Abstract
Rationale and objectives
Alcohol and nicotine are the most commonly abused drugs and they are often taken together. We have developed a procedure in which rats self-administer nicotine intravenously and alcohol orally during the same operant session.
Methods
Male Wistar rats were trained to self-administer alcohol (12% w/v, 0.19 ml/alcohol delivery) or implanted with jugular catheters and trained to self-administer nicotine (30 µg/kg i.v./infusion) by pressing a lever, or were trained to self-administer both drugs, some with alcohol first, and others with nicotine first. The effects of extinction of responding for either or both drugs in animals trained to co-administer alcohol and nicotine, and the effects of alcohol and nicotine primes on reinstatement was also determined
Results
Animals readily co-administered alcohol and nicotine concurrently. Access to alcohol reduced nicotine self-administration significantly. When responding for alcohol was extinguished with nicotine still available, extinction of alcohol seeking was slowed significantly. In rats trained to co-administer nicotine and alcohol, priming with nicotine or alcohol reinstated extinguished responding for both drugs. Reinstatement of extinguished nicotine or alcohol seeking by, respectively, nicotine or alcohol priming was unaffected by continued access to the other drug.
Conclusions
These results show that rats will self-administer relevant amounts of intravenous nicotine and oral alcohol concurrently. They also provide further support for the important relationship between nicotine and alcohol.
Alcohol and nicotine are often taken together. It has been estimated that 70–80% of alcoholics smoke, a rate three times higher than in the general population (DiFranza and Guerrera 1990). Non-alcoholic drinkers are also more likely to smoke (Kandel et al. 1997). Research using a variety of different approaches provide support for the idea that this relationship is biologically based (Funk et al. 2006; Little 2000). Genetic studies in humans have established that the liability to use either drug, and both drugs is co-inherited (Dani and Harris 2005). In agreement with this clinical work, we have shown that strains of rats selected for high alcohol drinking self-administer more nicotine than strains selected for low consumption (Le et al. 2006).
The effects of nicotine administration on alcohol intake has been investigated in laboratory rodents. The most common findings is that repeated or chronic administration of nicotine to rats increases home-cage intake and operant self-administration of alcohol (Le et al. 2000; Olausson et al. 2001). Other groups have, however, reported decreases in alcohol intake with repeated nicotine injection (Sharpe and Samson 2002). Using the reinstatement procedure, we reported that acute administration of nicotine reinstates extinguished responding for alcohol (Le et al. 2003). The effects of alcohol administration on nicotine intake in rodents is not known.
Although these studies demonstrate a relationship between nicotine and alcohol self-administration, their relevance is limited. In humans, both drugs are voluntarily self-administered. With the exception of one study that examined oral consumption of alcohol and nicotine solutions in a two bottle choice procedure (Marshall et al. 2003), the animal studies to date have determined the effects of passive administration of nicotine or alcohol on self-administration of the other. Nicotine, alcohol and other drugs of abuse are known to have different behavioral and biological effects depending on whether they are self-administered, or administered passively (Donny et al. 2000; Stefanski et al. 1999; Wilson et al. 1994).
Since alcohol and nicotine interact in the production of their rewarding effects, there is a challenge in terms of treatment design in cases of co morbidity. The few clinical studies that have addressed this have produced mixed results (Hays et al. 1999; Kozlowski et al. 1989; Stuyt 1997). Another important issue that has received little attention is how alcohol-dependent patients respond to pharmacological agents used to treat nicotine addiction and vice versa. For example, the opiate antagonist naltrexone, used to treat alcohol dependence (O'Brien et al. 1996), has been shown to have conflicting effects on smoking with some studies reporting reductions (Wong et al. 1999), while others report increases in the urge to smoke (Krishnan-Sarin et al. 1999).
To help address these issues, we have developed a procedure in which rats have the opportunity to self-administer nicotine intravenously and alcohol orally during the same operant sessions. We first compare responding and intake in animals trained to self-administer alcohol or nicotine alone with animals trained to co-administer both drugs. We also determine whether the order of training (alcohol or nicotine first) affects co-administration. Then, in animals trained to co-administer alcohol and nicotine, we determine the patterns of extinction of responding when one or both drugs are withheld. Lastly, we examine the effects of priming doses of alcohol or nicotine on reinstatement in animals trained to co-administer nicotine and alcohol that were subject to these different extinction conditions.
METHODS
Animals
Male Wistar rats, 150–200 g, were obtained from Charles River, Montreal, and allowed to acclimatize to the animal facility for 1 wk prior to the experiments. Animals were individually housed and given free access to standard lab chow and tap water. The vivarium temperature was 21°C and lights were on from 7 a.m. to 7 p.m. The experimental procedures followed the “Principles of laboratory animal care” (NIH publication no. 85-23, 1996) and were approved by the local animal care committee.
Implantation of intravenous catheters
Rats were anaesthetized using a ketamine/xylazine mixture (75 mg/kg ketamine/10 mg/kg xylazine). Incision sites were treated with a local anesthetic (0.1 ml bupivacaine, 0.125%, s.c.). Buprenorphine (0.01 mg/kg, s.c.) was administered as an analgesic and penicillin (30 000 U, i.m.) was used as antibiotic treatment. Catheters were implanted into the right jugular vein as previously described (Corrigall and Coen 1989; Le et al. 2006). The catheter exited between the scapulae and was attached to the modified 22-gauge cannula that connected to the fluid swivel system during nicotine self-administration. The rats were allowed to recover from surgery for 6–8 days. Catheters were flushed daily with 0.1 ml of a sterile heparin-saline solution (50 U/ml) to maintain patency. Rats received weekly tests of catheter patency with i.v. injections of sodium methohexital (0.15 mg/kg).
Apparatus
Self administration of nicotine or alcohol alone or co-administration was done in 16 chambers housed in sound-attenuating boxes operated by a Med Associates (Georgia, VT) interface system. The interior dimensions of the plexiglas chambers were 30 × 21 × 21 cm. In the case of self-administration of alcohol or nicotine alone, only one lever was extended. Appropriate responding activated an infusion pump (Razel Sci., Stamford, CT) , in the case of alcohol, for 5 sec., delivering 0.19 ml of 12% (w/v) alcohol into a drinking receptacle, or for 0.5 s delivering 30 µg/kg nicotine/infusion via the intravenous catheter. The chambers were also equipped with a red house light located near the top of the chamber opposite the lever and a white cue light located above the lever. During co-administration sessions, the chambers were equipped with two infusion pumps, one that delivered alcohol into the drinking receptacle and one that delivered i.v. nicotine. The two levers were extended during co-administration sessions. Appropriate responding on one of the levers resulted in i.v. nicotine delivery, and on the other, alcohol delivery into the receptacle. In all of the experiments, nicotine reinforcements were accompanied by a continuously illuminated white cue light, while alcohol reinforcements were accompanied by a flashing white cue light (0.5 s on; 0.5 s off) during the timeout periods (described below). The orientation of the nicotine and alcohol-associated levers (right or left) was counterbalanced across animals in the co-administration groups.
Drugs
Nicotine solutions (Sigma) were prepared daily using sterile saline, and pH was adjusted to 6.8–7.2. The unit doses for nicotine self-administration were 30 µg /kg/infusion, expressed as base (Corrigall and Coen 1989; Le et al. 2006; Shoaib and Stolerman 1999). Alcohol (Commercial Alcohols Incorporated, Tiverton, ON) was diluted with tap water. The self-administration dose of alcohol was 0.19 ml of 12% w/v alcohol per reinforcement. These doses of nicotine and alcohol are commonly used in studies on self-administration (Caggiula et al. 2002; Corrigall and Coen 1989; Heyser et al. 2003; Le et al. 2000). For the reinstatement study, the priming dose of nicotine used was 0.15 mg/kg s.c. (Shram et al. 2008) and for alcohol was one non-contingent delivery of alcohol in the same volume and concentration as used during self-administration.
Data analysis and presentation
In Experiments 1 and 2, nicotine and alcohol reinforcements were analyzed with mixed ANOVA, using the between factor of Group and within factor of Day. In Experiment 3, nicotine and alcohol infusions during the self-administration phase were analyzed with mixed ANOVAs with the between factor of Group and the within factor of Training phase and Day, or in the case of analysis of responding in 5 min intervals, the within factor of Interval. In the studies involving the effects of drug access on extinction or reinstatement (Experiments 3a and 3b), nicotine or alcohol infusions and active lever presses were analyzed separately with mixed two-way ANOVAs with the between factor of Extinction condition and within factor of Day, Significant effects from ANOVA (p values < 0.05) were followed by post-hoc tests using the Newman-Keuls procedure.
In the experiments involving the self-administration of alcohol, the volume of unconsumed alcohol left in the drinking well was recorded and used to calculate the numbers of reinforcements consumed, and these values were used for presentation and analysis. We note here that only a small minority of rats do not consume all of the delivered alcohol. The overall mean volume of unconsumed alcohol measured in the experiments involving alcohol self-administration was 0.2 ± 0.05 ml. Twelve rats were excluded from analysis as they consumed less than 0.4 g/kg/h alcohol during the alcohol training phases of the experiments.
Experimental procedures
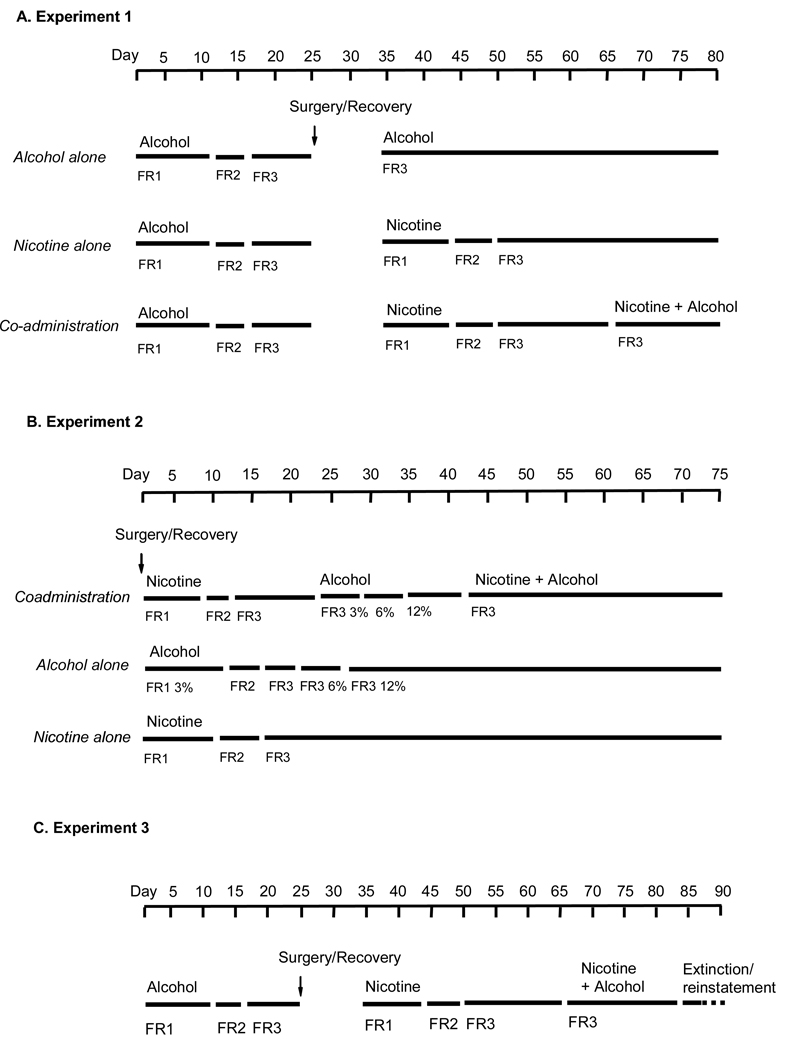
The designs of the three experiments are shown in Figure 1.
Figure 1.
Illustration of the nicotine and/or alcohol training regimens used in Experiments 1(A), 2(B) and 3(C). Animals receiving nicotine training self-administered it at a dose of 30 µg/kg/infusion at a final ratio of FR-3. For animals receiving training with alcohol, the final self-administered dose was 0.19 ml of 12% (w/v) alcohol/delivery at FR-3.
Experiment 1: Co-administration of nicotine and alcohol in animals trained to self administer alcohol first
Forty-four rats were assigned to one of three groups. Alcohol alone: Rats received daily limited access sessions (30 min) with the choice between water and alcohol in Richter tubes, in distinctive chambers in a separate room. Alcohol was provided in escalating concentrations: 3% for the first 5 d, 6% for the next 5 d and 12% for the last 10 d. Rats were then trained to lever press for alcohol (12% w/v) in the self-administration chambers during daily 1-h sessions. Lever presses were initially reinforced under a fixed-ratio 1 (FR-1) reinforcement schedule for 10 d. Subsequently, the response requirement was increased to an FR-2 for 5 d, and than to an FR-3 for 10 d. At the start of the sessions, the red house light was turned on and one of the retractable levers was extended. Appropriate responding on it resulted in alcohol delivery, after which a timeout period occurred, during which further responding was recorded but was without consequence. Alcohol deliveries were accompanied by a flashing white cue light above the lever that persisted through the timeout period. During the initial training at FR-1, in order to speed training, the timeout period was 5 s. At FR-2 and FR-3, the timeout was lengthened to 30 seconds. After responding for alcohol stabilized, rats in this group were given sham i.v. surgery, where they were anesthetized, received incisions on the neck and overlying the scapular region that were then sutured, as in animals catheterized for nicotine self-administration. After 7–10 d of recovery, they received further training with alcohol self-administration at FR-3 for 15 d.
Nicotine alone: Animals were trained to self-administer alcohol as above, and after stable responding at FR-3 were implanted with intravenous catheters. After recovery, the rats were trained to self-administer nicotine (30 µg/kg per infusion) for 1 h/d for 24 d. For the first 10 d, active lever responding was reinforced under a FR-1 schedule, the next 6 d at FR-2, and then under FR-3 for 15 d. At the start of the sessions, the red house light was turned on, one of the retractable levers was extended, and a white cue light above the lever was continuously illuminated for 30 sec. Each nicotine infusion was accompanied by a 30-sec timeout during which responses were recorded but did not lead to drug delivery; during the timeout, the white cue light remained illuminated.
For both of these experimental groups, after delivery of alcohol or nicotine the levers retracted for the duration of the 30 s timeout period.
Co-administration: All rats were first trained to first self-administer alcohol as described above. Once stable responding for alcohol self-administration at FR-3 was obtained, they were implanted with i.v. catheters, allowed to recover for 7–10 d, and then were trained to self-administer nicotine to a final ratio of FR-3 as described above for the nicotine alone group. Animals then started nicotine and alcohol co-administration, each at FR-3. During this phase, a 30 s timeout period also occurred after delivery of nicotine or alcohol; during the timeout, both levers retracted. The co-administration phase was conducted daily for 15 d.
Experiment 2: Co-administration of nicotine and alcohol in animals trained to self administer nicotine first
Forty rats were trained to self-administer sucrose orally in 5 daily 1 hr sessions, in order to speed the learning of the operant response. They were then assigned to one of three groups. Co-administration: animals received i.v. surgery, and after 7–10 d recovery, were trained to self-administer nicotine (30 µg/kg per infusion) at FR-1, FR-2 and FR-3 for, respectively, 8, 3 and 11 days. They were then trained to self-administer alcohol alone beginning with 3% alcohol at FR-1 (8 d), FR-2 (3 d) and FR-3 (6 d), followed by 6% alcohol at FR-3 (5 d), and then 12% alcohol at FR-3 for the rest of the experiment. After animals showed stable responding for 12% alcohol at FR-3, they received sessions with concurrent access to alcohol and nicotine (both at FR-3). Animals were run under these co-administration conditions in daily sessions for 2 weeks. Alcohol alone: animals received sham i.v. surgery, and after 7–10 d of recovery, were trained to self-administer 3% alcohol at FR-1, FR-2 and FR-3 for 8, 3 and 3 days, the 6% alcohol at FR-3 for 5 days, and finally 12% alcohol at FR-3 for the rest of the experiment. Nicotine alone: animals were implanted with i.v. catheters and after recovery, were trained to self-administer nicotine (30 µg/kg per infusion) according using the same parameters and schedule described for nicotine training in Group 1.
Experiment 3: Extinction and reinstatement in rats with concurrent access to alcohol and nicotine
Training for co-administration: Forty-eight rats were first trained to self-administer alcohol (0.19 ml of 12% w/v alcohol), received i.v. surgery and recovery, and were then trained to self-administer nicotine alone (30 µg/kg per infusion) as described for Experiment 1. After animals showed stable responding for nicotine at FR-3, they received sessions with concurrent access to alcohol and nicotine. After delivery of each alcohol or nicotine infusion, a 30 s timeout period occurred; during the timeout, both levers retracted. Alcohol reinforcements were paired with a flashing white cue light and nicotine with a continuously illuminated light for the duration of the timeout period. Animals were run under these co-administration conditions in daily sessions for 3 weeks prior to the start of extinction.
Experiment 3a: Extinction of nicotine and/or alcohol self-administration in animals trained to self-administer both drugs concurrently
Animals were assigned to one of three groups matched on responding for alcohol and nicotine. In the first group, alcohol self-administration was extinguished while nicotine self-administration was maintained. In the second, nicotine self-administration was extinguished while alcohol self-administration was maintained. In the third group, both alcohol and nicotine self-administration was extinguished. The conditions during this phase were the same as during co-administration, except that drug infusions were withheld in the groups where nicotine and/or alcohol responding was extinguished. The drug-associated cue lights were also present during these extinction sessions. Animals were run under these extinction conditions in daily sessions for 15 d.
Experiment 3b: Reinstatement induced by priming with nicotine or alcohol
After the 15 d of extinction, the animals in Experiment 2a, the effects of priming injections of nicotine or alcohol on reinstatement of responding for the extinguished drug and on self-administration of the remaining drug were examined.
Nicotine (0.15 mg/kg, s.c.) was injected 15 min prior to the test sessions in rats in the group in which both nicotine and alcohol was extinguished, and in the group with nicotine extinguished that continued to have alcohol available. A single non-contingent infusion of alcohol (0.19 ml, 12% w/v) was delivered into the drinking cup at the beginning of the test sessions in the group in which responding for both drugs was extinguished, and the group where only alcohol was extinguished, with nicotine available.
Nicotine or alcohol was given in a counterbalanced manner with 2–3 extinction days between each test. Prior to beginning the priming tests, rats received three daily s.c. injections of saline in order to habituate them to the injection procedure. Before testing the effects of each drug on self-administration and/or reinstatement of extinguished responding, a baseline test after a saline vehicle injection was determined. The cues previously associated with nicotine or alcohol self-administration were present during extinction and the reinstatement tests.
Results
Table 1 shows the alcohol and nicotine intake of animals in the three experiments.
Table 1.
Mean (± sem) nicotine and alcohol intake, and volumes of unconsumed alcohol remaining in the drinking receptacles in rats under the different self-administration conditions in the three experiments.
| Drug Intake/ 1h | ||
|---|---|---|
| Alcohol (g/kg) | Nicotine (mg/kg) | |
| Experiment 1 | ||
| Group | ||
| Alcohol alone | 1.32 ± 0.03 | --- |
| Nicotine alone | --- | 1.35 ± 0.14 |
| Co-admin. | 1.28 ± 0.09 | 0.92 ± 0.15 |
| Experiment 2 | ||
| Group | ||
| Alcohol alone | 0.91 ± 0.11 | --- |
| Nicotine alone | --- | 1.42 ± 0.28 |
| Co-admin. | 0.70 ± 0.14 | 0.92 ± 0.16 |
| Experiment 3 | ||
| Training phase | ||
| Alcohol | 1.40 ± 0.07 | --- |
| Nicotine | --- | 1.68 ± 0.18 |
| Co-admin. | 1.18 ± 0.06 | 1.19 ± 0.19 |
Experiment 1: Co-administration of nicotine and alcohol in animals trained to self administer alcohol first
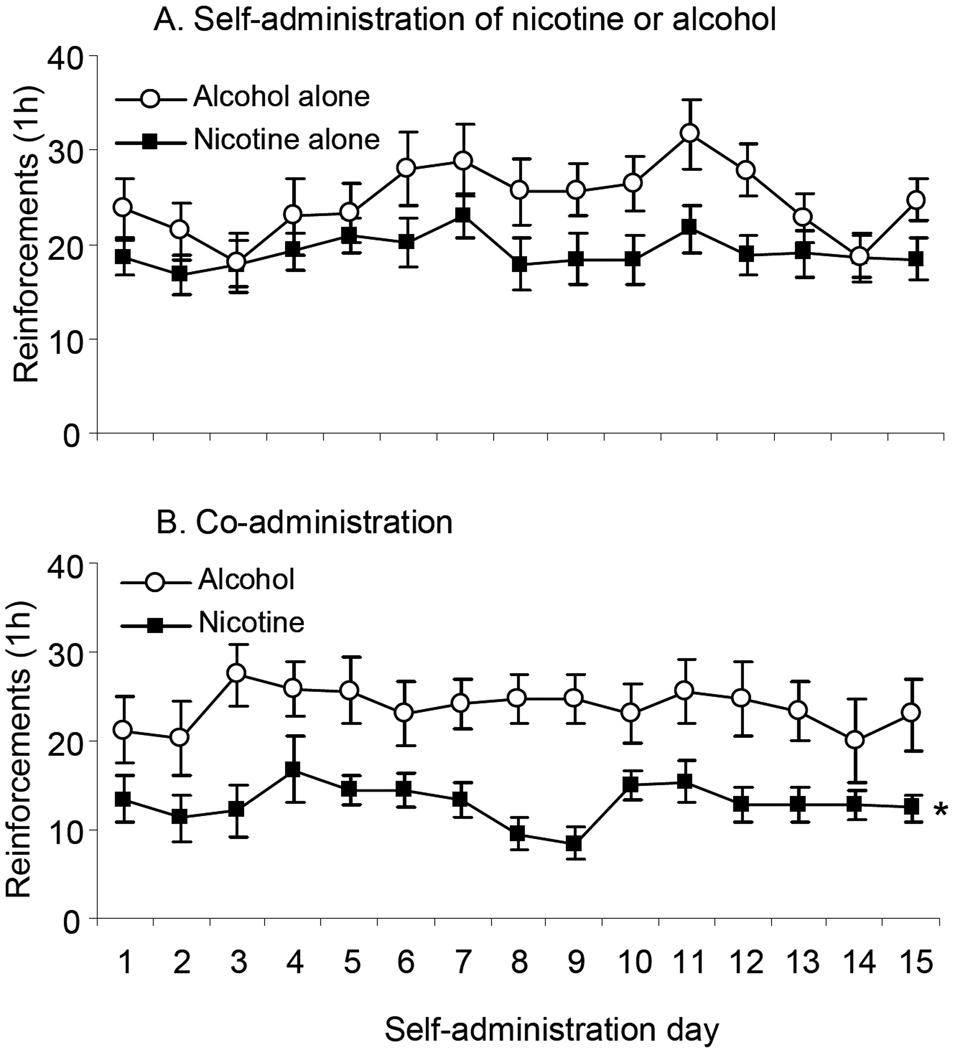
Figure 2 shows the daily responding of animals trained to self-administer nicotine or alcohol alone (A), or to co-administer nicotine and alcohol (B). A mixed ANOVA with the within factor of Day (1 to 15) and between factor of Group (Nicotine alone, Nicotine with alcohol access) done on numbers of nicotine reinforcements revealed a significant main effect of Day (F(14,392)=3.377, p<0.05), and Group (F(1,28)=5.146, p<0.05), as animals co-administering nicotine and alcohol received significantly fewer nicotine reinforcements than those self-administering nicotine alone. The complementary analysis done on alcohol reinforcements showed a significant effect of Day (F(14,350)=1.82, p<0.05), but no effect of Group or interaction, indicating that concurrent access to nicotine did not affect the numbers of alcohol reinforcements received and consumed.
Figure 2. Nicotine or alcohol self-administration and co-administration of both drugs in animals trained to self-administer alcohol first.
A. Mean (± sem) numbers of reinforcements earned by animals trained to self-administer nicotine (30 µg/kg/infusion, N=11) or alcohol (0.19 ml of 12% w/v, N=11) alone at fixed ratio (FR)-3 in 1 h daily sessions. B. Mean reinforcements in animals co-administering both drugs (N=12). In this experiment, 8 rats were excluded due to catheter blockage, and 1 due to low alcohol consumption. * Significant main effect of Group (p<0.05).
Experiment 2: Co-administration of nicotine and alcohol in animals trained to self administer nicotine first
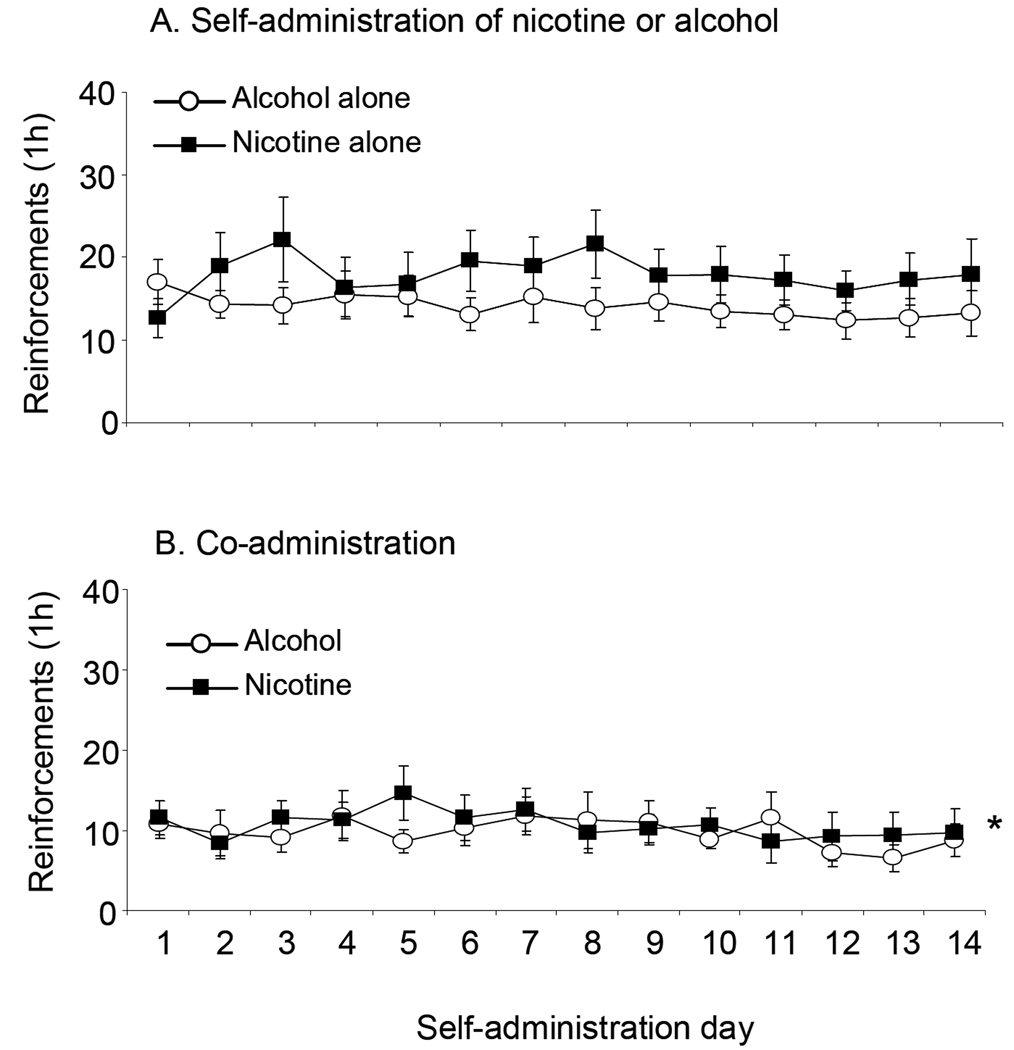
Figure 3 shows the reinforcements obtained by animals trained to self-administer nicotine or alcohol alone (A), or to self-administer nicotine and then alcohol, and then given access to both (B) over 14 self-administration sessions. The mixed ANOVA with the within factor of Day (1 to 14) and between factor of Group (Nicotine alone, Nicotine with alcohol access) done on numbers of nicotine reinforcements revealed a significant main effect of Group (F(1,19)=4.88, p<0.05), as overall, animals co-administering alcohol and nicotine self-administered less nicotine than those self-administering it alone. There were no significant main effects of Day or Group or interaction in the mixed ANOVA done on alcohol reinforcements in animals trained to self-administer alcohol alone or to co-administer both drugs.
Figure 3. Nicotine or alcohol self-administration and co-administration of both drugs in animals trained to self-administer nicotine first.
A. Mean (± sem) reinforcements earned by animals trained to self-administer nicotine (N=10) or alcohol (N=12) alone at FR-3 in 1 h daily sessions. B. Mean einforcements in animals co-administering both drugs (N=12). Six animals were excluded from analysis due to catheter blockage. * Significant main effect of Group (p<0.05).
Experiment 3: Extinction and reinstatement in rats trained to self-administer alcohol and nicotine concurrently
Figure 4A shows responding for alcohol and nicotine during the last three days of training for, respectively, self-administration of alcohol and nicotine alone and then when animals were given the opportunity to co-administer both drugs. The reintroduction of alcohol significantly reduced responding for nicotine, as shown by a repeated measures analysis done on the mean numbers of nicotine reinforcements obtained by rats in the 3 days prior to the reintroduction of alcohol and the 15 days after (F(15,542)=4.11, p<0.05). Post hoc analysis revealed that this was due to significant reductions in responding for nicotine on each of the 15 days after alcohol was reintroduced (p’s <0.05). Analysis of alcohol reinforcements in the 15 days of co-administration revealed a significant effect of Day, as there was a decrease in the numbers of reinforcements across the co-administration sessions (F(14,508)=7.27, p<0.05).
Figure 4. Training for co-administration of nicotine and alcohol.
A. Mean (± sem) reinforcements of nicotine and alcohol obtained prior to (Day −3 to 3) and after (Day 4 to 18) initiation of co-administration sessions. Days −3 to −1, show the numbers of alcohol reinforcements on the last three days of training with alcohol (0.19 ml, 12% w/v) alone at FR-3 are presented. On Days 1–3, the mean numbers of nicotine reinforcements (30 µg/kg/infusion) earned on the last three days of training for nicotine self-administration at FR-3 are shown. The break in the graph depicts the interval during which i.v. surgery, recovery and the initial training for nicotine self-administration occurred (30 days). B. Mean alcohol and nicotine reinforcements obtained by animals on Day −2 (alcohol) and 3 (nicotine), depicted in 5 min intervals. C. Mean reinforcements obtained by animals trained to co-administer alcohol and nicotine on Day 11 of co-administration, in 5 min intervals. For all graphs, N=34. * Significantly different from the average number of nicotine reinforcements on Days 1–3 (p<0.05). + Significantly different from preceding intervals for alcohol (p<0.05). # Significantly different from preceding intervals in nicotine alone group (p<0.05).
In order to provide more detail on the relationship between nicotine and alcohol self-administration in animals co-administering the two drugs, we show in Figure 4B and C the numbers of nicotine and alcohol reinforcements in 5 minute intervals earned by animals self-administering alcohol (Day -2) or nicotine (Day 3) alone (B), and by animals self-administering both concurrently (Day 11) (C). They were selected to be representative of days involving self-administration of single drugs and of co-administration. Due to a problem with the data extraction program, only these days had interval data from all animals.
Mixed ANOVAs with the within factor of Interval (5–60 min) and between factor of Group (Single drug alone, Co-administration) were done separately on nicotine and alcohol reinforcements. For both nicotine and alcohol reinforcements, there was a significant effect of group (Nicotine: F(1,65)=7.45; Alcohol: F(1,66)=24.69, p’s<0.05), as overall, animals co-administering both drugs received fewer reinforcements than when they were self-administering either drug alone. There were also significant Group × Interval interactions in these analyses (Nicotine: F(11,65)=1.98; Alcohol: F(1,66)=17.92, p’s<0.05), which were followed by separate ANOVAs on alcohol and nicotine reinforcements for each of the different drug and co-administration conditions with the within factor of interval. The factor of Interval was significant for both nicotine and alcohol alone (Alcohol: F(11,33)=62.17, p<0.05; Nicotine: F(11,32)=4.46, p<0.05). Post-hoc analyses showed that for alcohol, the 5, 10 and 15 min intervals were significantly higher than all succeeding intervals, while for nicotine alone, responding was higher in the first 5 min of the session compared to intervals 10 to 50 min (B).
Overall, animals received fewer nicotine and alcohol reinforcements under conditions of co-administration (Figure 4C). Animals received the highest numbers of alcohol reinforcements during the first 10 minutes of the session; this was reflected in a significant effect of Interval (F(11,33)=9.47, p<0.05). Post hoc analysis showed that numbers of alcohol reinforcements at 5 and 10 min were significantly higher than all succeeding intervals (p’s<0.05). The numbers of nicotine reinforcements earned per 5 min period did not vary significantly as a function of interval during co-administration.
Experiment 3a Extinction of nicotine and/or alcohol seeking in rats with concurrent access to the two drugs
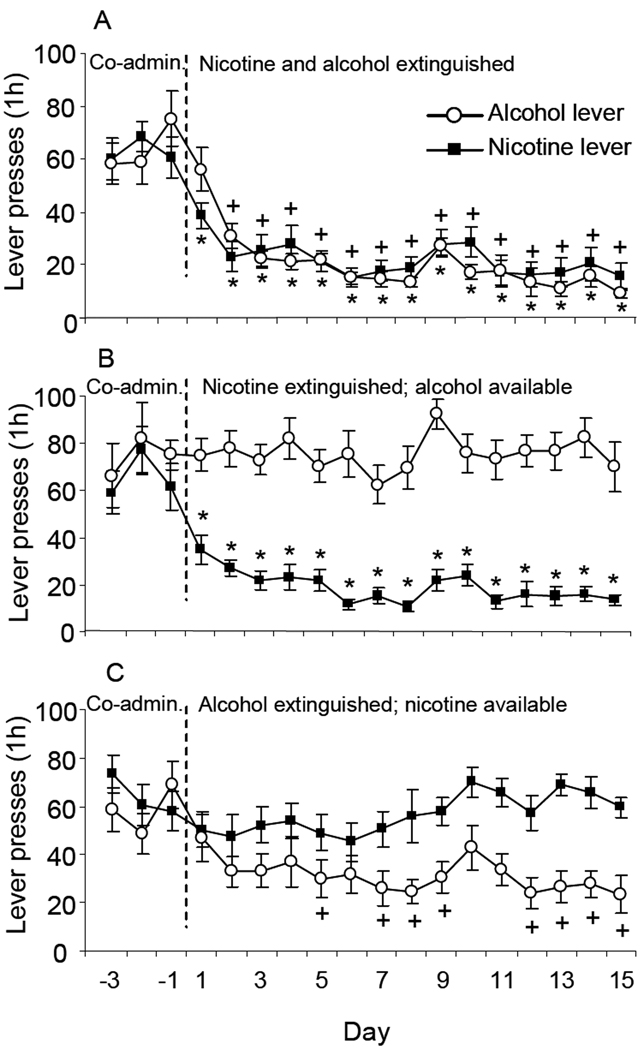
Extinction of responding for both drugs resulted in comparable and significant decreases in numbers of lever presses on the nicotine and alcohol associated levers (Figure 5A), as shown by separate ANOVAs with the within factor of Day (Baseline, Extinction days 1–15) done on numbers of responses made on the levers previously associated with nicotine and alcohol (Nicotine lever: F(15,191)=11.61; Alcohol lever: F(15,191)=16.14, p’s<0.05).
Figure 5. Responding of animals trained to co-administer nicotine and alcohol subject to different extinction conditions.
A. Mean (± sem) responses on the nicotine and alcohol-associated levers in animals subject to extinction of responding for nicotine and alcohol (N=12). B. Extinction of responding for nicotine with continued access to alcohol (N=11). C. Extinction of responding for alcohol with continued access to nicotine (N=11). + Significantly different from the mean responses on the alcohol lever during the last 3 days of co-administration. * Significantly different from the mean responses on the nicotine lever during the last 3 days of co-administration.
In the group in which responding for nicotine was extinguished, but alcohol access was maintained, numbers of lever responses for nicotine decreased significantly (Figure 5B). An ANOVA with the within factor of Day (Baseline, Extinction days 1–15) and between factor of Group (Nicotine extinguished with alcohol available, Both extinguished) revealed a significant effect of Day (F(1,367)=24.43, p<0.05), but not of Group or an interaction, suggesting that animals receiving extinction of both drugs extinguished at a rate similar to those receiving extinction of only nicotine.
In the group in which responding for alcohol was extinguished, but nicotine access was maintained, numbers of alcohol lever responses decreased significantly (Figure 5C). The rate of extinction of responding for alcohol was significantly slower in these animals compared to those receiving simultaneous extinction of both drugs. An ANOVA done with the within factor of Day (Baseline, Extinction days 1–15) and between factor of Group (Alcohol extinguished with nicotine available, Both extinguished) revealed a significant effect of Day (F(15,366)=13.64, p<0.05) and a significant Day × Group interaction (F(15,366)=2.359, p<0.05). Post hoc analyses revealed significantly decreased responding on the alcohol associated lever on extinction days 2 to 15 in the group receiving extinction of both drugs, but only on extinction days 5, 7–9 and 12–15 in the group with only alcohol extinguished.
Experiment 3b: Effects of different extinction conditions on reinstatement of responding by priming injections of alcohol or nicotine in rats trained to co-administer both drugs
In animals where responding for both alcohol and nicotine was extinguished, nicotine induced a significant reinstatement of responding on the levers previously associated with each drug, compared to the saline baseline (Figure 6, panel A) (Nicotine lever: F(1,21)=29.35, p<0.05, Alcohol lever: F(1,21)=22.42, p<0.05). Priming injections of alcohol in these animals induced a significant reinstatement of responding on both the nicotine and alcohol-associated levers (B) (Nicotine lever: F(1,19)=5.87, p<0.05; Alcohol lever: F(1,19)=15.11, p<0.05).
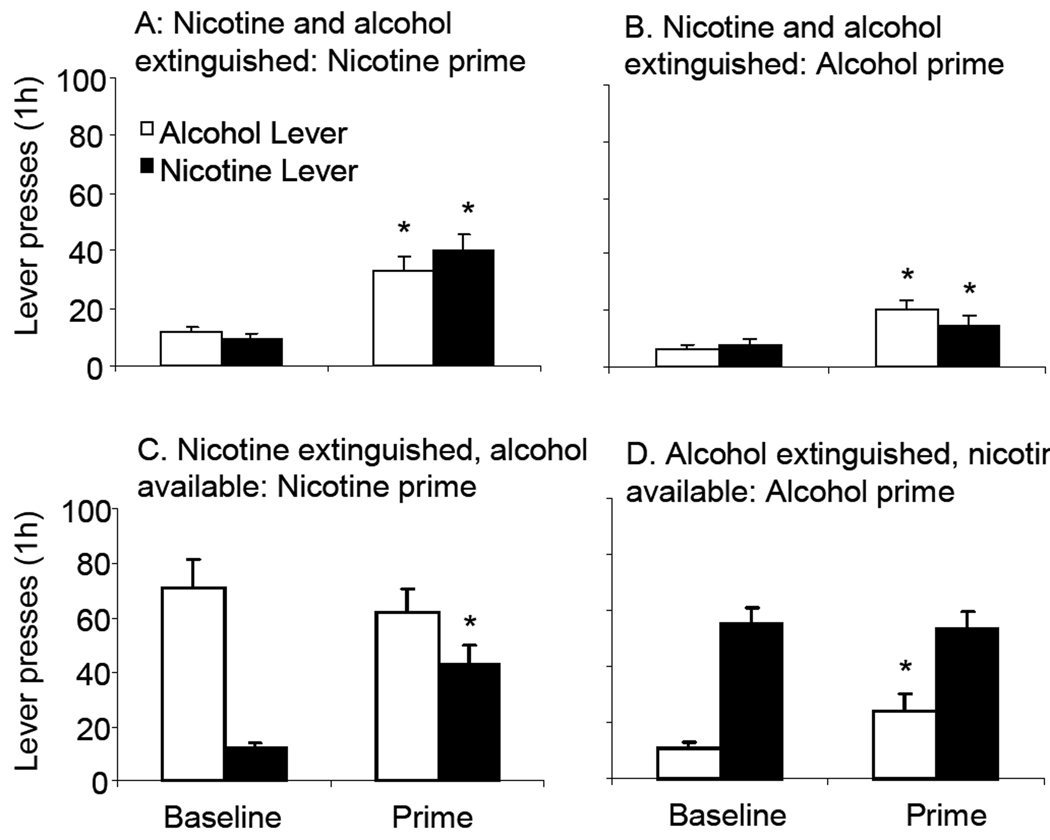
Figure 6. Reinstatement induced by nicotine or alcohol primes in rats trained to co-administer nicotine and alcohol subject to different extinction conditions.
Effects of priming with nicotine (A) or alcohol (B) in rats subject to extinction of responding for nicotine and alcohol. C. Effects of a nicotine prime in rats that received extinction of nicotine, but with continued access to alcohol (N=11). D. Effects of an alcohol prime in rats that received extinction of alcohol, with continued access to nicotine (N=9). Mean (± sem) numbers of lever presses on the nicotine and alcohol-associated levers are presented. In each graph, the left bars represent baseline responding on the day prior to the priming test, and the right bars responding on the priming test day. Nicotine (0.15 mg/kg, s.c.) was administered 15 min before the test. The alcohol prime comprised a non-contingent presentation of 0.19 ml of alcohol (12% w/v) into the drinking cup at the onset of the test session. Ten rats were excluded from analysis due to catheter blockage and four that failed to meet the extinction criterion. In Experiment 3, ten rats were excluded from analysis due to catheter blockage and four due to failure to meet the extinction criterion. * Significantly different from baseline (p<0.05).
In animals whose responding for nicotine was extinguished, but received continued access to alcohol, injections of nicotine significantly reinstated responding for nicotine (F(1,21)=26.72, p<0.05) (C). Alcohol self-administration was not affected by priming injections of nicotine.
Priming injections of alcohol significantly reinstated responding on the lever previously associated with alcohol in alcohol-extinguished animals that received continued access to nicotine (F(1,17)=7.57, p<0.05)(D). Nicotine self-administration was unaffected by the alcohol prime.
Discussion
These experiments were done in order to determine whether rats would concurrently self-administer intravenous nicotine and oral alcohol. Previously, the relationship between nicotine and alcohol self-administration has been investigated only with the technique of non-contingent injections of nicotine or alcohol in animals trained to self-administer the other drug. We found in the present series of studies that when both drugs were concurrently available, both nicotine and alcohol was self-administered in a stable manner, amounts comparable to animals self-administering either of the drugs alone.
One of our goals was to determine how access to one drug affected responding for the other. We observed significant decreases in nicotine self-administration as a function of concurrent access to alcohol in animals trained to self-administer alcohol prior to nicotine, and also when the order of training was reversed. Even though alcohol access resulted in significantly reduced responding for nicotine, animals continued to self-administer nicotine in amounts comparable to what is seen in animals self-administering nicotine alone.
The decrements in nicotine self-administration were greater than for alcohol during co-administration. Results obtained using the progressive ratio schedule would have predicted the opposite, since they show that alcohol is a weaker reinforcer than nicotine (Donny et al. 1999; Rodd et al. 2003; Shram et al. 2008). A possible reason for this may be that the sedative or motor-impairing properties of alcohol reduce responding for nicotine (White et al. 2002). This may also summate with the mild sedative properties that have been described for nicotine (Brielmaier et al. 2007).
Interestingly our findings are consistent with those from a study in rats trained to self-administer nicotine and cocaine concurrently (Manzardo et al. 2002). Access to cocaine was found to reduce nicotine self-administration. It is not known whether this pattern would occur with nicotine in cases when it is co-administered with other drugs.
Previous work has demonstrated increased alcohol self-administration induced by injections of nicotine (Le et al. 2000; Le et al. 2003; Olausson et al. 2001; Smith et al. 1999). We did not observe any evidence for nicotine-induced increases in alcohol self-administration when it was co-administered. One interpretation of this is that increases are observed when nicotine is administered non-contingently, but not when voluntarily self-administered. Another is that the doses used in the studies demonstrating nicotine-induced increases in alcohol self-administration are higher than those self-administered by the rats in our studies, although comparisons are difficult due to the different routes of administration.
Differences in the within-session pattern of responding for the two drugs may also help to explain why nicotine did not affect alcohol self-administration in animals with concurrent access. We found that the most of the alcohol reinforcements were earned during the first 10–15 min of the 1 h session, while nicotine reinforcements were earned more steadily across the session. These results are in agreement with previous descriptions of alcohol “loading” in the early portions of operant alcohol self-administration sessions (Williams and Broadbridge 2009). Given this pattern, nicotine would not be expected to have a marked effect on alcohol intake. The use of longer session durations may help to reveal effects of self-administered alcohol or nicotine on the other drug.
We also determined how the continued availability of alcohol and nicotine affect extinction of responding for the other drug, and compared these conditions to one where responding for both was extinguished. This was done to help address the question of whether alcoholism or nicotine addiction or both should be treated when they occur co morbidly in humans. The few clinical studies on this issue have produced mixed results. Discontinuation of smoking may increases the chances of relapse to alcohol use (Kozlowski et al. 1989). Another study found that alcoholics who continue to smoke may be more likely to relapse to alcohol (Stuyt 1997). Others have reported that smoking cessation programs do not interfere with the success of alcohol treatment (De Soto et al. 1989) or that unaided smoking cessation might actually enhance the response to treatment for alcoholism (Karam-Hage et al. 2005). The reasons for these discrepant results are not known.
We found that animals trained to co-administer nicotine and alcohol showed a progressive decline in responding when the delivery of both drugs was withheld. Likewise, animals showed a decline in responding for nicotine when it was withheld, when alcohol was still available. Interestingly, when alcohol was withheld with nicotine still available, the extinction of responding for alcohol was delayed. One interpretation of this is that continued self-administration of nicotine prolongs the motivation to seek alcohol. This is consistent with work showing that nicotine users have lower recovery rates from alcoholism (Stuyt 1997).
A potential explanation for the reduction in the rate of extinction of responding for alcohol in animals with continued access to nicotine deals with the interaction of nicotine with cues. Nicotine self-administration has been shown to be greatly potentiated when response-contingent cues are present (Caggiula et al. 2002), and moreover, non-contingent injections of nicotine can increase responding for visual cues alone (Donny et al. 2003). In our studies, the response contingent visual and auditory cues were present during self-administration, extinction and reinstatement. In this light, it may be that the self-administered nicotine in this group potentiated responding for the cue previously associated with alcohol during the extinction sessions. Given the importance of response-contingent cues in nicotine self-administration, it would be of interest in future studies to specifically examine their role in co-administration and in extinction and reinstatement after concurrent access.
Relapse or reinstatement in response to priming with the abused drug is another hallmark of addiction. Little is known about the effects of priming doses of nicotine or alcohol when their abuse is co morbid. We therefore determined the effects of nicotine or alcohol primes or responding in animals trained to co-administer whose lever pressing for nicotine, alcohol or both was extinguished. In co-administering animals whose responding for both alcohol and nicotine were extinguished, priming injections of nicotine or presentation of alcohol reinstated responding on both the nicotine and alcohol-associated levers. Continued access to nicotine or alcohol, while extinguishing responding for the other drug, did not make animals more or less susceptible to reinstatement by, respectively, alcohol or nicotine primes. These results are consistent with findings in experimental animals that nicotine can reinstate extinguished responding for alcohol or nicotine, and that alcohol priming induces reinstatement of responding for alcohol (Le et al. 1999; Le et al. 1998; Le et al. 2003). They also show that alcohol can induce a modest reinstatement of responding for nicotine.
An important limitation of these results is that the alcohol and nicotine training experiences differed among the various groups in Experiments 1 and 2, making the interpretation of the observed between-group differences in responding problematic. A number of studies have shown that prior experience with drug may modify responding for that or another drug (Shoaib et al. 1997; Valadez and Schenk 1994). In order to make unambiguous between-group comparisons, all animals should have identical nicotine and alcohol experience prior to assignment to the alcohol, nicotine or co-administration groups. Future studies using the co-administration procedure are planned that will include groups whose drug experiences are rigorously equated in this way. Although this limitation may impact the interpretation of group comparisons, it does not diminish the primary goal achieved by these studies, the demonstration that rats will reliably self-administer alcohol and nicotine concurrently.
Groups of animals were run that received experience with only nicotine or alcohol, and their responding was compared with co-administering animals. These “single drug” groups were trained using the routine training procedures of our laboratory. This comparison is important as it allows the comparison of the behavior of co-administering animals to our previously published findings with alcohol or nicotine alone.
Additionally, although training differences among the groups may impact between-group comparisons, it is less of a problem for contrasts made within a particular self-administration group. A major effect we demonstrated in terms of the relationship between alcohol and nicotine was a reduction in nicotine self-administration when alcohol was made available concurrently. We found this using both within- and between-group methodologies.
Another limitation is that the blood alcohol levels of animals co-administering nicotine and alcohol were not compared with those self-administering alcohol alone. As mentioned earlier, we recorded the numbers of reinforcements obtained and determined reinforcements consumed by measuring the alcohol left in the receptacle at the termination of all co- and self-administration sessions involving alcohol. We have noted that the consumption of 16–18 reinforcements (each 0.19 ml of 12% w/v alcohol) yields blood alcohol concentrations of about 70 mg/dl.
These studies employed a relatively short session length (1 h). It could be argued that this duration does not adequately model human nicotine and alcohol consumption, as humans typically co-administer these drugs over much longer time periods. An extension, therefore, would be to examine co-administration of the two drugs in longer sessions. We have collected preliminary data, comparing co-administration in 1 and 2 h sessions, and find that animals do self-administer significantly more alcohol and nicotine in sessions of the longer duration.
In the studies presented here, single doses of i.v. nicotine and oral alcohol for self-administration were used. One extension of the present work, therefore, is to determine how different self-administration doses of alcohol and nicotine influence the relationship between the two drugs during co-administration. Such studies may help shed light on the reductions in nicotine self-administration observed when alcohol was concurrently available. Another extension would be to test the effects of drugs used to treat alcohol or nicotine addiction on the co-administration of these drugs. This model would be advantageous in determining the most effective means to treat co morbid nicotine and alcohol addiction.
To summarize, the most important finding in these studies is that rats will self-administer significant amounts of nicotine intravenously and alcohol orally when the two drugs are concurrently available. The results of these experiments lay the groundwork for the development of an animal model of nicotine and alcohol co abuse.
Acknowledgments
This work was supported by grants from the Canadian Psychiatric Research Foundation and the NIAAA (AA13108) and a donation from the Shane McMullen Foundation to A.D. Lê.
References
- Brielmaier JM, McDonald CG, Smith RF. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicol Teratol. 2007;29:74–80. doi: 10.1016/j.ntt.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology. 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8:1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- De Soto CB, O'Donnell WE, De Soto JL. Long-term recovery in alcoholics. Alcohol Clin Exp Res. 1989;13:693–697. doi: 10.1111/j.1530-0277.1989.tb00406.x. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147:135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rose C, Jacobs KS, Mielke MM, Sved AF. Differential effects of response-contingent and response-independent nicotine in rats. Eur J Pharmacol. 2000;402:231–240. doi: 10.1016/s0014-2999(00)00532-x. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Funk D, Marinelli PW, Le AD. Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Res Health. 2006;29:186–192. [PMC free article] [PubMed] [Google Scholar]
- Hays JT, Schroeder DR, Offord KP, Croghan IT, Patten CA, Hurt RD, Jorenby DE, Fiore MC. Response to nicotine dependence treatment in smokers with current and past alcohol problems. Ann Behav Med. 1999;21:244–250. doi: 10.1007/BF02884841. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Moc K, Koob GF. Effects of naltrexone alone and in combination with acamprosate on the alcohol deprivation effect in rats. Neuropsychopharmacology. 2003;28:1463–1471. doi: 10.1038/sj.npp.1300175. [DOI] [PubMed] [Google Scholar]
- Kandel D, Chen K, Warner LA, Kessler RC, Grant B. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the U.S. population. Drug Alcohol Depend. 1997;44:11–29. doi: 10.1016/s0376-8716(96)01315-4. [DOI] [PubMed] [Google Scholar]
- Karam-Hage M, Pomerleau CS, Pomerleau OF, Brower KJ. Unaided smoking cessation among smokers in treatment for alcohol dependence. Addict Behav. 2005;30:1247–1253. doi: 10.1016/j.addbeh.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Skinner W, Kent C, Pope MA. Prospects for smoking treatment in individuals seeking treatment for alcohol and other drug problems. Addict Behav. 1989;14:273–278. doi: 10.1016/0306-4603(89)90058-0. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Rosen MI, O'Malley SS. Naloxone challenge in smokers. Preliminary evidence of an opioid component in nicotine dependence. Arch Gen Psychiatry. 1999;56:663–668. doi: 10.1001/archpsyc.56.7.663. [DOI] [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin. Exp. Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26:1872–1879. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus W, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress in rats. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Le AD, Quan B, Juzystch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Le AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology. 2003;168:216. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- Little HJ. Behavioral mechanisms underlying the link between smoking and drinking. Alcohol Res Health. 2000;24:215–224. [PMC free article] [PubMed] [Google Scholar]
- Manzardo AM, Stein L, Belluzzi JD. Rats prefer cocaine over nicotine in a two-lever self-administration choice test. Brain Res. 2002;924:10–19. doi: 10.1016/s0006-8993(01)03215-2. [DOI] [PubMed] [Google Scholar]
- Marshall CE, Dadmarz M, Hofford JM, Gottheil E, Vogel WH. Self-administration of both ethanol and nicotine in rats. Pharmacology. 2003;67:143–149. doi: 10.1159/000067801. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treatment of alcoholism: a clinical review. Alcohol. 1996;13:35. doi: 10.1016/0741-8329(95)02038-1. [DOI] [PubMed] [Google Scholar]
- Olausson P, Ericson M, Lof E, Engel JA, Soderpalm B. Nicotine-induced behavioral disinhibition and ethanol preference correlate after repeated nicotine treatment. Eur. J. Pharmacol. 2001;417:117–123. doi: 10.1016/s0014-2999(01)00903-7. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li TK, McBride WJ. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28:1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Samson HH. Repeated nicotine injections decrease operant ethanol self-administration. Alcohol. 2002;28:1–7. doi: 10.1016/s0741-8329(02)00238-0. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology. 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Stolerman IP. Plasma nicotine and cotinine levels following intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 1999;143:318–321. doi: 10.1007/s002130050954. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2008;33:739–748. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- Smith BR, Horan JT, Gaskin S, Amit Z. Exposure to nicotine enhances acquisition of ethanol drinking by laboratory rats in a limited access paradigm. Psychopharmacology. 1999;142:408–412. doi: 10.1007/s002130050906. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur. J. Pharmacol. 1999;371:123–135. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- Stuyt EB. Recovery rates after treatment for alcohol/drug dependence. Tobacco users vs. non-tobacco users. Am J Addict. 1997;6:159–167. [PubMed] [Google Scholar]
- Valadez A, Schenk S. Persistence of the ability of amphetamine preexposure to facilitate acquisition of cocaine self-administration. Pharmacol Biochem Behav. 1994;47:203–205. doi: 10.1016/0091-3057(94)90132-5. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Williams KL, Broadbridge CL. Potency of naltrexone to reduce ethanol self-administration in rats is greater for subcutaneous versus intraperitoneal injection. Alcohol. 2009;43:119–126. doi: 10.1016/j.alcohol.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Nobrega JN, Corrigall WA, Coen KM, Shannak K, Kish SJ. Amygdala dopamine levels are markedly elevated after self- but not passive-administration of cocaine. Brain Res. 1994;668:39–45. doi: 10.1016/0006-8993(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Wong GY, Wolter TD, Croghan GA, Croghan IT, Offord KP, Hurt RD. A randomized trial of naltrexone for smoking cessation. Addiction. 1999;94:1227–1237. doi: 10.1046/j.1360-0443.1999.948122713.x. [DOI] [PubMed] [Google Scholar]