Abstract
Coull and Nobre (2008) suggested that tasks that employ temporal cues might be divided on the basis of whether these cues are explicitly or implicitly processed. Furthermore, they suggested that implicit timing preferentially engages the left cerebral hemisphere. We tested this hypothesis by conducting a quantitative meta-analysis of eleven neuroimaging studies of implicit timing using the activation-likelihood estimation (ALE) algorithm (Turkeltaub, et al. 2002). Our analysis revealed a single but robust cluster of activation-likelihood in the left inferior parietal cortex (supramarginal gyrus). This result is in accord with the hypothesis that the left hemisphere subserves implicit timing mechanisms. Furthermore, in conjunction with a previously reported meta-analysis of explicit timing tasks, our data support the claim that implicit and explicit timing are supported by at least partially distinct neural structures.
1. Introduction
Behavioral and cognitive investigations in animals and humans over the past century have explored our shared ability to perceive time. Most experiments involve the presentation of temporal cues, such as a light or tone stimulus, about which subjects are to make a judgment. In the course of normal activities, however, timing procedures are often recruited during complex tasks, such as the perception of velocity or decoding speech. Timing is also crucial for action, as complex movements typically require temporally precise activations of agonists and antagonist muscles.
In a recent review of the literature on time perception, Coull and Nobre (2008) fractionated timing tasks on the basis of whether the timing mechanisms were explicitly or implicitly engaged. In explicit timing, the participant is instructed to attend to the duration of a stimulus. In contrast, implicit timing requires subjects to perform tasks for which timing is crucial, but not the primary focus of the task; implicit timing may be engaged during collision judgments or temporal cueing paradigms, in which a cue predicts the arrival time of a target (see Appendix). In a collision judgment task, for example, subjects must determine the speed at which one or more objects are moving – a process that is dependent on time estimation – and the predicted location of the object in the future. Thus, temporal processing is central to a collision judgment task but time is not the focus of the task. Similarly, in temporal cuing paradigms, temporal information conveyed by cues improves performance, although the temporal relationship between the cue and target stimulus is never made explicit and is not the subject’s focus.
Coull and Nobre (2008) hypothesized that explicit and implicit timing tasks engage distinct neural networks. Support for this claim came from functional imaging studies classified as engaging implicit timing mechanisms. Activation foci from these studies led to the suggestion that explicit timing tasks preferentially activate the right hemisphere as well as the supplementary motor area (SMA) and basal ganglia, whereas the left hemisphere is predominantly activated by implicit timing tasks. However, no quantitative basis for this claim was provided.
Data relevant to this question comes from our recent quantitative meta-analysis of neuroimaging studies of explicit timing (Wiener, Turkeltaub & Coslett, 2010). We found a differential activity that was dependent on stimulus duration and whether the task was “motor” or “perceptual” in nature. We found foci of activation in the SMA and right frontal region across all types of task. Additionally, the basal ganglia showed strong activation-likelihood, particularly in sub-second studies, These results provide support for the involvement of the SMA and basal ganglia in explicit timing, as suggested by Coull and Nobre (2008) and provide partial support for a right-hemispheric bias, However, the findings do not speak to the hypothesis that implicit timing engages a left-hemispheric network, as relevant tasks were not included in the meta-analysis. In order to identify the neural correlates of implicit timing, we report a meta-analysis of implicit timing neuroimaging studies classified by the criteria set forth by Coull and Nobre (2008).
2. Materials and Methods
2.1 Included Studies
The following selection criteria were employed to identify studies assessing implicit timing: 1) the study must have employed a cognitive task for which time is a necessary, but not overtly attended, dimension of task performance, 2) the durations employed were predictable 3) participants were not asked to provide an estimate of duration, 4) task activations were contrasted with an appropriate control task rather than rest or a passive viewing condition, 5) task contrasts were intended to demonstrate timing-related activations 6) results were reported in stereotactic 3-dimensional coordinates, 7) a-priori region-of-interest (ROI) analyses were not used for the reported results. Literature searches were conducted using PubMed and Medline databases, as well as by searching the reference sections of relevant studies and reviews (Coull & Nobre, 2008; Oliveri, Koch & Caltagirone, 2009). Using these criteria, we identified eleven studies for our meta-analysis. These studies are listed in Table 1 and a brief synopsis of each study is included in the Appendix.
Table 1.
Published studies investigating implicit timing mechanisms
| Study | Imaging | N Subjects | Task | Contrast (control) | Foci | Study # |
|---|---|---|---|---|---|---|
| Assmus et al. 2003 | fMRI | 12 | Allocentric Collision | Size judgment | 1 | 1 |
| Assmus et al. 2005 | fMRI | 12 | Allocentric Collision | Size judgment | 3 | 2 |
| Coull et al. 1998a | PET | 7 | Temporal Cueing | Spatial Cueing | 4 | 3 |
| Coull et al. 1998b | fMRI | 8 | Temporal Cueing | Spatial Cueing | 3 | 4 |
| Coull et al. 2000 | fMRI | 6 | Temporal Cueing | Trial Validity | 6 | 5 |
| Coull et al. 2001 | fMRI | 10 | Temporal Cueing | Neutral Cueing | 9 | 6 |
| Coull et al. 2008 | fMRI | 12 | Allocentric Collision Egocentnc Collision |
Allocentric Color judgment Egocentnc Collision Egocentric Color judgment |
8 | 7 |
| Dreher et al. 2002 | fMRI | 8 | Letter discrimination | Predictable - Unpredictable timed task switching | 11 | 8 |
| Field & Wann. 2005 | fMRI | 12 | Allocentric Collision | Inflation judgment | 9 | 9 |
| Geiser et al. 2008 | fMRI | 25 | Prosody | Isochronous - Nonisochronous | l | 10 |
| O’Reilly et al. 2008 | fMRI | 12 | Temporal Expectancy | Spatial Expectancy | 11 | 11 |
| Straube & Chatterjee, 2010 | fMRI | 16 | Causality Judgment | Increasing Time Delay | 1 | 12 |
2.2 Activation Likelihood Estimation
ALE models activation likelihood of published activation foci as Gaussian probability density distributions. The width of the Gaussians is calculated from the N of each experiment, using empirical estimates of the relationship between N and localization uncertainty (Eickhoff et al., 2009). In the current version of ALE (GingerALE 2.0 software; www.brainmap.org), experiment-level ALE maps (called modeled activation or MA maps) are calculated as the voxelwise union of probabilities associated with all the foci reported by an experiment, and ALE values are calculated as the voxelwise union of all MA maps in a dataset. This is mathematically equivalent to taking the union of probabilities associated with all foci in a dataset regardless of its source, and allows experiments to influence ALE values in proportion to the number of foci they report. To prevent this, we used a new version of ALE in which MA values are calculated as the maximum probability associated with any one focus reported by the experiment. The voxelwise union of MA maps is then taken to yield an ALE map which is unbiased by the number of foci reported by each experiment (Turkeltaub, et al. submitted). The modified algorithm was implemented by adjusting GingerALE 2.0 Java code (www.brainmap.org). A false-discovery rate (FDR) threshold of q < 0.01 was set for all significant voxels; minimum cluster size > 100 mm3 (Laird, et al. 2005). Additionally, we masked our results with a study contribution threshold (Wiener, et al. 2010; Turkeltaub & Coslett, 2010). In order for voxels to be considered significant, they must have been contributed by at least 3 separate experiments. Voxelwise ALE maps were rendered on the standard Colin brain; anatomical labels were assigned using the Talairach atlas daemon (www.talairach.org). The final ALE meta-analysis included 67 foci from across 12 separate experiments. All foci were in standardized Talairach coordinates; foci reported in MNI format were converted to Talairach space using the Lancaster transform (Lancaster, et al. 2007).
3. Results
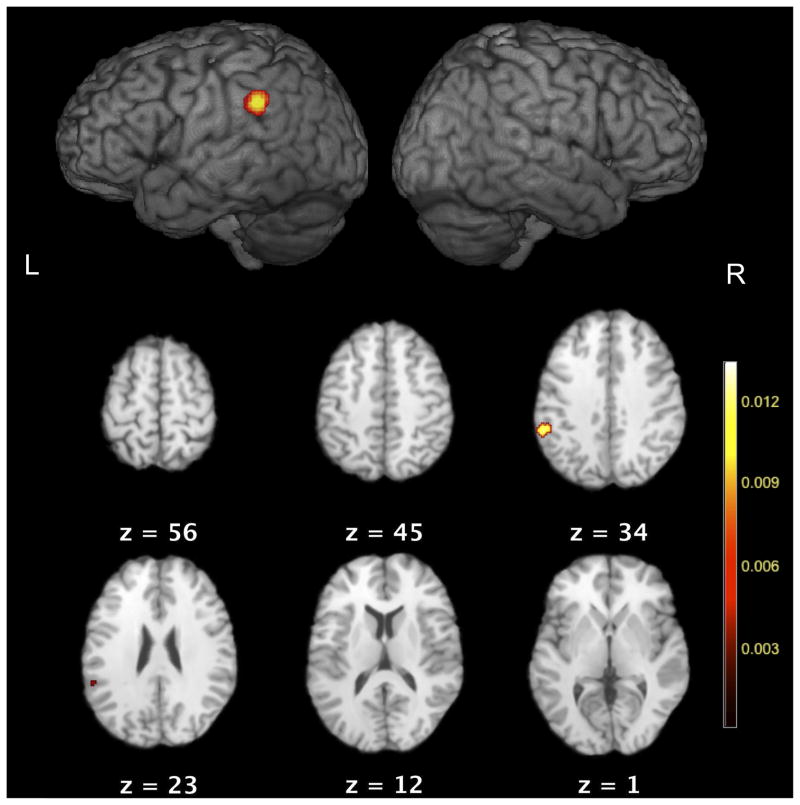
The results of the implicit timing meta-analysis revealed a single cluster of significant activation-likelihood located in the left inferior parietal cortex (supramarginal gyrus, x = −56, y = −42, z = 30, Table 2 and Figure 1). The volume of the cluster was 1328 mm3. Five separate experiments were found to contribute to this cluster, with a peak ALE value of 0.013. The extent of the cluster ranged from x = −50 to x = −62, z = 36 to z = 24 and y = −36 to y = −50, within the region of Brodmann area 40. A total of 7 out of 12 reported foci within 15mm of the left supramarginal gyrus cluster peak, two of which did not contribute to this cluster (Coull & Nobre, 1998; Geiser, et al. 2008).
Table 2.
Significant activation-likelihood clusters for implicit timing
| Location | x | y | z | ALE Value | Volume (mm3) | Studies |
|---|---|---|---|---|---|---|
| Left supramarginal gyrus (BA 40) | −56 | −42 | 30 | 0.013 | 1328 | [1], [2], [5], [6], [7] |
Figure 1.
ALE map of implicit timing studies. Surface renderings and axial slices with ALE value overlays are displayed. Values are significant at FDR q < 0.01, 3 or more experiments were required to contribute, cluster size > 100 mm3.
4. Discussion
The identification of a single left hemisphere cluster of voxels in the left supramarginal gyrus is in agreement with the hypothesis that implicit timing tasks preferentially activate the left hemisphere. Furthermore, the fact that the cluster is located in the inferior parietal lobe is in accordance with the hypothesis that implicit timing crucially depends on sensorimotor processes (Coull, 2004).
It is noteworthy that tasks in the left supramarginal cluster involved judgments of collision and temporal expectation. The fact that tasks with substantially different processing requirements generate a single cluster of voxels is consistent with the hypothesis that the voxels common to these tasks reflect a common component, the processes involved in implicit timing. Although all of the experiments that contributed to the left supramarginal gyrus cluster employed a motor response, we believe that the results are not likely to reflect the effect of motor processing because the studies employed suitable control tasks to minimize the effects of speeded motor responses.
The present results provide partial support for the claim that different brain regions support explicit and implicit timing. Although a right-hemispheric bias for explicit timing has only been partially confirmed (Wiener, et al. 2010), the present results speak to a left-hemispheric bias for implicit timing. The cluster in the present study was not observed in our explicit timing meta-analysis, further supporting the claim that the neural mechanisms for both tasks are distinct. Perhaps more interesting is the observation that we previously stimulated the left supramarginal gyrus using transcranial magnetic stimulation (TMS) during an explicit timing task, with no effect (Wiener, et al. 2009). Rather, a disruption of explicit timing task performance was only observed following stimulation of the right supramarginal gyrus, an area with significant activation-likelihood in our explicit timing meta-analysis.
One account of the involvement of the left supramarginal cortex in implicit timing appeals to the role of this brain region in motor processing. Data from functional imaging tasks demonstrates that supramarginal cortex is important for preparation of motor responses (e.g., Rushworth, Krams & Passingham, 2001). Additionally, investigations involving subjects with brain lesions or “virtual lesions” induced by TMS demonstrate that lesions or TMS of the left, but not right inferior parietal cortex induce deficits in motor attention tasks (Rushworth et al, 1997; Rushworth, Ellison & Walsh, 2001). Furthermore, posterior parietal cortex may be involved in the processes by which the sensory consequences of action are modeled and predicted (Medina, Jax & Coslett, 2009). Thus, on this account the involvement of the supramarginal cortex reflects its role in attending to and/or modeling action.
An alternative but closely related account is that the left supramarginal cortex is crucial not only for modeling action but for prediction more generally (see Schubotz, 2007). Many recent accounts of motor planning emphasize the role of “forward models” that predict the motor and sensory consequences of an action plan. Schubotz and colleagues (e.g., Bubic, von Cramon & Schubotz, 2010) have argued, however, that premotor and posterior parietal cortex support not only forward models of action but also predictions of external events more generally. Predictive events may be intrinsically tied to linguistic and spatiotemporal factors, as action verbs conjugated in the future, but not past tense can increase the excitability of motor cortex (Candidi, et al. 2010), and future-tense verbs are processed faster when displayed in the right visual hemifield (Oliveri, et al. 2009). Data from our meta-analysis suggests that the supramarginal cortex may be central to these predictions.
In conclusion, we conducted a quantitative meta-analysis of neuroimaging studies exploring implicit timing mechanisms. We found a single cluster of significant activation likelihood in the left supramarginal gyrus of the inferior parietal cortex. This result supports the hypothesis that implicit timing is a left-hemisphere mediated phenomenon. The specific role of this structure in implicit timing however, will need to be further verified with additional experimental paradigms, such as lesion studies or TMS.
Acknowledgments
This work was supported by the American Academy of Neurology Foundation (CRTF) awarded to PET and RO1MH076227 awarded to HBC. We thank Mick Fox for modifying the GingerALE 2.0 code to implement the algorithm used here. Additionally, the authors would like to thank Dr. Elaine Wencil for her insightful comments on a version of this manuscript.
5. Appendix
Coull and Nobre, 1998
This study was the first to employ a temporal cueing paradigm, an analog to the spatial cueing paradigm first developed by Posner (1980). Participants were required to respond as quickly as possible to the presentation of a visual target; a visual cue was presented for 100ms at trial onset, indicating where in space, time or both space and time the cue would appear. Time cues indicated whether the target would appear after a short (300ms) or long (1500ms) delay. Separate fMRI and PET experiments were conducted with two participant groups.
Coull et al. 20001
A follow-up fMRI study utilized the same temporal cueing paradigm as before, except the delay durations were set to 600ms or 1400ms. Also, the cues invalidly predicted the delay on 20% of the trials.
Coull, Nobre & Frith, 2001*
fMRI study of participants on or off the noradrenergic α2 agonist Clonidine. Participants performed the temporal cueing paradigm utilized previously, with delay durations of 600ms or 1400ms. Foci used in the meta-analysis came from analysis of placebo session data.
Dreher, et al. 2002
Participants performed two separate letter discrimination tasks, in which they were required to identify whether a presented letter was a vowel or consonant, or upper or lower case; both tasks required participants to respond as quickly as possible. Both tasks were presented in the same block of trials, with the tasks switching in a predictable or unpredictable manner between blocks. Also, the experimenters manipulated the timing with which the next trial was initiated as either being fixed (every 2500ms) or unpredictable. Foci used in the meta-analysis came from the main effect of predictable timing.
Assmus, et al. 2003*
fMRI study examining allocentric collision judgments. Participants viewed visual stimuli passing behind an occluder and were asked whether the stimuli would have collided.
Assmus, et al. 2005*
A follow-up fMRI study to Assmus et al. (2003). Participants again performed the same allocentric collision and size judgment task as before; task difficulty was varied parametrically between trials.
Field and Wann, 2005
fMRI study examining allocentric and egocentric collision judgments. participants viewed two visual stimuli approaching a point of observation, and were asked which of the two stimuli would arrive first. In egocentric trials, the point of observation was the observer.
Coull et al. 2008*
An fMRI study similar to Field and Wan (2005), in which participants made allocentric or egocentric collision judgments. However, the collision stimuli decelerated and disappeared prior to reaching the point of observation; participants were required to judge whether the moving stimuli would have collided with the point of observation.
O’Reilly, Mesulam & Nobre, 2008
In this fMRI study participants viewed a visual stimulus moving behind an occluder and re-appearing on the other side. Either the velocity or the direction of the visual stimulus was manipulated, such that the stimulus arrived on the other side of the occluder at an expected or unexpected position. Participants were required to make judgments about the velocity or direction of the stimulus in separate trials.
Geiser, et al. 2008
A speech-processing fMRI study in which participants listened to German sentences spoken either isochronously or non-isochronously. Separate groups of participants judged whether the sentences were isochronous or non-isochronous (rhythm), or whether the sentence was a question or not (prosody). The same contrast (isochronous – non-isochronous) was run for rhythm and prosody conditions with the intention of elucidating implicit timing mechanisms during the prosody task.
Straube & Chatterjee, 2010
An fMRI study of causality-judgment. Subjects viewed a moving visual stimulus collide with a stationary visual stimulus, which then continued moving. The angle at which the second visual stimulus moved, as well as the duration prior to moving after contact by the first visual stimulus were separately manipulated. Subjects were required to judge whether the first stimulus caused the second stimulus to move. The result used in the present meta-analysis comes from the main effect of increasing delay duration.
Footnotes
Contribute to the left supramarginal gyrus cluster
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Assmus A, Marshall JC, Ritzi A, Noth J, Zilles K, Fink GR. Left inferior parietal cortex integrates time and space during collision judgments. Neuroimage. 2003;20(Suppl 1):S82–8. doi: 10.1016/j.neuroimage.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Assmus A, Marshall JC, Noth J, Zilles K, Fink GR. Difficulty of perceptual spatiotemporal integration modulates the neural activity of left inferior parietal cortex. Neuroscience. 2005;132:923–927. doi: 10.1016/j.neuroscience.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Bubic A, von Cramon DY, Schubotz RI. Prediction, cognition and the brain. Frontiers in Human Neuroscience. 2010;4 doi: 10.3389/fnhum.2010.00025. article 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candidi M, Leone-Fernandez B, Barber HA, Carreiras M, Aglioti SM. Hands on the future: facilitation of cortico-spinal hand-representation when reading the future tense of hand-related action verbs. European Journal of Neuroscience. 2010;32(4):677–683. doi: 10.1111/j.1460-9568.2010.07305.x. [DOI] [PubMed] [Google Scholar]
- Coull J. fMRI studies of temporal attention: allocating attention within, or towards, time. Brain Research Cognitive Brain Research. 2004;21(2):216–226. doi: 10.1016/j.cogbrainres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Coull J, Nobre A. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. Journal of Neuroscience. 1998;18(18):7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J, Frith CD, Buchel C, Nobre A. Orienting attention in time: behavioural and neuroanatomical distinction between exogenous and endogenous shifts. Neuropsychologia. 2000;38(6):808–819. doi: 10.1016/s0028-3932(99)00132-3. [DOI] [PubMed] [Google Scholar]
- Coull J, Nobre AC, Frith CD. The noradrenergic α2 agonist clonidine modulates behavioural and neuroanatomical correlates of human attentional orienting and alerting. Cerebral Cortex. 2001;11(1):73–84. doi: 10.1093/cercor/11.1.73. [DOI] [PubMed] [Google Scholar]
- Coull J, Nobre A. Dissociating explicit timing from temporal expectation with fMRI. Current Opinion in Neurobiology. 2008;18(2):137–144. doi: 10.1016/j.conb.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Coull J, Vidal F, Goulon C, Nazarian B, Craig C. Using time-to-contact information to assess potential collision modulates both visual and temporal prediction networks. Frontiers in Human Neuroscience. 2008;2:10. doi: 10.3389/neuro.09.010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Koechlin E, Ali SO, Grafman J. The roles of timing and task order during task switching. Neuroimage. 2002;17(1):95–109. doi: 10.1006/nimg.2002.1169. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field DT, Wann JP. Perceiving time to collision activates the sensorimotor cortex. Current Biology. 2005;15:453–458. doi: 10.1016/j.cub.2004.12.081. [DOI] [PubMed] [Google Scholar]
- Geiser E, Zaehle T, Jancke L, Meyer M. The neural correlate of speech rhythm as evidenced by metrical speech processing. Journal of Cognitive Neuroscience. 2008;20(3):541–552. doi: 10.1162/jocn.2008.20029. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlledtime measurement: evidence from neuroimaging. Curr Opin Neurobiol. 2003;13(2):250–255. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Koch G, Caltagirone C. Spatial-temporal interactions in the human brain. Experimental Brain Research. 2009;195(4):489–497. doi: 10.1007/s00221-009-1834-1. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Bonni S, Turriziani P, Koch G, Lo Gerfo E, Torriero S, Vicario CM, Petrosini L, Caltagirone C. Motor and linguistic linking of space and time in the cerebellum. PLoS One. 2009;4(11):e7933. doi: 10.1371/journal.pone.0007933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JX, Mesulam MM, Nobre A. The cerebellum predicts the timing of perceptual events. Journal of Neuroscience. 2008;28(9):2252–2260. doi: 10.1523/JNEUROSCI.2742-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Nixon PD, Renowden S, Wade DT, Passingham RE. The left parietal cortex and motor attention. Neuropsychologia. 1997;35(9):1261–1273. doi: 10.1016/s0028-3932(97)00050-x. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Ellison A, Walsh V. Complementary localization and lateralization of orienting and motor attention. Nature Neuroscience. 2001;4(6):656–661. doi: 10.1038/88492. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Krams M, Passingham RE. The attentional role of the left parietal cortex: the distinct lateralization and localization of motor attention in the human brain. Journal of Cognitive Neuroscience. 2001;13(5):698–710. doi: 10.1162/089892901750363244. [DOI] [PubMed] [Google Scholar]
- Schubotz RI. Prediction of external events with our motor system: towards a new framework. Trends in Cognitive Science. 2007;11(5):211–218. doi: 10.1016/j.tics.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Straube B, Chatterjee A. Space and time in perceptual causality. Frontiers in Human Neuroscience. 2010;4:28. doi: 10.3389/fnhum.2010.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J, Jax SA, Coslett HB. Two-component models of reaching: evidence from deafferentation in a Fitts’ law task. Neuroscience Letters. 2009;451(3):222–226. doi: 10.1016/j.neulet.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16(3):765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Wiener M, Turkeltaub PE, Coslett HB. The image of time: a voxel-wise meta-analysis. Neuroimage. 2010a;49(2):1728–1740. doi: 10.1016/j.neuroimage.2009.09.064. [DOI] [PubMed] [Google Scholar]
- Wiener M, Hamilton R, Turkeltaub PE, Matell MS, Coslett HB. Fast forward: supramarginal gyrus stimulation alters time measurement. J Cogn Neurosci. 2010b;22(1):23–31. doi: 10.1162/jocn.2009.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]