Abstract
Context:
Acute decreases in strength have been associated with risky biomechanical strategies that might predispose one to injury. Whether acute changes in thigh muscle torque occur across the menstrual cycle remains equivocal.
Objective:
We compared maximal voluntary isometric contraction (MVIC) torque of the knee flexors and extensors between the early follicular (EF) and either the early luteal (EL) or midluteal (ML) phases, which were confirmed by serum hormone concentrations. We expected that MVIC torques would increase from the EF to the EL phase after estradiol peaked and before increased exposure to progesterone.
Design:
Cohort study.
Setting:
Applied Neuromechanics Research Laboratory.
Patients or Other Participants:
Seventy-one recreationally active women (age range, 18–30 years).
Intervention(s):
The MVICs were measured 1 day during menses and 1 day during the 8 days after ovulation. Participants were grouped by the hormone profile of their luteal test days as EL phase, ML phase, or anovulatory cycle.
Main Outcome Measure(s):
The MVIC torque of knee flexors and extensors (Nm/kg), estradiol (pg/mL), progesterone (ng/mL), and testosterone (ng/dL).
Results:
We tested 29 women during their EL phases, 32 during their ML phases, and 10 during anovulatory cycles. Although we observed relatively large individual changes in sex hormone concentrations and MVIC torques across the 2 test sessions, we observed no difference in MVIC torque between test phases (F1,68 = 1.17, P = .28) or among groups by test phase (F2,68 = 0.31, P = .74).
Conclusions:
Thigh MVIC torque did not change from time of menses (when estradiol and progesterone were lowest) to time in the luteal phase after an unopposed estradiol rise or combined estradiol and progesterone rise. However, these findings were limited to MVIC torque production measured at 2 different times, and further research examining these relationships at multiple times and using other measures of neuromuscular function is needed.
Keywords: muscles, strength, hormone, menstrual cycle
Key Points
The influence of hormone changes on muscle strength across the menstrual cycle remains equivocal.
Interindividual variations in magnitude and timing of hormonal fluctuations necessitate serial serum hormone measurements to accurately determine menstrual cycle phase.
Maximal isometric thigh strength did not change from the early follicular to the early or midluteal phases of the menstrual cycle or during anovulatory cycles, as determined by serum hormone concentrations.
More work is needed to fully elucidate the relationships among cyclic hormone fluctuations, neuromuscular control, functional movement mechanics, and injury risk.
Female athletes are at greater risk for noncontact anterior cruciate ligament (ACL) injuries than are their male counterparts.1,2 Therefore, researchers have attempted to identify the factors underlying this phenomenon. Recently, authors3,4 of 2 expert consensus statements acknowledged hormonal influences as potential risk factors for female athletes. This acknowledgment was based on reviews of multiple studies5–7 in which a larger proportion of injuries was reported during the preovulatory phase than during the postovulatory phases. However, the mechanisms by which hormones might influence injury risk remain unknown. Although most researchers have focused on the hormonal influence on the ligament itself (eg, joint laxity), they know much less about potential hormonal effects on the surrounding musculature.
The thigh musculature plays a crucial role in dynamic knee joint stability by effectively controlling joint motion and protecting the passive joint structures from excessive externally applied loads. Fundamental to this protective response is the muscle's ability to produce sufficient torque in a timely fashion. Researchers8,9 have suggested that the relative weakness of the quadriceps and hamstrings in women compared with men might explain some of the sex differences observed in landing mechanics that are thought to contribute to lower extremity injury risk. Specific to the knee flexors and extensors, researchers have investigated the effects of both absolute thigh muscle strength and acute alterations in thigh muscle strength on lower extremity mechanics during sport-related tasks. When examining absolute differences in thigh muscle strength, researchers found that greater maximal isometric strength was a predictor of greater muscle energy absorption (women)10 and a weak (men) to moderate (women) predictor of lower quadriceps activation amplitude9 at the knee during the deceleration phase of a drop-jump task. When examining the effects of strength training on jumping and landing performance, Hewett et al11 reported that increased hamstrings isokinetic torques after an intervention program resulted in lower impact forces during landing from a volleyball block jump, whereas Herman et al12 did not identify more desirable lower extremity mechanics during a stop-jump task after a training-induced increase in maximal voluntary isometric contraction (MVIC) strength of hip and knee muscles. Researchers examining acute reductions in strength have observed potentially injurious mechanics. After a fatigue-induced decrease in muscle function (measured with vertical-jump height), more shallow knee-flexion angles and greater anterior tibial shear forces were observed during a stop-jump task, indicating a decreased ability to absorb the forces of ground contact.13 Similarly, inhibition of the quadriceps' force-producing capability in response to an artificially induced knee effusion (as evidenced by a reduced knee-extension moment and lower activation amplitude than the control condition) resulted in participants performing a single-leg drop landing with a more extended knee position and greater vertical ground reaction forces.14 Collectively, these studies indicate that acute changes in muscle strength and function might induce functional alterations in landing strategies (eg, smaller knee-flexion angles, higher ground reaction forces) that, in turn, might increase the potential for injury.15 Although the magnitude of muscle strength needed to ensure proper lower extremity mechanics is not clear from these studies, women might operate closer to this threshold because absolute strength was a strong predictor of knee joint function in women compared with men during the same drop-jump task from a 45-cm height.9,10 Hence, understanding factors that might acutely alter thigh muscle strength is important because smaller strength alterations subsequently might affect dynamic knee stabilization in women more than in men.
Given recent findings16–18 that human skeletal muscle expresses receptors for estrogen and testosterone, hormones might interact with the muscle in a way that could acutely affect its force-producing capability across the menstrual cycle. The early theory that hormones play a role in muscle strength was largely based on anecdotal evidence from women who reported a sense of decreased strength and athletic performance during the days immediately before and after menses.19 More recently, the chronic effects of hormone concentrations on strength have been documented with the findings that administering estrogen and/or progesterone hormone-replacement therapy can ameliorate the large decrease in isometric thigh20 and hand21 strength that most women experience after menopause, when hormone levels decrease dramatically. Although these findings indicate that estrogen, with or without progesterone, exerts positive effects on muscle strength, the acute effect on muscle strength of normal cyclic variations in hormone concentrations across the menstrual cycle remains equivocal.
Several investigators have examined the effects of female sex hormones on measures of muscle strength by taking periodic measurements of strength during different phases of the menstrual cycle in eumenorrheic women. Specific to the knee musculature, knee-flexor and knee-extensor strength have been tested using isometric22,23 and isokinetic22,24,25 contractions and field tests known to be correlated with leg strength.26,27 Some researchers have reported cyclic changes in quadriceps and hamstrings strength across the cycle,23,28 but others have not.22,24–26 Sarwar et al23 reported a midcycle (days 12–18) peak in maximal quadriceps isometric torque in sedentary women compared with the early follicular (days 1–7), midfollicular (days 7–12), midluteal (days 18–21), and late luteal (days 21–32) phases.23 Similarly, Bambaeichi et al28 tested isometric and isokinetic contractions of the knee flexors and extensors in 8 sedentary women on multiple days. Although peak knee-extensor torques at 3.14 rad/s were greater when measured on a day near ovulation (around the day of urinary luteinizing hormone [LH] peak) and a day during the midluteal phase (days 19–21) than when measured on a day during menses (days 1–4) and a day during the midfollicular phase (days 7–9), peak knee-flexor torques (isometric and 1.05 rad/s) were greater during a day of the ovulatory phase than during a day of the midfollicular and midluteal phases. Together, these findings indicate that rising estrogen concentrations, with or without a combined rise in progesterone concentrations, might result in improved muscle strength in the postovulatory phase compared with the preovulatory phase of the menstrual cycle. However, other studies do not support these findings. Gür25 measured serum hormone concentrations and quadriceps and hamstrings isokinetic torques at 60°/s in sedentary women during 1 of 3 days for each of the menstrual (days 1–3), follicular (days 8–10), and luteal (days 19–21) phases and reported no change in concentric or eccentric muscle torques. Similar findings were reported by Fridén et al26 and Lebrun et al24 when comparing isokinetic thigh torques between menses and days 19 through 21 or days 4 through 9, respectively, after predicted ovulation using basal body temperature (BBT) in trained women. Furthermore, Janse de Jonge et al22 compared both isometric and isokinetic contractions of the quadriceps during the menstrual (days 1–3), late follicular, and luteal phases, as determined by BBT, and did not observe differences in knee-extension torques between phases.
In part, the inconsistency in findings across studies might be a result of varying methods for determining the menstrual cycle phase (and thus the hormone environment) during which testing occurred. Most commonly, particular days of the menstrual cycle have been chosen in an attempt to isolate the effects of estrogen and progesterone based on known hormone fluctuations. These chosen times are based on the days of a typical 28-day menstrual cycle when both hormones generally are thought to be at their nadirs (days of menses/early follicular phase), when estradiol peaks immediately before ovulation (periovulatory), and after progesterone is produced by the corpus luteum and peaks rapidly, along with a second smaller peak in estrogen (midluteal [ML] phase).29 Most commonly, these cycle phases are determined by counting days forward from menses22–24,26,30 and, at times, by using a positive urinary ovulation test to detect the LH surge to more accurately identify the time of impending ovulation.26,28 However, research29,31 has shown considerable variability among women in terms of the timing and magnitude of these hormone concentration changes. Thus, testing all participants on a predetermined day of the cycle might not adequately capture a common hormonal environment in all women. Moreover, although some investigators have documented hormone concentrations on the day of testing to confirm that an individual is within an expected range for the intended testing phase,22,25,26 it is difficult to determine from this single-day measurement whether hormone levels are rising, peaking, or falling.4 Hence, a more precise characterization of cycle phase might clarify further whether a particular hormone environment leads to acute changes in muscle strength.
Therefore, the purpose of our study was to compare MVIC torque of the knee flexors and extensors at known times of the menstrual cycle when estrogen and progesterone are at their nadirs (early follicular phase) and after either an unopposed rise in estradiol levels (early luteal [EL] phase) or substantial rises in both estradiol and progesterone (ML phase), as confirmed by serum hormone concentrations. Based on the literature in which researchers generally observed greater strength in postovulatory than in preovulatory phases of the cycle, we expected to observe increases in MVIC torques from the early follicular to the EL and ML phases of the menstrual cycle and to observe the greatest increase with testing in the EL phase shortly after the estradiol peak at ovulation. Although research is conflicting on the relationship between MVIC strength and functional performance32 and although MVIC strength has not been linked specifically to ACL injury risk, we believed it was important to first assess isometric contractions as a measure of muscle strength so that we could best isolate the basic force-producing capability of each muscle group while minimizing the potentially confounding effects of larger amounts of antagonist activity33 often observed with higher-speed isokinetic contractions.34
METHODS
Participants
Participants included all 74 women who participated in a larger study in which we investigated the effect of hormone-mediated changes in knee laxity and their effects on knee joint neuromechanics. Participants were included in the larger study if they were between 18 and 30 years of age, had a body mass index (weight/height2) equal to or less than 30, had been recreationally active (self-reported that they engaged in mild-intensity, moderate-intensity, or high-intensity physical activity for at least 2.5 h/wk but not more than 10 h/wk) for the 3 months before the study, reported consistent menstrual cycles lasting 26 to 32 days, and had not used exogenous hormones for the 6 months before the study, had never been pregnant, were nonsmokers, and had no history of ligament or cartilage injury to the knee. All participants provided informed consent, and the university's Institutional Review Board for the Protection of Human Subjects approved the study.
Data Collection
As part of the larger study, isometric strength and hormone data were obtained for each woman at 2 predetermined times during her menstrual cycle: a day during the first 6 days of menses (onset based on self-reported data), when hormones were at their nadirs, and a day during the first 8 days of the luteal phase (onset based on evidence of ovulation). These times were based on the aims of the larger study and coincided with the 2 days of the cycle during which the participant's knee laxity was at the minimum (first 6 days of menses) and maximum (first 8 days of luteal phase). To determine these 2 measurement times, participants were measured daily over 2 consecutive months with regard to their laxity and serum hormone concentrations. This provided the unique opportunity to examine strength changes at 2 times in the cycle (early follicular, postovulatory) based on a more accurate characterization of hormone concentrations that surrounded these times. We describe the procedures for obtaining the serum samples and isometric strength measurements used in this study and for categorizing the hormone environment at the time of testing.
Tracking Phase
For 2 months before strength assessment, serum was obtained on 6 consecutive mornings after the onset of menstrual bleeding (early follicular phase) and for 8 consecutive mornings after a positive home ovulation test (CVS One Step Ovulation Predictor; CVS Corporation, Woonsocket, RI) (luteal phase). Morning testing sessions typically took place between 6:30 am and 9:00 am to control for diurnal hormonal fluctuations. During each data collection session, serum was collected from a vein in the antecubital region of the arm in a 10-mL red-top Vacutainer tube (BD Vacutainer; BD, Franklin Lakes, NJ) using standard venipuncture techniques. Samples were allowed to clot at room temperature for 15 minutes before being centrifuged at 2500 rpm at 5°C for 15 minutes. The serum was pipetted into 3 separate 1-mL aliquots and frozen at −80°C until transported and assayed at the Ligand Assay and Analysis Core Laboratory (Center for Research in Reproduction, University of Virginia, Charlottesville, VA). Estradiol concentration was analyzed with a double-antibody radioimmunoassay (DSL-4400; Beckman Coulter, Webster, TX), and progesterone and testosterone concentrations were analyzed using Coat-A-Count radioimmunoassay (TKPG-2 and TKTT-2; Siemens Medical Solutions Diagnostics, Los Angeles, CA). The detection sensitivity (mean intra-assay and interassay coefficients of variation) were 10 pg/mL (5.2% and 10.6%) for estradiol, 0.1 ng/mL (4.1% and 6.4%) for progesterone, and 10 ng/dL (3.4% and 8.1%) for testosterone.
Maximal Voluntary Isometric Contraction Testing
After the 2 tracking months, each participant reported to the laboratory on 1 predetermined day during the early follicular block of days and on 1 day during the luteal block of days. Serum hormone concentrations were obtained in the morning hours using methods consistent with the tracking months. For each test day (menses and luteal), each participant performed knee-extensor and knee-flexor MVICs with the dominant leg (designated as the stance leg when kicking a ball). To control for possible diurnal fluctuations in hormone concentrations35 and muscle strength,36 the luteal test session was performed within 1 hour of the time at which the early follicular test session occurred. Participants also were instructed to refrain from physical activity that would cause undue muscle soreness or fatigue during the 48 hours preceding each testing session. Participants were familiarized with the MVIC task approximately 2 weeks before the first testing session.
Before strength assessment, participants performed a 5-minute warm-up on a standard cycle ergometer before being positioned in the Biodex System 3 isokinetic dynamometer (Biodex Medical Systems Inc, Shirley, NY). Participants were positioned using standard protocol, and straps were used to secure the chest, hips, thigh, and distal shank (2 fingers' breadth proximal to the calcaneal insertion of the Achilles tendon); the lateral femoral epicondyle was aligned with the dynamometer axis of rotation. Knee position was fixed in 20° of flexion. Participants then performed practice trials at 25%, 50%, 75%, and 100% of perceived maximal effort before performing three 3-second MVICs of the knee extensors with a 30-second rest period between trials. This procedure was repeated for the knee flexors. The investigators provided strong verbal encouragement during each trial to maximize consistent effort across trials. Each participant's peak knee-flexor and knee-extensor torques (Nm) across the 3 trials were recorded and normalized to body mass (kg) for analysis.
Data Reduction
Serum estradiol and progesterone concentrations from the 2 tracking months were used to create an individual hormone profile for each participant (Figure). To place each participant's luteal test day in the appropriate phase (hormone environment), the hormone concentrations from the luteal test day and the day of testing after ovulation (ie, number of days from the day when a positive ovulation test was identified) were compared with the individual's profiles, obtained during the 2 tracking months. Participants then were placed into 1 of 3 groups based on their luteal test-day hormone concentrations. Participants who were tested within the first 3 days of their initial progesterone rise (2 ng/mL threshold)37 were classified as being tested during the EL phase; participants who were tested near or at their progesterone peak were classified as being tested during the ML phase. If the participant's progesterone concentration did not rise above 3 ng/mL during the luteal test date, as expected based on the hormone patterns of the 2 tracking months, the participant was classified as being tested during an anovulatory cycle (AN).37 If the participant's phase could not be determined (ie, her hormone concentrations during the 2 tracking months were inconsistent with the day and values obtained in the strength testing month), she was excluded from the analysis.
Figure.
data from 1 participant shows the hormone concentrations obtained during the menses and luteal days over 2 cycles during the tracking months: A, cycle 1 and B, cycle 2. Participant-specific hormone profiles were used to determine group assignment based on the hormone profile of the participant's luteal phase test date.
Data Analysis
We used separate 3 × 2 analyses of variance (ANOVAs) with repeated measures on group (EL, ML, AN) and test phase (menses, luteal) to compare menses and luteal hormone concentrations across the 3 groups for estradiol, testosterone, and progesterone. We used a 3 × 2 × 2 ANOVA with repeated measures on group, test phase, and muscle group (quadriceps, hamstrings) to examine mean differences in knee-flexor and knee-extensor torques from the menses to luteal test sessions between groups. Group main effects and interactions were examined further using pairwise comparisons with Bonferroni corrections. To further explore potential relationships between changes in sex hormone concentrations and changes in thigh MVIC torque between the menses and luteal test sessions, we used separate stepwise multiple regressions to examine the extent to which the nadir hormone concentrations measured at the menses test date (estradiol, progesterone, testosterone) and absolute change in hormone concentrations from the menses to luteal test dates (Δ = luteal − menses) predicted individual changes in knee-extensor and knee-flexor MVIC torques from the menses to luteal test date (Δ = luteal − menses). All analyses were performed with SPSS software (version 15.0; SPSS Inc, Chicago, IL). The α level was set a priori at .05.
RESULTS
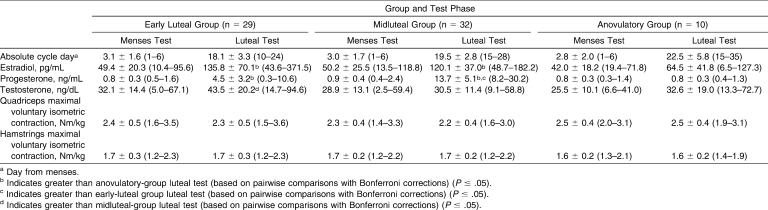
Of the 74 women who participated in the study, 2 women were excluded because they did not complete the luteal test date, and 1 woman was excluded because we could not determine the hormone environment of the luteal test date based on the hormones obtained during the 2-month tracking phase. Of the remaining 71 participants, the luteal test day occurred in the EL phase for 29 participants (age = 21.5 ± 2.7 years, height = 164.9 ± 7.2 cm, mass = 61.7 ± 9.3 kg) and in the ML phase for 32 participants (age = 21.2 ± 2.4 years, height = 164.0 ± 6.6 cm, mass = 60.8 ± 9.1 kg). Ten participants were tested during AN cycles (age = 22.3 ± 3.0 years, height = 163.6 ± 5.1 cm, mass = 61.9 ± 8.2 kg). Table 1 provides the sex hormone concentrations and the quadriceps and hamstrings isometric torques by group and test phase. We identified group-by-test phase interactions for estradiol (F2,68 = 7.3, P = .001), progesterone (F2,68 = 59.9, P < .001), and testosterone (F2,68 = 3.6, P = .03) concentrations. Post hoc analyses revealed that hormone concentrations were similar among the 3 groups at the menses test phase but differed at the luteal test phase; estradiol concentrations were greater in the EL (P = .002) and ML (P = .02) groups than in the AN group, progesterone concentrations were greater in the EL (P = .04) and ML (P < .001) groups than in the AN group, and testosterone concentrations were greater in the EL than in the ML group (P = .01). When examining thigh MVIC torque differences among groups and test sessions, the repeated-measures ANOVA revealed no differences in muscle torque by test phase (F1,68 = 1.17, P = .28), test phase by group (F2,68 = 0.31, P = .74), test phase by muscle (F1,68 = 0.01, P = .92), or muscle by group (F2,68 = 2.33, P = .11). As expected, participants produced greater quadriceps MVIC torque than hamstrings MVIC torque (F1,68 = 186.2, P < .001), and this muscle group difference was consistent across groups (P = .11).
Table 1.
Hormone Concentrations and Maximal Voluntary Isometric Contraction Torques of the Quadriceps and Hamstrings Muscles for the Menses and Luteal Test Phases (Group Means ± SDs [Ranges])
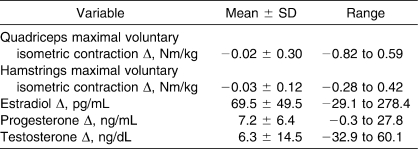
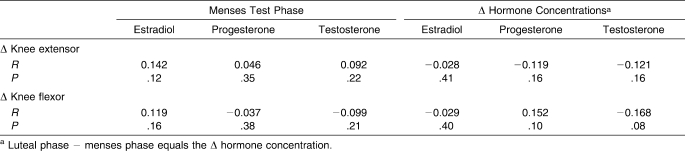
Table 2 lists the means, SDs, and ranges for individual Δ values for changes in sex hormone concentrations and quadriceps and hamstrings MVIC torques from menses to luteal test dates. Table 3 lists the Pearson r correlations between menses level and Δ hormone concentrations with the Δ quadriceps and hamstrings MVIC torques. Although relatively large individual changes in sex hormone concentrations and quadriceps and hamstrings torques were observed across the 2 test sessions, multiple linear regressions further confirmed the findings of the ANOVA results, revealing no relationship between the absolute level and magnitude of change in an individual's sex hormone concentrations and the change in quadriceps (R2 = 0.071, P = .56) or hamstrings (R2 = 0.075, P = .53) MVIC torques.
Table 2.
Individual Values for Changes in Sex Hormone Concentrations and Quadriceps and Hamstrings Maximal Voluntary Isometric Contraction Torques From Menses to Luteal Test Dates
Table 3.
Pearson Product Moment Correlations and P Values Between Menses Test Phase and Δ (Luteal − Menses) Hormone Concentrations with Δ Knee-Extensor and Δ Knee-Flexor Maximal Voluntary Isometric Contraction Torques
DISCUSSION
Based on previous findings23,28 that women produce larger torque values from midcycle and beyond, we hypothesized that thigh MVIC torques would be lower when hormone concentrations were at their nadirs during the early follicular phase than during the latter part of the cycle. To improve on previous methods and better capture the hormone environments in which participants' MVIC torques were tested postovulation, we tracked serum hormone concentrations for 2 months before testing. Because we tracked each participant's hormone concentrations for 2 months around times when we expected hormones to be at their nadirs (menses) and at substantially higher levels (luteal), we could create individual hormone profiles for each participant and more accurately determine whether we tested each participant shortly after the estradiol peak (EL phase), after exposure to both estradiol and progesterone (ML phase), or with little to no rise in sex hormone concentration levels (AN cycle). Our primary findings revealed no changes in thigh MVIC torques of the knee extensors and flexors from the early follicular to luteal phases of the menstrual cycle, regardless of whether participants were tested in the EL, ML, or AN cycles.
Although the practicality of tracking daily hormone concentrations for 2 months is not always realistic, we believe that this design provided an opportunity to more accurately document hormone characteristics and to build on previous findings. Our findings are consistent with findings of most previous studies,22,25,26 in which researchers reported no differences in thigh MVIC torques between cycle phases. In one of the most comprehensive studies, Janse de Jonge et al22 measured isometric strength and contractility (with superimposed electric stimulation to ensure maximal contraction) and isokinetic strength and fatigability of the quadriceps in 19 normally menstruating women during 3 phases of the menstrual cycle when estrogen and progesterone were in different combinations: menstrual (days 1–3; low estrogen–low progesterone), late follicular (high estrogen–low progesterone), and luteal (high estrogen–high progesterone). They determined cycle phase with BBT, which was measured orally each morning, whereby nadir BBT identifies the late follicular phase 1 to 2 days before the LH surge and is followed by a 0.5°F to 1.0°F (0.28°C–0.56°C) increase after ovulation.38 Cycle phase then was confirmed with single-day serum hormone measurements on the day of strength testing. Janse de Jonge et al22 observed no differences in their measures between cycle phases, and their analysis did not reveal correlations between serum estradiol or progesterone and any of the muscle measurements.
Conversely, our findings do not agree with those of Sarwar et al23 and Bambaeichi et al,28 who observed higher thigh torque at midcycle (days 12–18) and after evidence of an LH surge, respectively. A strength of both studies is that the authors used a superimposed twitch during the MVIC, which ensured that participants were able to fully activate their thigh muscles during each phase of the cycle; this was consistent with measurements obtained by Janse de Jonge et al.22 However, several fundamental differences between these studies and ours and that of Janse de Jonge et al22 might explain the disparate findings. First, in both studies, the investigators examined a relatively small sample size of normal, sedentary, menstruating women (828 and 1023 women, respectively). Because researchers39 have suggested that active women might have lower estradiol levels than sedentary women, the acute effects of estradiol exposure might not be as pronounced in physically active women and, therefore, might explain the lack of strength changes in physically active women. Second, in both studies, the investigators determined cycle phase based primarily on day of the cycle and did not obtain actual hormone levels to confirm cycle phase. In part, our data showed the variability in the days of the cycle during which particular hormone events occur. For example, the mean test day that captured hormone concentrations consistent with the EL phase was 18.1, but this day ranged from day 10 to day 24, and the mean test day that measured ML events was 19.5, although this day ranged from day 15 to day 28. Given the large variability in the timing and magnitude of hormone concentrations among individual women in our study and those previously observed,31 directly relating the findings to the assumed hormone events is difficult. Of interest, the strength changes reported by Bambaeichi et al28 were inconsistent because maximal isometric strength of the knee extensors did not change across the cycle, but maximal isometric strength of the knee flexors did. One would suspect that if hormone changes were responsible for strength changes, both muscle groups would have demonstrated changes.
An additional strength of our study was that we included participants who were tested during AN cycles. Because we had individual profiles from the 2 tracking months, we could identify those participants who did not experience a rise in their progesterone levels, indicating an AN cycle. These AN cycles were further substantiated by much lower estradiol levels. In our study, 20% of the participants experienced at least 1 AN cycle over the course of 3 consecutive months. This is consistent with the findings of Janse de Jonge et al,22 who reported that 4 of 19 participants (whom they excluded from their study) did not exceed a progesterone threshold required to confirm ovulation. Spontaneous AN cycles are common in recreationally active athletes despite a eumenorrheic cycle, during which the menstrual periods occur regularly,40 and these participants do not experience a dramatically different hormone profile in the luteal phase, compared with menses. Although AN cycles traditionally have been excluded from analysis because the altered hormone levels potentially can confound results, we analyzed this group separately to gain a better understanding of how deviations (or lack thereof) in hormone profiles can affect the force-producing capability of the thigh muscles across the cycle.4 Our findings that thigh MVIC torque changes between menses and luteal test dates did not differ by groups who had different luteal phase hormone concentrations provide stronger evidence that acute hormone fluctuations have little effect on the force-producing capability of the muscle.
We noted no acute changes in knee-extensor or knee-flexor isometric strength during the early follicular to EL and ML phases of the menstrual cycle. However, our data were limited to a single test during the first 6 days postmenses and a single test representing either EL or ML hormone events. Because our luteal-phase MVIC strength measures always were obtained 1 or more days postovulation, we rarely captured the estradiol peak that typically occurs just before ovulation. As such, we cannot rule out more immediate acute effects of an unopposed rise or peak in estrogen concentrations on muscle strength. Furthermore, the lack of correlation between changes in hormone concentrations and changes in isometric thigh torques observed by Janse de Jonge et al22 and us might be due to a delayed effect related to when hormones change and when thigh-strength changes occur.41 Unfortunately, the limited times we examined did not allow us to perform the type of analyses necessary to examine these potential time delays. In the future, researchers should extend these findings to examine more time periods across a woman's cycle, including a more comprehensive assessment around periovulatory events, when hormone concentrations are changing rapidly.
In addition, these data were limited to the measurement of absolute torque production during an MVIC, which is not fully representative of the dynamic muscle actions that occur during sport activity. As stated, we purposely examined an isometric contraction in an effort to isolate the force-producing ability of the muscle from the other components of neuromuscular control. Although we believed this was an important first step, we readily acknowledge that a static contraction is not adequately reflective of the complex interaction of neural activation strategies (both planned and unplanned) and intrinsic muscle properties (eg, musculotendinous stiffness, electromechanical delay) inherent in dynamic tasks when injuries actually occur. Although MVIC strength might shed some light on dynamic movement, only low to moderate correlations have been reported between MVICs of the quadriceps and squat jump32,42 and countermovement jump in trained male athletes32,42 and between greater MVIC thigh strength and greater muscular energy absorption during a drop-jump landing in women.10 Hence, although our findings revealed no acute changes in isometric torque production across the cycle, we cannot rule out possible hormone effects on other aspects of neuromuscular control that contribute to dynamic movement. Clearly, more work is needed to fully elucidate the relationships among cyclic hormone effects, neuromuscular control, functional movement mechanics, and injury risk. To advance these findings, valid methods of determining cycle phase based on actual hormone concentrations in the days surrounding the test date are critical.
Acknowledgments
This study was supported by the National Institutes of Health and National Institute of Arthritis and Musculoskeletal Diseases research grant R01 AR53172 and by the National Institute of Child Health and Human Development and National Institutes of Health cooperative agreement U54 HD28934 as part of the Specialized Cooperative Centers Program in Reproductive Research. We thank Anh-Dung Nguyen, PhD, ATC, and Hyunsoo Kim, MS, ATC, for their assistance with data collection.
REFERENCES
- 1.Bjordal J. M., Arnly F., Hannestad B., Strand T. Epidemiology of anterior cruciate ligament injuries in soccer. Am J Sports Med. 1997;25(3):341–345. doi: 10.1177/036354659702500312. [DOI] [PubMed] [Google Scholar]
- 2.Engstrom B., Johansson C., Tornkvist H. Soccer injuries among elite female players. Am J Sports Med. 1991;19(4):372–375. doi: 10.1177/036354659101900408. [DOI] [PubMed] [Google Scholar]
- 3.Renstrom P., Ljungqvist A., Arendt E., et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42(6):394–412. doi: 10.1136/bjsm.2008.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shultz S. J., Schmitz R. J., Nguyen A. D. Research Retreat IV: ACL injuries—the gender bias: April 3–5, 2008, Greensboro, NC. J Athl Train. 2008;43(5):530–531. doi: 10.4085/1062-6050-43.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arendt E. A., Bershadsky B., Agel J. Periodicity of noncontact anterior cruciate ligament injuries during the menstrual cycle. J Gender Specific Med. 2002;5(2):19–26. [PubMed] [Google Scholar]
- 6.Beynnon B. D., Johnson R. J., Braun S., et al. The relationship between menstrual cycle phase and anterior cruciate ligament injury: a case-control study of recreational alpine skiers. Am J Sports Med. 2006;34(5):757–764. doi: 10.1177/0363546505282624. [DOI] [PubMed] [Google Scholar]
- 7.Wojtys E. M., Huston L. J., Boynton M. D., Spindler K. P., Lindenfeld T. N. The effect of the menstrual cycle on anterior cruciate ligament injuries in women as determined by hormone levels. Am J Sports Med. 2002;30(2):182–188. doi: 10.1177/03635465020300020601. [DOI] [PubMed] [Google Scholar]
- 8.Lephart S. M., Ferris C. M., Riemann B. L., Myers J. B., Fu F. H. Gender differences in strength and lower extremity kinematics during landing. Clin Orthop Relat Res. 2002;401:162–169. doi: 10.1097/00003086-200208000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Shultz S. J., Nguyen A. D., Leonard M. D., Schmitz R. J. Thigh strength and activation as predictors of knee biomechanics during a drop jump task. Med Sci Sports Exerc. 2009;41(4):857–866. doi: 10.1249/MSS.0b013e3181e3b3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz R., Shultz S. Contribution of knee flexor and extensor strength on sex-specific energy absorption and torsional joint stiffness patterns during drop jumping. J Athl Train. 2010;45(5):445–452. doi: 10.4085/1062-6050-45.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hewett T. E., Stroupe A. L., Nance T. A., Noyes F. R. Plyometric training in female athletes: decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24(6):765–773. doi: 10.1177/036354659602400611. [DOI] [PubMed] [Google Scholar]
- 12.Herman D. C., Weinhold P. S., Guskiewicz K. M., Garrett W. E., Yu B., Padua D. A. The effects of strength training on the lower extremity biomechanics of female recreational athletes during a stop-jump task. Am J Sports Med. 2008;36(4):733–740. doi: 10.1177/0363546507311602. [DOI] [PubMed] [Google Scholar]
- 13.Chappell J. D., Herman D. C., Knight B. S., Kirkendall D. T., Garrett W. E., Yu B. Effect of fatigue on knee kinetics and kinematics in stop-jump tasks. Am J Sports Med. 2005;33(7):1022–1029. doi: 10.1177/0363546504273047. [DOI] [PubMed] [Google Scholar]
- 14.Palmieri-Smith R. M., Kreinbrink J., Ashton-Miller J. A., Wojtys E. M. Quadriceps inhibition induced by an experimental knee joint effusion affects knee joint mechanics during a single-legged drop landing. Am J Sports Med. 2007;35(8):1269–1275. doi: 10.1177/0363546506296417. [DOI] [PubMed] [Google Scholar]
- 15.Decker M. J., Torry M. R., Wyland D. J., Sterett W. I., Steadman R. J. Gender differences in lower extremity kinematics, kinetics and energy absorption during landing. Clin Biomech (Bristol, Avon) 2003;18(7):662–669. doi: 10.1016/s0268-0033(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 16.Lemoine S., Granier P., Tiffoche C., Rannou-Bekono F., Thieulant M. L., Delamarche P. Estrogen receptor alpha mRNA in human skeletal muscles. Med Sci Sports Exerc. 2003;35(3):439–443. doi: 10.1249/01.MSS.0000053654.14410.78. [DOI] [PubMed] [Google Scholar]
- 17.Sinha-Hikim I., Taylor W. E., Gonzalez-Cadavid N. F., Zheng W., Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab. 2004;89(10):5245–5255. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- 18.Wiik A., Glenmark B., Ekman M., et al. Oestrogen receptor beta is expressed in adult human skeletal muscle both at the mRNA and protein level. Acta Physiol Scand. 2003;179(4):381–387. doi: 10.1046/j.0001-6772.2003.01186.x. [DOI] [PubMed] [Google Scholar]
- 19.Erdelyi G. J. Gynecological survey of female athletes. J Sports Med Phys Fitness. 1962;2:174–179. [Google Scholar]
- 20.Greeves J. P., Cable N. T., Reilly T., Kingsland C. Changes in muscle strength in women following the menopause: a longitudinal assessment of the efficacy of hormone replacement therapy. Clin Sci (Lond) 1999;97(1):79–84. [PubMed] [Google Scholar]
- 21.Phillips S. K., Rook K. M., Siddle N. C., Bruce S. A., Woledge R. C. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond) 1993;84(1):95–98. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- 22.Janse de Jonge X. A., Boot C. R., Thom J. M., Ruell P. A., Thompson M. W. The influence of menstrual cycle phase on skeletal muscle contractile characteristics in humans. J Physiol. 2001;530(pt 1):161–166. doi: 10.1111/j.1469-7793.2001.0161m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarwar R., Niclos B. B., Rutherford O. M. Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. J Physiol. 1996;493(pt 1):267–272. doi: 10.1113/jphysiol.1996.sp021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebrun C. M., McKenzie D. C., Prior J. C., Taunton J. E. Effects of menstrual cycle phase on athletic performance. Med Sci Sports Exerc. 1995;27(3):437–444. [PubMed] [Google Scholar]
- 25.Gür H. Concentric and eccentric isokinetic measurements in knee muscles during the menstrual cycle: a special reference to reciprocal moment ratios. Arch Phys Med Rehabil. 1997;78(5):501–505. doi: 10.1016/s0003-9993(97)90164-7. [DOI] [PubMed] [Google Scholar]
- 26.Fridén C., Hirschberg A. L., Saartok T. Muscle strength and endurance do not significantly vary across 3 phases of the menstrual cycle in moderately active premenopausal women. Clin J Sport Med. 2003;13(4):238–241. doi: 10.1097/00042752-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Davies B. N., Elford J. C., Jamieson K. F. Variations in performance in simple muscle tests at different phases of the menstrual cycle. J Sports Med Phys Fitness. 1991;31(4):532–537. [PubMed] [Google Scholar]
- 28.Bambaeichi E., Reilly T., Cable N. T., Giacomoni M. The isolated and combined effects of menstrual cycle phase and time-of-day on muscle strength of eumenorrheic females. Chronobiol Int. 2004;21(4–5):645–660. doi: 10.1081/cbi-120039206. [DOI] [PubMed] [Google Scholar]
- 29.Landgren B. M., Unden A. L., Diczfalusy E. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinol (Copenh) 1980;94(1):89–98. doi: 10.1530/acta.0.0940089. [DOI] [PubMed] [Google Scholar]
- 30.Elliott K. J., Cable N. T., Reilly T., Diver M. J. Effect of menstrual cycle phase on the concentration of bioavailable 17-beta oestradiol and testosterone and muscle strength. Clin Sci (Lond) 2003;105(6):663–669. doi: 10.1042/CS20020360. [DOI] [PubMed] [Google Scholar]
- 31.Shultz S. J., Kirk S. E., Johnson M. L., Sander T. C., Perrin D. H. Relationship between sex hormones and anterior knee laxity across the menstrual cycle. Med Sci Sports Exerc. 2004;36(7):1165–1174. doi: 10.1249/01.MSS.0000132270.43579.1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Requena B., Gonzalez-Badillo J. J., de Villareal E. S., et al. Functional performance, maximal strength, and power characteristics in isometric and dynamic actions of lower extremities in soccer players. J Strength Cond Res. 2009;23(5):1391–1401. doi: 10.1519/JSC.0b013e3181a4e88e. [DOI] [PubMed] [Google Scholar]
- 33.Grabiner M. D., Campbell K. R., Hawthorne D. L., Hawkins D. A. Electromyographic study of the anterior cruciate ligament-hamstrings synergy during isometric knee extension. J Orthop Res. 1989;7(1):152–155. doi: 10.1002/jor.1100070122. [DOI] [PubMed] [Google Scholar]
- 34.Hagood S., Solomonow M., Baratta R., Zhou B. H., D'Ambrosia R. The effect of joint velocity on the contribution of the antagonist musculature to knee stiffness and laxity. Am J Sports Med. 1990;18(2):182–187. doi: 10.1177/036354659001800212. [DOI] [PubMed] [Google Scholar]
- 35.Bao A. M., Liu R. Y., van Someren E. J., Hofman M. A., Cao Y. X., Zhou J. N. Diurnal rhythm of free estradiol during the menstrual cycle. Eur J Endocrinol. 2003;148(2):227–232. doi: 10.1530/eje.0.1480227. [DOI] [PubMed] [Google Scholar]
- 36.Birch K., Reilly T. The diurnal rhythm in isometric muscular performance differs with eumenorrheic menstrual cycle phase. Chronobiol Int. 2002;19(4):731–742. doi: 10.1081/cbi-120006083. [DOI] [PubMed] [Google Scholar]
- 37.Israel R., Mishell D. R., Jr, Stone S. C., Thorneycroft I. H., Moyer D. L. Single luteal phase serum progesterone assay as an indicator of ovulation. Am J Obstet Gynecol. 1972;112(8):1043–1046. doi: 10.1016/0002-9378(72)90178-0. [DOI] [PubMed] [Google Scholar]
- 38.Barron M. L., Fehring R. J. Basal body temperature assessment: is it useful to couples seeking pregnancy? MCN Am J Matern Child Nurs. 2005;30(5):290–298. doi: 10.1097/00005721-200509000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Russell J. B., Mitchell D., Musey P. I., Collins D. C. The relationship of exercise to anovulatory cycles in female athletes: hormonal and physical characteristics. Obstet Gynecol. 1984;63(4):452–456. [PubMed] [Google Scholar]
- 40.De Souza M. J., Miller B. E., Loucks A. B., et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83(12):4220–4232. doi: 10.1210/jcem.83.12.5334. [DOI] [PubMed] [Google Scholar]
- 41.Phillips S. K., Sanderson A. G., Birch K., Bruce S. A., Woledge R. C. Changes in maximal voluntary force of human adductor pollicis muscle during the menstrual cycle. J Physiol. 1996;496(pt 2):551–557. doi: 10.1113/jphysiol.1996.sp021706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakkinen K. Force production characteristics of leg extensor, trunk flexor and extensor muscles in male and female basketball players. J Sports Med Phys Fitness. 1991;31(3):325–331. [PubMed] [Google Scholar]