Abstract
Alpha-dystroglycan is a cell-surface glycoprotein that acts as a receptor for both extracellular matrix proteins containing laminin-G domains and certain arenaviruses. Receptor binding is thought to be mediated by a post-translational modification, and defective binding with laminin underlies a subclass of congenital muscular dystrophy. Here, using mass spectrometry- and NMR-based structural analyses, we identified a phosphorylated O-mannosyl glycan on the mucin-like domain of recombinant alpha-dystroglycan, which was required for laminin binding. We demonstrated that patients with muscle-eye-brain disease and Fukuyama congenital muscular dystrophy, as well as mice with myodystrophy, commonly have defects in a post-phosphoryl modification of this phosphorylated O-linked mannose, and that this modification is mediated by the like-acetylglucosaminyltransferase (LARGE) protein. Our findings expand our understanding of the mechanisms that underlie congenital muscular dystrophy.
Diverse post-translational modifications influence the structure and function of many proteins. Dystroglycan (DG) is a membrane protein that requires extensive post-translational processing in order to function as an extracellular matrix receptor. It is comprised of an extracellular α-DG subunit and a transmembrane β-DG subunit (1). α-DG serves as a receptor for extracellular matrix laminin G domain-containing ligands such as laminin (1) and agrin (2) in both muscle and brain, and these interactions depend on an unidentified post-translational α-DG modification. α-DG is also the cellular receptor for lymphocytic choriomeningitis virus (LCMV), Lassa fever virus (LFV), and clade C New World arenaviruses (3, 4). Although the binding sites for LCMV and LFV on α-DG have not yet been identified, they are thought to overlap with the modification recognized by laminin (5, 6).
Glycosyltransferase-mediated glycosylation is one form of post-translational modification that can modulate protein structure and function. The main forms in mammals are N- and O-glycosylation, and these are distinguished by how the oligosaccharide moiety links to the amino acid. Mutations in six known or putative glycosyltransferase genes—POMT1 (7), POMT2 (8), POMGnT1 (9), fukutin (10), FKRP (11), and LARGE (12)—have been identified in patients with congenital muscular dystrophy (CMD). These disorders cover a spectrum of abnormalities affecting the brain, eye, and skeletal muscle, and show a dramatic gradient of phenotypic severity ranging from the most devastating in Walker-Warburg syndrome (WWS; OMIM# 236670), to less severe in muscle-eye-brain disease (MEB; OMIM# 253280) and Fukuyama CMD (FCMD; OMIM# 253800), and to mild limb-girdle muscular dystrophies. In these diseases, the ability of α-DG to bind laminin is markedly reduced (13), suggesting that these (putative) glycosyltransferases participate in the post-translational modification that enables α-DG to bind laminin. Whereas the molecular functions of LARGE, fukutin, and FKRP remain unclear, POMT1/2 (14) and POMGnT1 (9) are known to catalyze two steps in the biosynthesis of an O-mannosyl tetrasaccharide (NeuNAc-α-2,3-Gal-β-1,4-GlcNAc-β-1,2-Man) that is found in high abundance on both brain and muscle α-DG (15, 16). However, this glycan itself is not likely the laminin-binding moiety, since glycosidase-mediated removal of all or most of the three sugars at the non-reducing terminus does not reduce α-DG binding to laminin (17).
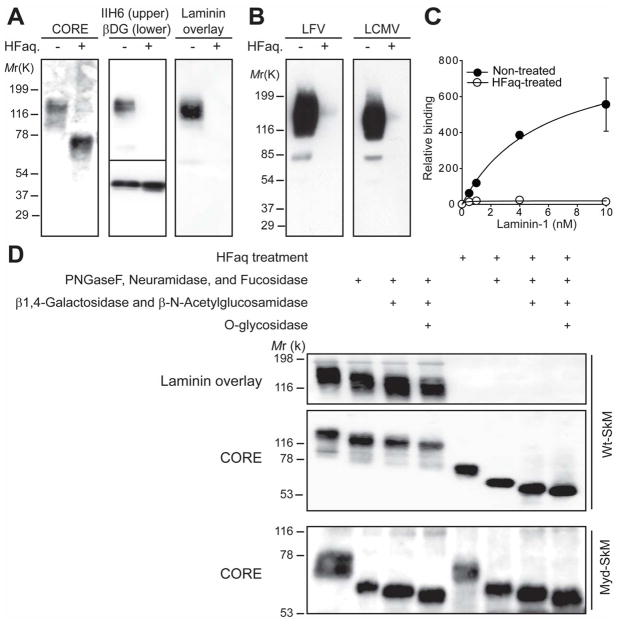
To determine which post-translational modification is necessary for the α-DG/laminin interaction, we processed wheat germ agglutinin-enriched proteins (glycoproteins) from C57BL/6J (wild type, Wt) muscle using various enzymatic and chemical treatments. We then analyzed the products by immunoblotting with antibodies against α-DG core protein (CORE), the laminin-binding form of α-DG (IIH6), and β-DG core protein (AP83), as well as by laminin overlay assay. Treatment with cold aqueous hydrofluoric acid (HFaq), which specifically cleaves phosphoester linkages (18) resulted in reduction of the α-DG Mr from 150- to 70-kDa, loss of IIH6 immunoreactivity and laminin binding (Fig. 1A), and loss of binding to LFV and LCMV (Fig. 1B). As the Mr of N-glycosylated β-DG did not change (Fig. 1A), these effects were not caused by the degradation of either peptide or glycosyl linkages. A quantitative solid-phase assay revealed that a 97% reduction in total high-affinity binding to laminin was achieved (Fig. 1C). HFaq treatment also abolished laminin-receptor activity of α-DG in heart, brain and kidney (fig. S1A). We next tested whether N-glycan and/or the two O-glycans known to modify the laminin-binding form of α-DG—Core1 O-glycan and the O-mannosyl tetrasaccharide (in either the sialylated or fucosylated form) (15, 16)—are sensitive to HFaq treatment. Immunoblotting of Wt-muscle glycoproteins treated with several cocktails of glycosidases that degrade these three glycans showed that the glycosidase-mediated reduction in α-DG glycosylation was impervious to HFaq treatment (Fig. 1D). Similar experiment using muscle glycoproteins from the CMD-model mouse LARGEmyd, in which a mutation in LARGE prevents the α-DG modification necessary for laminin-binding (19), revealed that HFaq treatment did not significantly reduce the Mr of α-DG (Fig. 1D). Thus, HFaq degrades specifically the laminin-binding moiety on α-DG. Finally, we tested the type of phosphorylation involved by digesting Wt-muscle glycoproteins with alkaline phosphatase. The laminin-receptor activity of α-DG (fig. S1B) was not affected, suggesting that functional modification of α-DG involves an internal phosphoryl linkage rather than a monoester-linked phosphate.
Fig. 1.
Chemical dephosphorylation by HFaq treatment abolishes laminin and virus binding to α-DG in tissues from Wt mice. (A and B) Treated glycoproteins prepared from Wt muscle were subjected to: (A) immunoblotting with antibodies against the α-DG core protein (CORE) or the laminin-binding form α-DG epitope (IIH6) and β-DG (AP83), or to laminin overlay assay; (B) virus overlay assays with γ-inactivated LFV or LCMV cl-13. (C) Wt muscle glycoproteins with and without HFaq-treatment were subjected to a solid-phase laminin-binding assay (n=3). Open circles=treated; closed circles=untreated. Error bars indicate standard deviation. (D) Muscle glycoproteins from Wt and Largemyd (Myd) mice were digested using cocktails of glycosidases that degrade sialylated and/or fucosylated N-glycan, Core1 O-glycan, and O-mannosyl glycan, before (first 4 lanes) and after (last 4 lanes) HFaq treatment. The products were subjected to either immunoblotting with CORE antibody or laminin overlay assay.
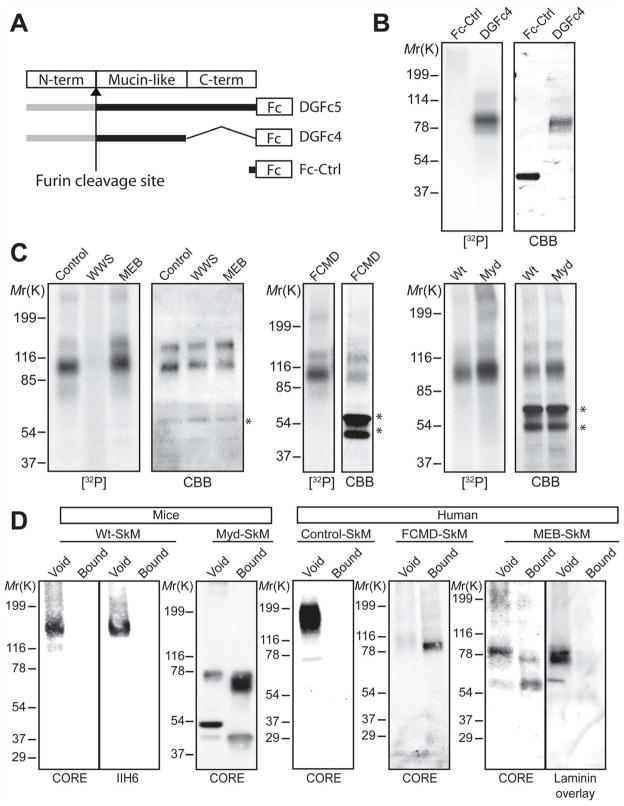
To verify that α-DG is phosphorylated, we labeled human embryonic kidney cells (HEK293) expressing Fc-tagged α-DG recombinants (Fig. 2A) that are secreted into the medium with [32P]-orthophosphate. Phosphor-imaging showed that secreted DGFc4, which contains only the mucin-like region of α-DG owing to cleavage of its N-terminus by a furin-like protease (20), was phosphorylated (Fig. 2B). When [32P]-DGFc4 was hydrolyzed under conditions conducive to the dissolution of polypeptide and phosphoester linkages to carbohydrates, but not to linkages to amino acids (21), inorganic phosphate but not phospho-amino acids were generated (fig. S2), suggesting that phosphorylation does not occur directly on the peptide. To test if the phosphorylation depends on glycosylation, we expressed DGFc5 in [32P]-orthophosphate-labeled human cells derived from POMT1-mutated WWS, POMGnT1-mutated MEB, fukutin-mutated FCMD, and control cells, as well as in fibroblasts from Largemyd and Wt mice (Fig. 2C). All except the POMT1-mutated WWS cells secreted [32P]-phosphorylated DGFc5 into the medium, strongly suggesting that phosphorylation occurs on the O-linked mannose of α-DG. By measuring inorganic phosphate following acid hydrolysis, we confirmed that native α-DG purified from rabbit skeletal muscle is phosphorylated, and further quantitated this modification at 4.7 mol phosphate per mol protein (SEM=0.22, n=3).
Fig. 2.
The mucin-like region of α-DG is phosphorylated in an O-mannosylation-dependent manner. (A) Structures of recombinant α-DG constructs used in the study. (B and C) [32P]-orthophosphate labeling of (B) Fc-Ctrl- or DGFc4-expressing HEK293 cells and (C) DGFc5-expressing cultured cells from CMD patients (WWS, MEB, FCMD) and control humans, and from Wt and Largemyd (Myd) mice. Fc-tagged recombinant α-DG was isolated from the culture medium using protein-A agarose, separated by SDS-PAGE, stained with Coomassie brilliant blue (CBB) and analyzed by phosphor-imaging ([32P]). Phosphorylation on α-DG required prior O-mannosylation. Asterisks indicate contaminating proteins derived from FBS. (D) IMAC-binding assay testing glycoproteins from Wt and Largemyd mice, and from FCMD, MEB, and control human muscle (SkM).
To assess if CMD cells that synthesize phosphorylated α-DG can further modify the phosphate residue, we immunoprecipitated glycoproteins from mouse Largemyd and Wt muscle, as well as from human POMGnT1-mutated MEB, fukutin-mutated FCMD, and control muscle, using immobilized metal affinity chromatography (IMAC)-beads that bind to monoester-linked, but not diester-linked, phosphorylated compounds. Only fukutin-mutated FCMD and Largemyd muscle α-DG were captured by the beads, revealing that the phosphate residue does not undergo further modification in these CMD cells (Fig. 2D). This suggests that fukutin and LARGE participate in a common pathway to assemble the laminin-binding moiety onto the phosphorylated O-linked mannose. This speculation is compatible with the fact that α-DG prepared from Largemyd muscle rescued by adenovirus-mediated expression of LARGE regains laminin-receptor activity and concomitantly loses its affinity for IMAC beads (fig. S3). In the case of POMGnT1-mutated MEB patient muscle, several forms of α-DG were observed; the majority of these were captured by the beads, although a certain amount of α-DG with laminin-binding activity was detected in the void fraction (Fig. 2D). This finding suggests that a defect in POMGnT1 partially inhibits modification on the phosphoryl branch chain of the O-mannosyl glycan on α-DG.
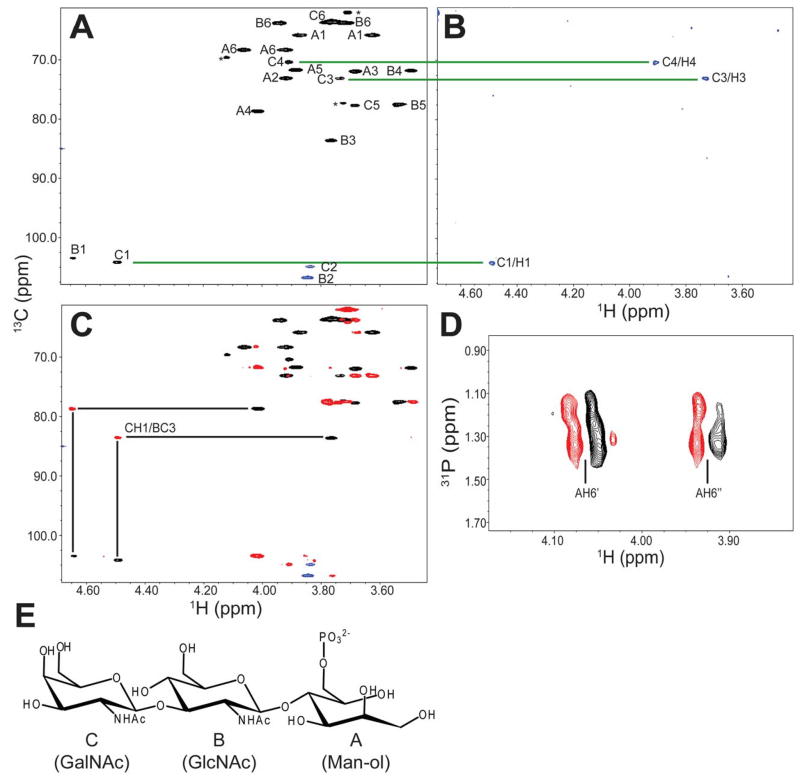
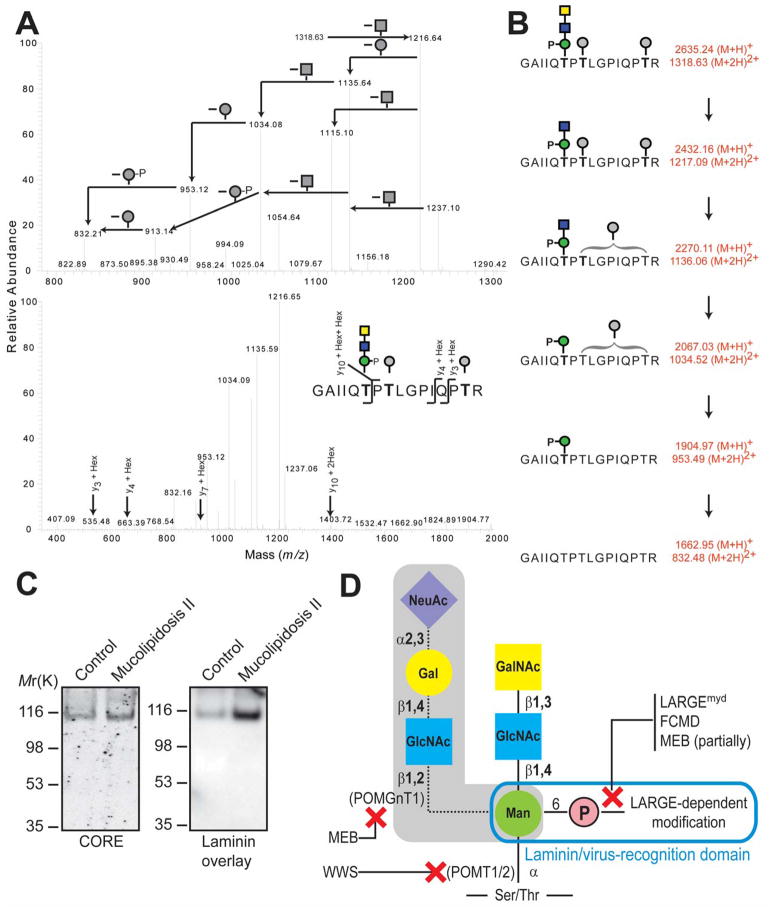
DGFc4 produced by HEK293 cells bound to IMAC-beads (fig. S4A) and gained laminin-binding activity when it was co-expressed with LARGE (fig. S4B); such a gain in activity has also been observed in FCMD, MEB, and Largemyd cells, both in this study and elsewhere (22). To determine the structure of the phosphorylated O-mannosyl glycan that appears to be necessary to assemble the laminin-binding moiety, we prepared O-glycans from HEK293-expressed DGFc4 by reductive β-elimination, and isolated the phosphorylated O-glycan using IMAC-beads. LTQ mass spectrometry-based analyses detected prominent ions at m/z 667 ([M-H]−) and m/z 333 ([M-2H]2−) which are assigned as a phosphorylated trisaccharide composed of HexNAc2Hexitol1 (fig. S5), and analysis by high performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) revealed the compositional sugars to be GlcNAc, GalNAc, and mannitol (fig. S6). Homo- and hetero-nuclear NMR techniques were used to assign the 13C/1H HMQC spectrum of the reduced O-glycan (Fig. 3A). The GlcNAc (subunit B) was assigned using DQF-COSY and TOCSY spectra with a series of mixing times (fig. S7). The GalNAc (subunit C) was partially assigned based on a selective-TOCSY-HSQC spectrum (Fig. 3B). The GalNAc (subunit C) is linked via a β1-3 linkage to the C3 position on GlcNAc (subunit B), which is in turn connected via a β1–4 linkage to the C4 position on mannitol (subunit A), as evidenced by the observed HMBC cross peaks CH1/BC3 and BH1/AC4 (Fig. 3C). The phosphate group is attached to the C6 position of the mannitol (subunit A) as determined from the cross peaks 31P/AH6’ and 31P/A6H” detected in the 31P/1H COSY spectrum (Fig. 3D). The complete NMR resonance assignments of the reduced O-glycan and its inter-residue correlations detected in the NOESY and HMBC spectra are summarized in table S1, and the determined structure is shown in Fig. 3E. To verify that this phosphorylated trisaccharide modifies the mucin-like domain of DGFc4, we enriched the trypsinized peptides using Wisteria Floribunda agglutinin-lectin and analyzed the GalNAc-terminated peptides by LCMS/MS. MS/MS fragmentation patterns at m/z 1318.63 (Fig. 4A), 1420.17 (fig. S9), and 1501.19 (fig. S10) identified a peptide (amino acids 374 to 389 of α-DG; Accession ID: CAA45732) bearing these modifications—the phosphorylated trisaccharide in conjunction with Hex-HexNAc-Hex, HexNAc-Hex or Hex. The presence of non-phosphorylated mannose-initiated structures on y3, y4, and y10 ions revealed that Thr379 is modified by the phosphorylated trisaccharide in all cases (Figs. 4AB, S9, and S10).
Fig. 3.
NMR analysis of phosphorylated O-glycan on HEK293-produced DGFc4. (A) HMQC spectrum where the assigned cross peaks are labeled with a letter for the subunit designated in (E), and a number for the position on that subunit. The folded cross peaks are indicated in red, and the cross peaks derived from sample impurities are marked by asterisks. (B) TOCSY-HSQC spectrum obtained using a selective excitation pulse at the subunit CH1 proton and a selective TOCSY mixing time of 113 ms. (C) HMBC (green) and HMQC (black) spectra for the assignment of interglycoside linkages. (D) 31P/1H COSY spectrum. (E) Structure of the O-glycan, with the sugar subunits labelled A–C.
Fig. 4.
Mapping of phosphorylated trisaccharide on HEK293-produced DGFc4, and characterization of mannosyl phosphorylation. (A) CID-MS/MS spectra from 780-1320 m/z (upper panel) and 375-2000 m/z (lower panel), revealing neutral loss pattern (upper panel) and peptide-derived b and y ions (lower panel) of the selected precursor ions at m/z 1318.63. Full FT mass spectrum is shown in fig. S8. Square: HexNAc; circle: Hexose. (B) Peptide and mononsaccharide unit identification based on fragmentation of the phosphorylated glycopeptide. (C) Glycoproteins prepared from cell lysates of fibroblasts derived from Mucolipidosis II patients and subjected to immunoblotting with CORE antibody, and to laminin overlay assay. (D) Schematic representation of O-mannosyl glycans on α-DG, with proteins involved in its biosynthesis, and diseases in which relevant steps are defective indicated. Shading indicates glycan identified in brain and muscle α-DG.
To assess where within the cell α-DG is phosphorylated, we separated the ER and Golgi of DGFc4-overexpressing HEK293 cells using an iodixanol gradient (fig. S11A). Immunoprecipitation with IMAC-beads showed that only the Golgi fraction contained phosphorylated DGFc4, suggesting that phosphorylation occurs in the Golgi (fig. S11B). Mannose-6-phosphorylation is known to occur on N-glycans during their passage through the Golgi, and to serve as the marker for targeting newly synthesized lysosomal enzymes to the lysosome (23). In this mannose-6-phosphate synthetic pathway, mannose is phosphorylated by the sequential actions of two enzymes: UDP-GlcNAc:lysosomal enzyme GlcNAc-1-phosphotransferase (OMIM# 607840), which transfers GlcNAc-1-phosphate to the C6 hydroxyl of mannose; and GlcNAc-1-phosphodiester α-N-acetylglucosaminidase (OMIM# 607985), which removes the terminal GlcNAc to form mannose-6-phosphate. Analysis of fibroblasts derived from patients with Mucolipidosis II (OMIM 252500), which have a defect in GlcNAc-1-phosphotransferase, revealed that they can synthesize the laminin-binding form of α-DG (Fig. 4C). Thus, an unidentified pathway phosphorylates the C6 hydroxyl of the O-mannosyl glycan.
We have identified a phosphorylated O-glycan on recombinant α-DG synthesized in HEK293 cells. Further, our comparative studies of α-DG in CMD and control muscle reveal that the phosphoryl chain on O-mannosyl glycan is required to assemble the laminin-binding moiety, and that LARGE directly participates in the post-phosphorylation biosynthetic pathway. MEB, FCMD and Largemyd cells, which have genetically distinct abnormalities, nevertheless show a similar defect in processing of the phosphoryl branch chain on the O-mannosyl glycan (Fig. 4D). These convergent mechanisms to pathology offer an explanation for previous reports that forced expression of LARGE can circumvent defects in α-DG modification in these CMD cells (22). We have shown that overexpressing LARGE, a putative glycosyltransferase with catalytic domains sharing homology with β-1,3-N-acetylglucosaminyltransferase and bacterial glycosyltransferase (19), increases the affinity of the cell surface for both the IIH6 antibody (Fig.S12A) and the Vicia villosa lectin (Fig. S12B). Thus, LARGE is likely to transfer glycosyl moiety to an O-linked mannose-6-phosphate.
To our knowledge, we provide the first evidence that a vertebrate non-GPI-anchored glycoprotein is modified by a phosphodiester linkage. Glycoproteins in the cell walls of yeasts and fungi bear phosphodiester-linked glycans generated by a process involving phosphorylation on the C6 hydroxyl of mannose (24). It is notable that α-DG, which is well conserved as an epithelial cell-surface protein in species ranging from lower vertebrates to mammals, is likewise modified by this ancient type of glycosylation. A recent study has shown that the most severe form of CMD—WWS—is a genetically heterogeneous disease. Moreover, only 40% of WWS cases are explained by mutations in known CMD-causative genes (24). Thus, a defect in the phosphorylation of an O-linked mannose may be responsible for severe CMD—in which case the discovery of mutations in novel genes responsible for WWS may not be far off.
Supplementary Material
Acknowledgments
25. We thank Michael Madson for insightful suggestions; M. E. Anderson and D. Venzke for technical assistance; L. M. Teesch for mass spectrometry, I. Nishino for providing WWS-patient cells, Paul D. Wellstone Muscular Dystrophy Cooperative Research Center Core B for providing patient cells and biopsies; the Gene Transfer Vector Core (UI, supported by NIH/NIDDK P30 DK 54759) for generating adenoviruses; the Glycotechnology Core Resource (UCSD) for HPAEC-PAD analysis; the Integrated Technology Resource for Biomedical Glycomics (UGA, supported by NIH/NCRR P41 RR018502) for tandem mass spectrometry; and past and present members of the Campbell lab for scientific contributions. This study was supported in part by a Paul D. Wellstone Muscular Dystrophy Cooperative Research Center Grant (1U54NS053672, K.P.C.), a U.S. Department of Defense Grant (W81XWH-05-1- 00794, K.P.C.), and AI55540 (to S.K. and M.B.A.O.). K.P.C. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
References and Notes
- 1.Ibraghimov-Beskrovnaya O, et al. Nature. 1992;355:696. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- 2.Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. Cell. 1994;77:675. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 3.Cao W, et al. Science. 1998;282:2079. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 4.Spiropoulou CF, Kunz S, Rollin PE, Campbell KP, Oldstone MB. J Virol. 2002;76:5140. doi: 10.1128/JVI.76.10.5140-5146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunz S, Sevilla N, McGavern DB, Campbell KP, Oldstone MB. J Cell Biol. 2001;155:301. doi: 10.1083/jcb.200104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunz S, Rojek JM, Perez M, Spiropoulou CF, Oldstone MB. J Virol. 2005;79:5979. doi: 10.1128/JVI.79.10.5979-5987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltran-Valero de Bernabe D, et al. Am J Hum Genet. 2002;71:1033. doi: 10.1086/342975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Reeuwijk J, et al. J Med Genet. 2005;42:907. doi: 10.1136/jmg.2005.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida A, et al. Dev Cell. 2001;1:717. doi: 10.1016/s1534-5807(01)00070-3. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi K, et al. Nature. 1998;394:388. doi: 10.1038/28653. [DOI] [PubMed] [Google Scholar]
- 11.Brockington M, et al. Am J Hum Genet. 2001;69:1198. doi: 10.1086/324412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longman C, et al. Hum Mol Genet. 2003;12:2853. doi: 10.1093/hmg/ddg307. [DOI] [PubMed] [Google Scholar]
- 13.Michele DE, et al. Nature. 2002;418:417. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 14.Manya H, et al. Proc Natl Acad Sci U S A. 2004;101:500. doi: 10.1073/pnas.0307228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki T, et al. Biochim Biophys Acta. 1998;1425:599. doi: 10.1016/s0304-4165(98)00114-7. [DOI] [PubMed] [Google Scholar]
- 16.Chiba A, et al. J Biol Chem. 1997;272:2156. doi: 10.1074/jbc.272.4.2156. [DOI] [PubMed] [Google Scholar]
- 17.Combs AC, Ervasti JM. Biochem J. 2005;390:303. doi: 10.1042/BJ20050375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilg T, et al. J Biol Chem. 1996;271:21583. doi: 10.1074/jbc.271.35.21583. [DOI] [PubMed] [Google Scholar]
- 19.Grewal PK, Holzfeind PJ, Bittner RE, Hewitt JE. Nat Genet. 2001;28:151. doi: 10.1038/88865. [DOI] [PubMed] [Google Scholar]
- 20.Kanagawa M, et al. Cell. 2004;117:953. doi: 10.1016/j.cell.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Ilg T, et al. J Biol Chem. 1994;269:24073. [PubMed] [Google Scholar]
- 22.Barresi R, et al. Nat Med. 2004;10:696. doi: 10.1038/nm1059. [DOI] [PubMed] [Google Scholar]
- 23.Dahms NM, Lobel P, Kornfeld S. J Biol Chem. 1989;264:12115. [PubMed] [Google Scholar]
- 24.Manzini MC, et al. Hum Mutat. 2008;29:E231. doi: 10.1002/humu.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.