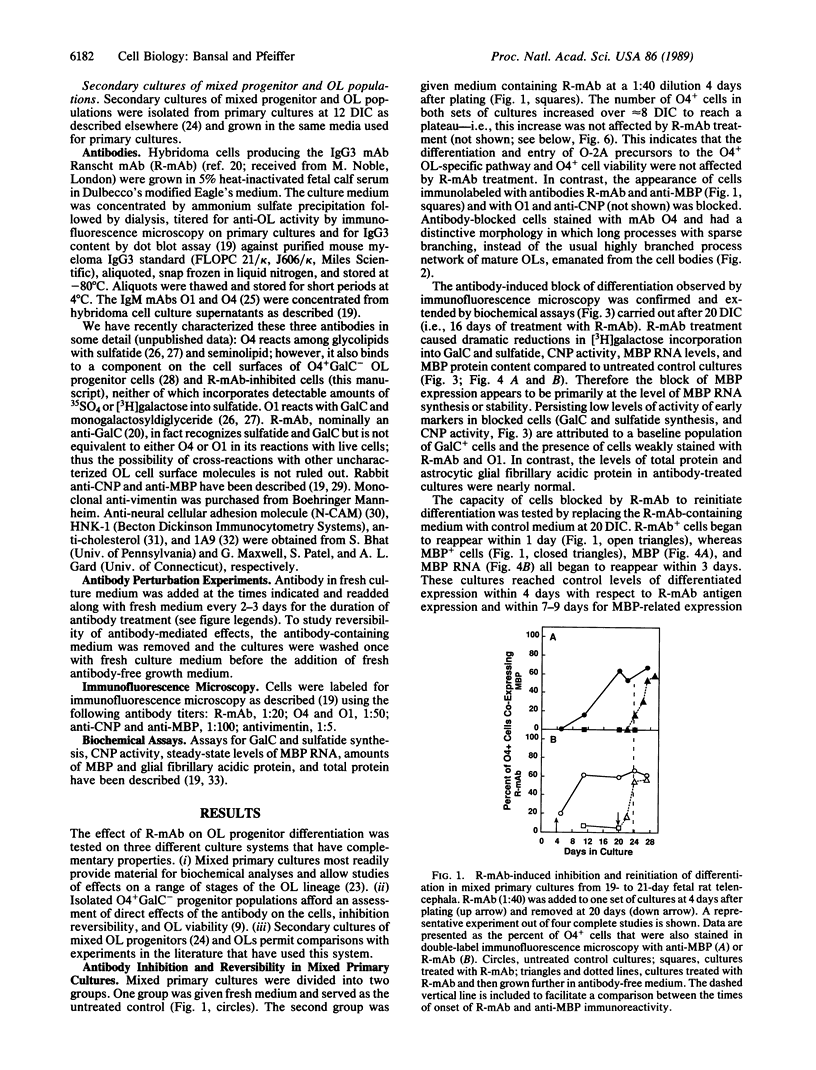
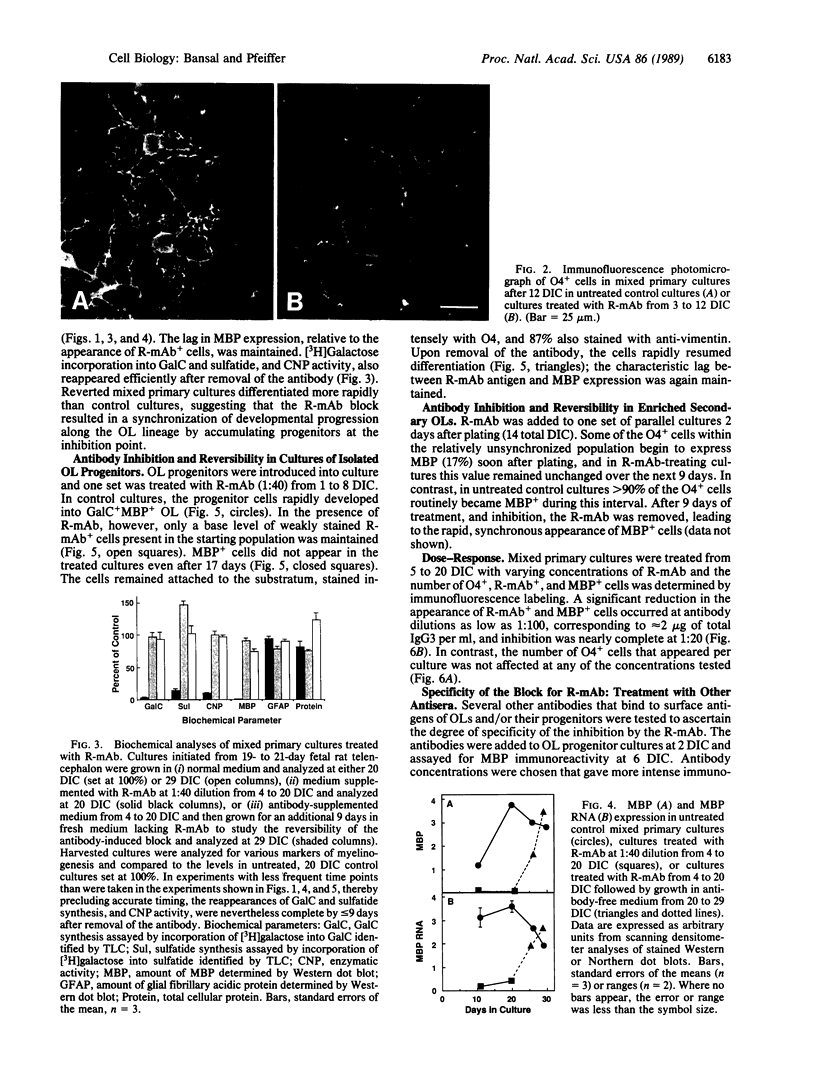
Abstract
We have hypothesized that oligodendrocyte (OL) surface glycolipids, specifically galactocerebroside and sulfatide, play a role in the regulation of OL development by acting as sensors/transmitters of environment information. In support of this hypothesis we report here a reversible inhibition of OL progenitor cell differentiation by a monoclonal antibody [Ranscht mAb (R-mAb); Ranscht, B., Clapshaw, P. A. & Seifert, W. (1982) Proc. Natl. Acad. Sci. USA 79, 2709-2713] that reacts with these glycolipids. When isolated OL progenitors or mixed primary cultures are grown in the presence of the antibody, myelinogenic development is blocked in a dose-dependent manner at concentrations as low as 2 micrograms of IgG per ml. The inhibited cells express the OL progenitor markers O4 and vimentin but are negative for galactosylcerebroside, sulfatide, 2',3'-cyclic nucleotide 3'-phosphohydrolase, myelin basic protein, and myelin basic protein RNA expression. In contrast, the levels of total cellular protein and the expression of astrocytic glial fibrillary acidic protein in mixed cultures are not affected. Antibody-blocked cells have a distinctive morphology in which long, sparsely branched processes emanate from round cell bodies. Upon removing the perturbing antibody, the cells rapidly resume differentiation. Reverted mixed primary cultures, in which OL progenitors of several sequential developmental stages are present at the time of plating, differentiate more rapidly than control cultures, suggesting that the antibody-induced block results in a synchronization of developmental progression along the OL lineage by accumulating cells at the inhibition point. However, the normal temporal sequence of marker expression is maintained. Control studies with several other antibodies recognizing OL cell surface antigens, including HNK-1, neural cellular adhesion molecule (N-CAM), 1A9, anticholesterol, and O1, did not inhibit development. Since the inhibition occurs in highly enriched populations of OL progenitors, the inhibition does not involve cell-cell interactions between OLs and other cell types but concerns interactions of OLs with themselves, soluble factors, or OL extracellular matrix molecules and adhesion factors that provide essential environmental signals required for normal myelinogenic development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bansal R., Gard A. L., Pfeiffer S. E. Stimulation of oligodendrocyte differentiation in culture by growth in the presence of a monoclonal antibody to sulfated glycolipid. J Neurosci Res. 1988 Oct-Dec;21(2-4):260–267. doi: 10.1002/jnr.490210218. [DOI] [PubMed] [Google Scholar]
- Bansal R., Pfeiffer S. E. Developmental expression of 2',3'-cyclic nucleotide 3'-phosphohydrolase in dissociated fetal rat brain cultures and rat brain. J Neurosci Res. 1985;14(1):21–34. doi: 10.1002/jnr.490140103. [DOI] [PubMed] [Google Scholar]
- Bansal R., Pfeiffer S. E. Regulated galactolipid synthesis and cell surface expression in Schwann cell line D6P2T. J Neurochem. 1987 Dec;49(6):1902–1911. doi: 10.1111/j.1471-4159.1987.tb02453.x. [DOI] [PubMed] [Google Scholar]
- Bhat S., Silberberg D. H. Rat oligodendrocytes have cell adhesion molecules. Brain Res. 1985 Mar;351(1):139–145. doi: 10.1016/0165-3806(85)90240-8. [DOI] [PubMed] [Google Scholar]
- Cardwell M. C., Rome L. H. RGD-containing peptides inhibit the synthesis of myelin-like membrane by cultured oligodendrocytes. J Cell Biol. 1988 Oct;107(4):1551–1559. doi: 10.1083/jcb.107.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R., Cohen J., Fok-Seang J., Hanley M. R., Gregson N. A., Reynolds R., Wilkin G. P. Development of macroglial cells in rat cerebellum. I. Use of antibodies to follow early in vivo development and migration of oligodendrocytes. J Neurocytol. 1988 Feb;17(1):43–54. doi: 10.1007/BF01735376. [DOI] [PubMed] [Google Scholar]
- Dyer C. A., Benjamins J. A. Antibody to galactocerebroside alters organization of oligodendroglial membrane sheets in culture. J Neurosci. 1988 Nov;8(11):4307–4318. doi: 10.1523/JNEUROSCI.08-11-04307.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer C. A., Benjamins J. A. Redistribution and internalization of antibodies to galactocerebroside by oligodendroglia. J Neurosci. 1988 Mar;8(3):883–891. doi: 10.1523/JNEUROSCI.08-03-00883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias S. B. Oligodendrocyte development and the natural history of multiple sclerosis. A new hypothesis for the pathogenesis of the disease. Arch Neurol. 1987 Dec;44(12):1294–1299. doi: 10.1001/archneur.1987.00520240066016. [DOI] [PubMed] [Google Scholar]
- Gard A. L., Dutton G. R. Myelin-specific domain on the plasmalemma of oligodendroglia: differential expression in the rat and hypomyelinating mouse mutants jimpy and quaking. J Neurosci Res. 1987;17(4):329–343. doi: 10.1002/jnr.490170403. [DOI] [PubMed] [Google Scholar]
- Gard A. L., Pfeiffer S. E. Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development. 1989 May;106(1):119–132. doi: 10.1242/dev.106.1.119. [DOI] [PubMed] [Google Scholar]
- Gard A. L., Warrington A. E., Pfeiffer S. E. Direct microculture enzyme-linked immunosorbent assay for studying neural cells: oligodendrocytes. J Neurosci Res. 1988 May;20(1):46–53. doi: 10.1002/jnr.490200108. [DOI] [PubMed] [Google Scholar]
- Grumet M., Hoffman S., Crossin K. L., Edelman G. M. Cytotactin, an extracellular matrix protein of neural and non-neural tissues that mediates glia-neuron interaction. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8075–8079. doi: 10.1073/pnas.82.23.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. M., Lillien L. E., Raff M. C., Rohrer H., Sendtner M. Ciliary neurotrophic factor induces type-2 astrocyte differentiation in culture. Nature. 1988 Sep 1;335(6185):70–73. doi: 10.1038/335070a0. [DOI] [PubMed] [Google Scholar]
- Künemund V., Jungalwala F. B., Fischer G., Chou D. K., Keilhauer G., Schachner M. The L2/HNK-1 carbohydrate of neural cell adhesion molecules is involved in cell interactions. J Cell Biol. 1988 Jan;106(1):213–223. doi: 10.1083/jcb.106.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine S. M., Goldman J. E. Embryonic divergence of oligodendrocyte and astrocyte lineages in developing rat cerebrum. J Neurosci. 1988 Nov;8(11):3992–4006. doi: 10.1523/JNEUROSCI.08-11-03992.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris F. A., Dubois-Dalcq M. Insulin-like growth factor I promotes cell proliferation and oligodendroglial commitment in rat glial progenitor cells developing in vitro. J Neurosci Res. 1988 Oct-Dec;21(2-4):199–209. doi: 10.1002/jnr.490210212. [DOI] [PubMed] [Google Scholar]
- Menko A. S., Boettiger D. Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell. 1987 Oct 9;51(1):51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Mirsky R., Winter J., Abney E. R., Pruss R. M., Gavrilovic J., Raff M. C. Myelin-specific proteins and glycolipids in rat Schwann cells and oligodendrocytes in culture. J Cell Biol. 1980 Mar;84(3):483–494. doi: 10.1083/jcb.84.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Leblond C. P. Electron microscopic identification of three classes of oligodendrocytes and a preliminary study of their proliferative activity in the corpus callosum of young rats. J Comp Neurol. 1970 May;139(1):1–28. doi: 10.1002/cne.901390102. [DOI] [PubMed] [Google Scholar]
- Noble M., Murray K., Stroobant P., Waterfield M. D., Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988 Jun 9;333(6173):560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Pesheva P., Juliano R. L., Schachner M. Expression and localization of the fibronectin receptor in the mouse nervous system. J Neurosci Res. 1988 Aug;20(4):420–430. doi: 10.1002/jnr.490200404. [DOI] [PubMed] [Google Scholar]
- Privat A. Postnatal gliogenesis in the mammalian brain. Int Rev Cytol. 1975;40:281–323. doi: 10.1016/s0074-7696(08)60955-9. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Lillien L. E., Richardson W. D., Burne J. F., Noble M. D. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988 Jun 9;333(6173):562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Mirsky R., Fields K. L., Lisak R. P., Dorfman S. H., Silberberg D. H., Gregson N. A., Leibowitz S., Kennedy M. C. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978 Aug 24;274(5673):813–816. [PubMed] [Google Scholar]
- Ranscht B., Clapshaw P. A., Price J., Noble M., Seifert W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2709–2713. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranscht B., Wood P. M., Bunge R. P. Inhibition of in vitro peripheral myelin formation by monoclonal anti-galactocerebroside. J Neurosci. 1987 Sep;7(9):2936–2947. doi: 10.1523/JNEUROSCI.07-09-02936.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R., Wilkin G. P. Development of macroglial cells in rat cerebellum. II. An in situ immunohistochemical study of oligodendroglial lineage from precursor to mature myelinating cell. Development. 1988 Feb;102(2):409–425. doi: 10.1242/dev.102.2.409. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Pringle N., Mosley M. J., Westermark B., Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988 Apr 22;53(2):309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Roberts D. D. Sulfatide-binding proteins. Chem Phys Lipids. 1986 Dec 15;42(1-3):173–183. doi: 10.1016/0009-3084(86)90051-4. [DOI] [PubMed] [Google Scholar]
- Schachner M. Cell type-specific surface antigens in the mammalian nervous system. J Neurochem. 1982 Jul;39(1):1–8. doi: 10.1111/j.1471-4159.1982.tb04694.x. [DOI] [PubMed] [Google Scholar]
- Singh H., Pfeiffer S. E. Myelin-associated galactolipids in primary cultures from dissociated fetal rat brain: biosynthesis, accumulation, and cell surface expression. J Neurochem. 1985 Nov;45(5):1371–1381. doi: 10.1111/j.1471-4159.1985.tb07202.x. [DOI] [PubMed] [Google Scholar]
- Skoff R. P., Price D. L., Stocks A. Electron microscopic autoradiographic studies of gliogenesis in rat optic nerve. II. Time of origin. J Comp Neurol. 1976 Oct 1;169(3):313–334. doi: 10.1002/cne.901690304. [DOI] [PubMed] [Google Scholar]
- Small R. K., Riddle P., Noble M. Evidence for migration of oligodendrocyte--type-2 astrocyte progenitor cells into the developing rat optic nerve. Nature. 1987 Jul 9;328(6126):155–157. doi: 10.1038/328155a0. [DOI] [PubMed] [Google Scholar]
- Sommer I., Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981 Apr 30;83(2):311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Swartz G. M., Jr, Gentry M. K., Amende L. M., Blanchette-Mackie E. J., Alving C. R. Antibodies to cholesterol. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1902–1906. doi: 10.1073/pnas.85.6.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian T., Sprinkle T. J., Dawson G., Szuchet S. Oligodendrocyte substratum adhesion modulates expression of adenylate cyclase-linked receptors. Proc Natl Acad Sci U S A. 1988 Feb;85(3):939–943. doi: 10.1073/pnas.85.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G., Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989 Feb;105(2):387–400. doi: 10.1242/dev.105.2.387. [DOI] [PubMed] [Google Scholar]
- Yim S. H., Szuchet S., Polak P. E. Cultured oligodendrocytes. A role for cell-substratum interaction in phenotypic expression. J Biol Chem. 1986 Sep 5;261(25):11808–11815. [PubMed] [Google Scholar]