.Mason-Brothers A, Ritvo ER, Guze B, et al. Pre-, peri-, and postnatal factors in 181 autistic patients from single and multiple incidence families. J Am Acad Child Adolesc Psychiatry. 1987;26(1):39–42. doi: 10.1097/00004583-198701000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Mason-Brothers A, Ritvo ER, Pingree C, et al. The UCLA-University of Utah epidemiologic survey of autism: prenatal, perinatal, and postnatal factors. Pediatrics. 1990;86(4):514–519. [PubMed] [Google Scholar]
- 26.Hultman CM, Sparen P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology. 2002;13(4):417–423. doi: 10.1097/00001648-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Deutsch CK, Joseph RM. Brief report: cognitive correlates of enlarged head circumference in children with autism. J Autism Dev Disord. 2003;33(2):209–215. doi: 10.1023/a:1022903913547. [DOI] [PubMed] [Google Scholar]
- 28.Fidler DJ, Bailey JN, Smalley SL. Macrocephaly in autism and other pervasive developmental disorders. Dev Med Child Neurol. 2000;42(11):737–740. doi: 10.1017/s0012162200001365. [DOI] [PubMed] [Google Scholar]
- 29.Fombonne E, Roge B, Claverie J, et al. Microcephaly and macrocephaly in autism. J Autism Dev Disord. 1999;29(2):113–119. doi: 10.1023/a:1023036509476. [DOI] [PubMed] [Google Scholar]
- 30.Ghaziuddin M, Zaccagnini J, Tsai L, Elardo S. Is megalencephaly specific to autism? J Intellect Disabil Res. 1999;43(4):279–282. doi: 10.1046/j.1365-2788.1999.00211.x. [DOI] [PubMed] [Google Scholar]
- 31.Miles JH, Hadden LL, Takahashi TN, Hillman RE. Head circumference is an independent clinical finding associated with autism. Am J Med Genet. 2000;95(4):339–350. [PubMed] [Google Scholar]
- 32.Stevenson RE, Schroer RJ, Skinner C, Fender D, Simensen RJ. Autism and macrocephaly. Lancet. 1997;349(9067):1744–1755. doi: 10.1016/S0140-6736(05)62956-X. [DOI] [PubMed] [Google Scholar]
- 33.Woodhouse W, Bailey A, Rutter M, et al. Head circumference in autism and other pervasive developmental disorders. J Child Psychol Psychiatry. 1996;37(6):665–671. doi: 10.1111/j.1469-7610.1996.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 34.Rodier PM, Bryson SE, Welch JP. Minor malformations and physical measurements in autism: data from Nova Scotia. Teratology. 1997;55(5):319–325. doi: 10.1002/(SICI)1096-9926(199705)55:5<319::AID-TERA4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 35.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 36.Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000;28(2):355–363. doi: 10.1016/s0896-6273(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 37.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 38.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]
- 39.Werner E, Dawson G. Validation of the phenomenon of autistic regression using home videotapes. Arch Gen Psychiatry. 2005;62(8):889–895. doi: 10.1001/archpsyc.62.8.889. [DOI] [PubMed] [Google Scholar]
- 40.Baranek GT. Autism during infancy: a retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. J Autism Dev Disord. 1999;29(3):213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- 41.Ong KK, Preece MA, Emmett PM, et al. Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: longitudinal birth cohort study and analysis. Pediatr Res. 2002;52(6):863–867. doi: 10.1203/00006450-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci. 2005;23(2–3):153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]