Abstract
The aggressive course of pancreatic cancer is believed to reflect its unusually invasive and metastatic nature, which is associated with epidermal growth factor receptor (EGFR) overexpression and NF-κB activation. MicroRNAs (miRNA) have been implicated in the regulation of various pathobiological processes in cancer, including metastasis in pancreatic cancer and in other human malignancies. In this study, we report lower expression of miR-146a in pancreatic cancer cells compared with normal human pancreatic duct epithelial cells. Reexpression of miR-146a inhibited the invasive capacity of pancreatic cancer cells with concomitant downregulation of EGFR and the NF-κB regulatory kinase interleukin 1 receptor–associated kinase 1 (IRAK-1). Cellular mechanism studies revealed crosstalk between EGFR, IRAK-1, IκBα, NF-κB, and MTA-2, a transcription factor that regulates metastasis. Treatment of pancreatic cancer cells with the natural products 3,3′-diinodolylmethane (DIM) or isoflavone, which increased miR-146a expression, caused a downregulation of EGFR, MTA-2, IRAK-1, and NF-κB, resulting in an inhibition of pancreatic cancer cell invasion. Our findings reveal DIM and isoflavone as nontoxic activators of a miRNA that can block pancreatic cancer cell invasion and metastasis, offering starting points to design novel anticancer agents.
Introduction
Pancreatic cancer is an aggressive malignancy with one of the worst outcomes among all cancers. For all stages combined, the 5-year relative survival rate is only 5% (1). The high mortality of pancreatic cancer could be partly due to the ability of pancreatic cancer cells to acquire invasive characteristics during the early stages of carcinogenesis. Thus, most patients diagnosed with pancreatic cancer are unresectable and usually die from metastatic disease even after treatment with existing therapies. Therefore, there is a dire need for understanding the molecular mechanism(s) involved in the aggressiveness of pancreatic cancer and its high propensity for metastasis, and further novel approaches are needed for developing novel therapeutic strategies for the inhibition of tumor aggressiveness and metastasis so that the patients diagnosed with pancreatic cancer could be treated with better outcome.
It has been reported that overexpression of epidermal growth factor receptor (EGFR) and activation of NF-κB are common in pancreatic cancer (2, 3). Elevated expression of EGFR and its ligand correlates with large tumor size, advanced clinical staging, and decreased survival period in pancreatic cancer (4). The constitutive activation of NF-κB, which regulates important genes and thereby affects many cellular processes, is also known to contribute to the aggressive behavior of pancreatic cancer (3, 5). However, the molecular mechanism(s) involved in the overexpression of EGFR and activation of NF-κB in pancreatic cancer have not been fully elucidated.
Recently, emerging evidence indicates the critical role of microRNAs (miRNA) in the regulation of various biological and pathologic processes, including metastasis (6). These small, noncoding molecules elicit their regulatory effects by imperfectly binding to the 3′ untranslated region of target mRNA, causing either degradation of mRNA or inhibition of their translation to functional proteins (7). The expression of miRNAs has been recognized as integral components of many normal biological processes involving cell proliferation, differentiation, apoptosis, and stress resistance (8). More importantly, it has been recently suggested that aberrant upregulation or downregulation of specific miRNAs and their targets in various types of cancer is associated with the development and progression of cancer (9–11). Moreover, miR-146a has been found to be lost in metastatic prostate cancer (12), and its expression reduces the metastatic potential of breast cancer cells through repression of interleukin 1 receptor–associated kinase 1 (IRAK-1) and subsequent inhibition of NF-κB activity (13, 14). In addition, miR-146a could target EGFR based on predicted base pairing by using miRBase analysis (15). Therefore, it is important to unravel the relationship between miR-146a and the signaling of EGFR and NF-κB. It is also important to find novel agents that could regulate miR-146, EGFR, and NF-κB, and that could be useful for the inhibition of pancreatic cancer progression and metastasis.
We have previously found that dietary compounds, including isoflavone genistein and 3,3′-diindolylmethane (DIM), could enhance the antitumor activity of chemotherapeutic agents through the inhibition of NF-κB (16, 17). However, the precise molecular mechanisms by which these dietary compounds regulate NF-κB have not been fully elucidated. In this study, we assessed the expression pattern of miRNAs in the normal pancreatic duct epithelial cells and pancreatic cancer cells. We also investigated whether the treatment of pancreatic cancer cells with either B-DIM (BioResponse formulated DIM with greater bioavailability) or G2535 (a mixture of genistein and other isoflavones) could alter the expression of miRNAs and their targets that are involved in the processes of invasion and metastasis. We found that B-DIM and G2535 could inhibit invasion of pancreatic cancer cells through the induction of miR-146a and with corresponding downregulation of its targets, including IRAK-1/NF-κB, EGFR, and metastasis-associated protein 2 (MTA-2), suggesting that reexpression of specific miRNA by nontoxic “natural agents” could be a useful strategy for the treatment of pancreatic cancer in the future.
Materials and Methods
Cell lines, reagents, and antibodies
Colo357 and Panc-1 pancreatic cancer cells were maintained in DMEM (Invitrogen) supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin in a 5% CO2 atmosphere at 37°C. Human pancreatic duct epithelial (HPDE) cells were obtained from M.D. Anderson Cancer Center (a generous gift of Dr. Paul J. Chiao) and cultured in keratinocyte serum-free medium supplied with 5 ng/mL of epidermal growth factor and 50 μg/mL of bovine pituitary extract (Invitrogen; ref. 18). The cell lines have been tested and authenticated in core facility Applied Genomics Technology Center at Wayne State University. The method used for testing was short tandem repeat profiling using the PowerPlex 16 System from Promega. B-DIM (BioResponse; known as BR-DIM and referred to as B-DIM with higher bioavailability in vivo; ref. 19) was generously provided by Dr. Michael Zeligs (BioResponse LLC, Boulder, CO) and was dissolved in DMSO to make a 50 mmol/L stock solution. Isoflavone mixture G2535 (70.54% genistein, 26.34% diadzin, and 0.31% glycitein, manufactured by Organic Technologies and obtained from the NIH) was dissolved in DMSO to make a stock solution containing 50 mmol/L equivalent to genistein. The concentration of isoflavone we described in this article all refer to the concentration of genistein in the isoflavone mixture. The EGFR tyrosine kinase inhibitor erlotinib was a generous gift from OSI Pharmaceuticals. Anti-EGFR (Cell Signaling), anti–phospho-EGFR Tyr992 (Cell Signaling), anti–IRAK-1 (Santa Cruz Biotechnology), anti–MTA-2 (Santa Cruz Biotechnology), anti–NF-κB p65 (Millipore), anti-IκBα (Cell Signaling), anti–phospho-IκBα (Cell Signaling), and anti–β-actin (Sigma) primary antibodies were used for Western blot analysis.
RNA isolation
Total RNA was extracted by using the mirVana miRNA Isolation Kit (Ambion, Inc.) following the protocol provided by the manufacturer. Briefly, Colo357 and Panc-1 cells were treated with 25 μmol/L B-DIM or 25 μmol/L G2535 for 48 h. Control cells received 0.05% DMSO. Then, the pancreatic cancer cells from each condition and the HPDE cells were subjected to RNA extraction following the procedure we reported previously (20).
miRNA array and data analysis
Five micrograms of each total RNA sample from HPDE, Colo357, and Panc-1 cells were sent to a service provider for completion of the miRNA arrays (LCSciences). In LCSciences, the total RNA samples were enriched for microRNAs and the miRNA arrays were performed on μParaFlo microfluidic chips (version 10.0), each of which has a miRNA probe region with multiple repeat regions that detect 711 miRNAs. Multiple control probes are also included on the arrays for assessing various chip and assay qualities, such as uniformity and specificity. Chips were scanned and the signal intensity data were obtained. Then, the data was analyzed by subtracting the background and normalizing the signals to balance the intensities of transcripts in normal, cancer, control, and treatment samples so that differential expression ratios can be calculated. The ratio of the two sets of detected signals, such as normal and cancer and control and treatment, was calculated. The t test was also performed to calculate the P value. The predicting target genes for various miRNAs were also analyzed using computerized analysis.3
miRNA real-time reverse transcriptase-PCR
To verify the alteration in the expression of miR-146a that was found in miRNA array analysis, we conducted real-time miRNA reverse transcriptase-PCR (RT-PCR) analysis using miR-146a TaqMan MicroRNA Assay Kit (Applied Biosystems) following the procedure we reported previously (20).
Real-time RT-PCR using mRNA
Real-time RT-PCR analysis was also conducted to measure the expression of EGFR in pancreatic cancer cells with and without 25 μmol/L B-DIM or 25 μmol/L G2535 treatment following the procedure we reported previously (20). The t test was also performed to calculate the P value for EGFR mRNA and miR-146a data.
Western blot analysis
We also conducted Western blot analysis to verify the alterations in the protein expression of genes, which are targets of miR-146a. Colo357 and Panc-1 pancreatic cancer cells were treated with or without 25 μmol/L B-DIM or 25 μmol/L G2535 for 48 h. After treatment, the cells were lysed in 62.5 mmol/L Tris-HCl and 2% SDS, and protein concentration was measured using BCA protein assay (Pierce). The proteins were subjected to 10% or 14% SDS-PAGE and electrophoretically transferred to nitrocellulose membrane. The membranes were incubated with specific primary antibodies and subsequently incubated with secondary antibody conjugated with peroxidase (Bio-Rad). The signal was detected using the chemiluminescent detection system (Pierce). The signal of each target protein expression was scanned and quantified by using AlphaEaseFC (Alpha Innotech). The ratios of detected target proteins against β-actin were calculated, with standardization of the ratio of each control to the unit value.
Reexpression and inhibition of miR-146a in pancreatic cancer cells
Colo357 and Panc-1 cells were seeded in six-well plates and transfected with miR-146a, miRNA-negative control, anti–miR-146, or anti–miR-negative control (Ambion) at a final concentration of 20 nmol/L using Dharma-Fect Transfection Reagent (Dharmacon). After 3 d of transfection, the cells were split and transfected repeatedly with the miRNA or anti-miRNA every 3 to 4 d for indicated periods of time. Total RNA from each samples were then extracted using the Trizol reagent (Invitrogen). One microgram of RNA was subjected to RT-PCR using the High Capacity RNA-to-cDNA Kit (Applied Biosystems) and SYBR Green PCR Master Mix (Applied Biosystems) as described earlier. Total proteins from each sample were also extracted and subjected to Western blot analysis as described previously. Cytoplasmic and nuclear proteins were also extracted and subjected to Western blot analysis and electrophoretic mobility shift assay (EMSA).
Inhibition of EGFR expression by siRNA in pancreatic cancer cells
Colo357 and Panc-1 cells were seeded in a six-well plate (1.2 × 105 cells per well) and incubated at 37°C for 24 h. The cells were then transfected with EGFR siRNA (Santa Cruz Biotechnology) or control RNA duplex by DharmaFect Transfection Reagent (Dharmacon) for 72 h. Then, the total, cytoplasmic, and nuclear proteins were extracted. The proteins were subjected to Western blot analysis.
Electrophoretic mobility shift assay
EMSA was conducted to measure the DNA-binding activity of NF-κB in miR-146a–transfected or B-DIM and G2535-treated Colo357 and Panc-1 cells. Briefly, after transfection or treatment, nuclear proteins from each sample were extracted and subjected to EMSA following the standard procedure we reported previously (17).
Invasion assay
The invasive capacity of Colo357 and Panc-1 cells with miR-146a or anti–miR-146 transfection or after different treatments were tested by using BD BioCoat Tumor Invasion Assay System (BD Biosciences) according to the manufacturer’s protocol with minor modification. Briefly, Colo357 and Panc-1 cells were transfected with miR-146a or anti–miR-146 for 5 d as described above and/or treated with 25 μmol/L B-DIM or 25 μmol/L G2535 for 2 d. Then, the transfected or treated Colo357 and Panc-1 cells (5 × 104) with serum-free medium were seeded into the upper chamber of the system. Bottom wells in the system were filled with complete medium. After 24 h of incubation, the cells in the upper chamber were removed, and the cells that invaded through the Matrigel matrix membrane were stained with 4 μg/mL Calcein AM in Hanks buffered saline at 37°C for 1 h. Then, fluorescence of the invaded cells was read in ULTRA Multifunctional Microplate Reader (Tecan) at excitation/emission wavelengths of 530/590 nm. These fluorescently labeled invasive cells were also photographed under a fluorescent microscope.
Growth inhibition assay
Panc-1 and Colo357 cells were transfected with miR-146a, anti–miR-146, or miRNA-negative control for 5 d as described. Then, the transfected cells were seeded in 96-well plates and treated with 1 μmol/L erlotinib, 12.5 μmol/L B-DIM, or 12.5 μmol/L G2535 for 2 d. After treatment, the cells were subjected to cell proliferation assay using Cell Proliferation Reagent WST-1 assay (Roche) following the manufacturer’s protocol. The spectrophotometric absorbance of the samples was determined by using Ultra Multifunctional Microplate Reader (Tecan) at 450 nm.
Results
Pancreatic cancer cells showed downregulation of miR-146a, and treatment of pancreatic cancer cells with B-DIM and G2535 showed increased expression of miR-146a
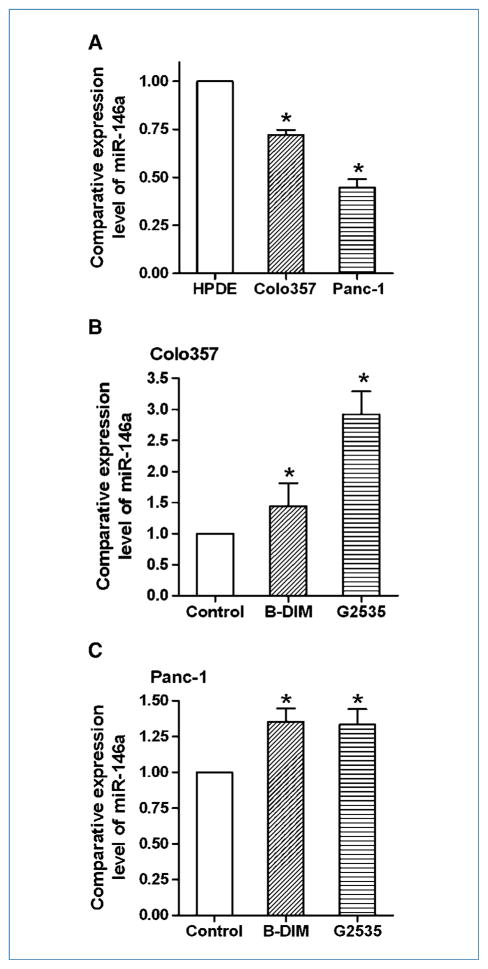
To investigate the difference in miRNA expression between normal HPDE and pancreatic cancer cells, we conducted a miRNA array. The results from miRNA array were validated by miRNA RT-PCR analysis. By miRNA array and RT-PCR, we found that the expression level of miR-146a in HPDE cells was higher than that in Colo357 and Panc-1 pancreatic cancer cells (Table 1; Fig. 1A), suggesting that pancreatic cancer cells had lower expression of miR-146a compared with normal cells. Importantly, we also found that the miR-146a expression was upregulated on 25 μmol/L B-DIM or 25 μmol/L G2535 treatments of Colo357 and Panc-1 cells (Table 1; Fig. 1B and C). These results suggest that miR-146a could be an inhibitory molecule for cancer development and progression, and that B-DIM and G2535 could inhibit the progression of pancreatic cancer through induction of miR-146a expression. Because miRNAs regulate expression of target genes, we further tested the expression of miR-146a target genes after reexpression or inhibition of miR-146a by transfection studies in pancreatic cancer cells.
Table 1.
Differential expression level (average signal) of miR-146a in pancreatic duct epithelial cells and pancreatic cancer cells treated with B-DIM or G2535 tested by miRNA array
| Cell line | Control | B-DIM treatment | G2535 treatment |
|---|---|---|---|
| Panc-1 | 1,829.54 ± 102.80* | 2,408.24 ± 97.82 | 2,732.86 ± 105.91 |
| Colo357 | 2,564.36 ± 133.84 | 4,541.72 ± 239.33 | 8,758.75 ± 429.05 |
| HPDE | 3,066.60 ± 145.00 |
Means ± SD.
Figure 1.
Real-time RT-PCR showed that the expression of miR-146a was higher in HPDE cells compared with pancreatic cancer cells (A) and that treatment of Colo357 (B) and Panc-1 (C) cells with 25 μmol/L B-DIM or 25 μmol/L isoflavone induced the expression of miR-146a compared with vehicle control. Columns, mean from three independent experiments; bars, SEM; *, P < 0.05.
Reexpression of miR-146a led to the inhibition of EGFR signaling in pancreatic cancer cells
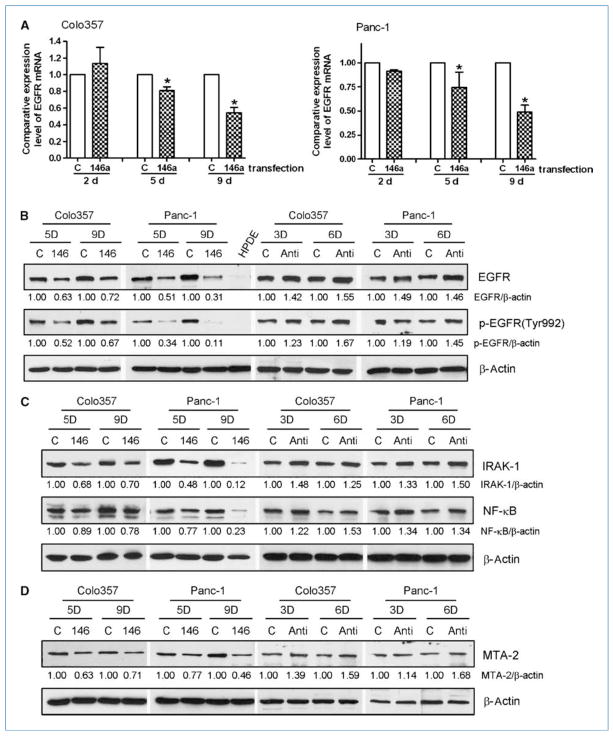
To investigate the role of miR-146a in the regulation of cellular signaling, we transfected Colo357 and Panc-1 cells with miR-146a pre-micro-RNAs and cultured for a period of up to 9 d. We found that the reexpression of miR-146a in the Colo357 and Panc-1 cells resulted in the downregulation of EGFR both at the mRNA and protein levels (Fig. 2A and B). Importantly, we found that phosphorylated EGFR at Tyr992, which is responsible for the binding of the Src homology 2–containing phosphotyrosine phosphatase and positively affects receptor tyrosine kinase signaling (21), was also downregulated after miR-146a transfection (Fig. 2B). To confirm the effects of miR-146a on EGFR, we transferred anti–miR-146 into pancreatic cancer cells and found that inhibition of miR-146 increased the levels of EGFR and p-EGFR (Fig. 2B). These results suggest the inhibitory effects of miR-146a on EGFR signaling. Because miR-146a could also regulate the expression of IRAK-1, which could activate NF-κB signaling, we investigated the effects of miR-146a on IRAK-1/NF-κB signaling pathways.
Figure 2.
Reexpression of miR-146a by transfection resulted in the downregulation of EGFR expression and phosphorylation in Colo357 and Panc-1 cells as assessed by real-time RT-PCR (A; columns, mean from three independent experiments; bars, SEM; *, P < 0.05.) and Western blot analysis (B). The expression of IRAK-1, NF-κB (C), and MTA-2 (D) was also downregulated in pancreatic cancer cells after miR-146a transfection. However, the expressions of EGFR, p-EGFR (B); IRAK-1, NF-κB (C); and MTA-2 (D) were upregulated by anti–miR-146 transfection. C, control miRNA transfection; 146, miR-146a transfection; Anti, anti–miR-146 transfection; 3D, 5D, 6D, 9D, transfection for 3, 5, 6, or 9 days, respectively; the ratios of detected target proteins against β-actin were shown under each Western blot image.
Reexpression of miR-146a led to the inhibition of IRAK-1/NF-κB signaling and MTA-2 expression in pancreatic cancer cells
After we transfected miR-146a into Colo357 and Panc-1 cells, we found that the reexpression of miR-146a also caused downregulation of IRAK-1 expression in the pancreatic cancer cells (Fig. 2C). Moreover, the level of NF-κB was also decreased after miR-146a transfection (Fig. 2C), suggesting that miR-146a could regulate IRAK-1/NF-κB signaling. Interestingly, we found that miR-146a inhibited the expression of MTA-2 (Fig. 2D), which is one of the computerized predicted miR-146a target genes and associated with cancer cell invasion and metastasis, suggesting that our findings are highly novel. These results were also confirmed by the observations that the expression of IRAK-1, NF-κB, and MTA-2 was upregulated by anti–miR-146 (Fig. 2C and D). Because we observed the upregulation of miR-146a after B-DIM and G2535 treatment, we further investigated whether the treatment of pancreatic cancer cells with B-DIM and G2535 could achieve similar effects as seen with reexpression of miR-146a by transfection experiments.
B-DIM and G2535 treatment suppressed EGFR, IRAK-1/NF-κB, and MTA-2 in pancreatic cancer cells
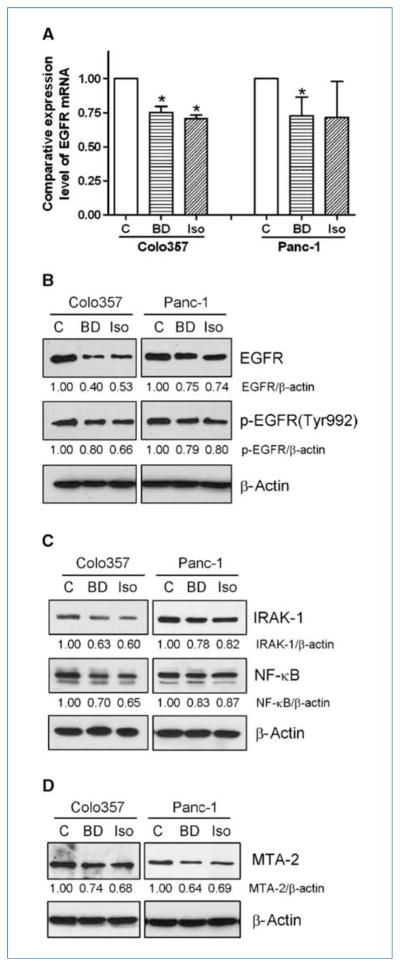
By RT-PCR and Western blot analysis, we found that 25 μmol/L B-DIM or 25 μmol/L G2535 inhibited the expression of EGFR at mRNA and protein levels in Colo357 and Panc-1 cells (Fig. 3A and B). Moreover, B-DIM and G2535 decreased the level of phosphorylated EGFR at the Tyr992 site in Colo357 and Panc-1 cells (Fig. 3B). Similar to the effects of miR-146a reexpression, B-DIM and G2535 also decreased the expression of IRAK-1, NF-κB, and MTA-2 in Colo357 and Panc-1 cells (Fig. 3C and D). These results suggest that the downregulation of EGFR, IRAK-1, NF-κB, and MTA-2 by B-DIM and G2535 could be mediated through the upregulation of miR-146a as seen earlier (Fig. 1B and C). Because EGFR and IRAK-1 could alter the activity of NF-κB, we further investigated the relationship between EGFR, IRAK-1, NF-κB, and MTA-2 as presented below.
Figure 3.
B-DIM (25 μmol/L) or isoflavone (G2535, 25 μmol/L) treatment for 2 d decreased EGFR expression and phosphorylation in pancreatic cancer cells as assessed by real-time RT-PCR (A; columns, mean from three independent experiments; bars, SEM; *, P < 0.05.) and Western blot analysis (B). The expression of IRAK-1, NF-κB (C), and MTA-2 (D) was also downregulated in pancreatic cancer cells after B-DIM or isoflavone treatment. C, control miRNA transfection; BD, B-DIM treatment; Iso, isoflavone treatment.
Cross-regulation between miR-146a, EGFR, IRAK-1, IκBα, NF-κB, and MTA-2
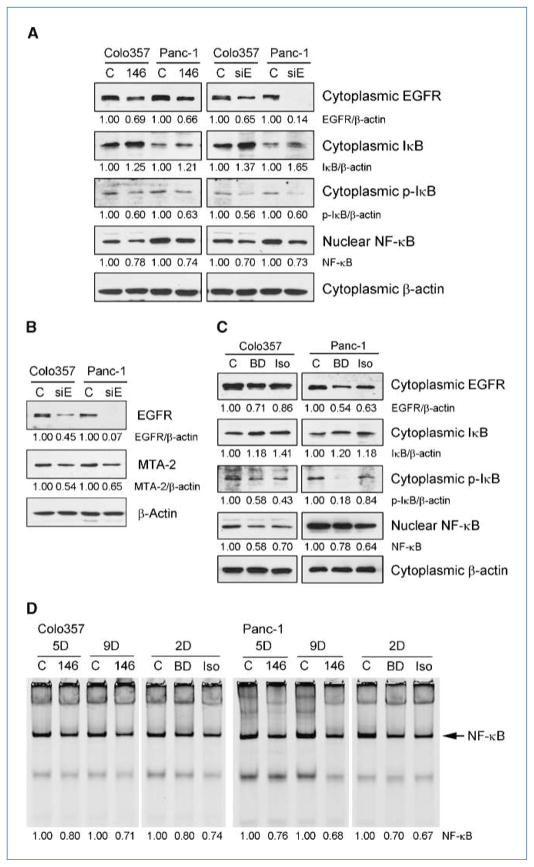
As shown in Fig. 4A, the reexpression of miR-146a inhibited the expression of cytoplasmic EGFR and decreased the level of cytoplasmic phosphorylated IκBα and nuclear NF-κB, whereas the expression of cytoplasmic IκBα was increased after miR-146a transfection. To confirm whether these effects of miR-146a were mediated through the downregulation of EGFR, we conducted EGFR siRNA silencing experiments. We found that downregulation of EGFR by EGFR siRNA also caused increased levels of cytoplasmic IκBα and decreased the levels of cytoplasmic phosphorylated IκBα and nuclear NF-κB (Fig. 4A). Interestingly, we also found inhibition of MTA-2 expression by EGFR siRNA (Fig. 4B). These results suggest that there are cross-regulations between EGFR, IκBα, NF-κB, and MTA-2 signaling. More importantly, we found that B-DIM and G2535 also increased the level of cytoplasmic IκBα and decreased the level of cytoplasmic EGFR, phosphorylated IκBα, and nuclear NF-κB (Fig. 4C). By EMSA, we observed that the NF-κB DNA-binding activity was inhibited by the reexpression of miR-146a, and similar findings were also obtained by the treatment of pancreatic cancer cells with B-DIM or G2535 (Fig. 4D), which is consistent with our data obtained from Western blot analysis. These results suggest that the inhibition of NF-κB by B-DIM and G2535 could be partly mediated through the upregulation of miR-146a and subsequent down-regulation of EGFR. Because EGFR, NF-κB, and MTA-2 all are associated with invasion and metastasis in cancer cells, we tested the invasive capacity of Colo357 and Panc-1 cells after reexpression or inhibition of miR-146a by transfection studies as well as after treatment of pancreatic cancer cells with B-DIM and G2535.
Figure 4.
miR-146a or EGFR siRNA transfection increased the level of IκBα (A) and decreased the levels of EGFR, p-IκBα, NF-κB, and MTA-2 (B) in pancreatic cancer cells. B-DIM (25 μmol/L) or isoflavone (25 μmol/L) treatment increased the level of IκBα and decreased the levels of EGFR, p-IκBα, and NF-κB in pancreatic cancer cells. EMSA (D) showed that NF-κB DNA-binding activity in pancreatic cancer cells was inhibited by the reexpression of miR-146a or the treatment of cells with 25 μmol/L B-DIM or 25 μmol/L isoflavone. C, control; 146, miR-146a transfection; siE, EGFR siRNA transfection; BD, B-DIM treatment; Iso, isoflavone treatment; 2D, treatment for 2 days; 5D, 9D, transfection for 5 or 9 days. The ratios of detected target proteins against β-actin were shown under each Western blot image.
miR-146a transfection or B-DIM and G2535 treatment inhibited pancreatic cancer cell invasion
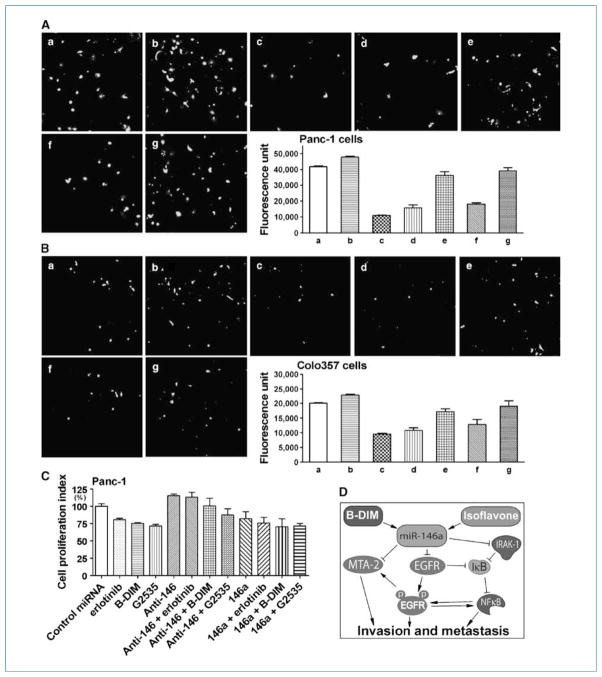
By invasion assay, we found that the invasive capacity of Colo357 and Panc-1 cells was significantly inhibited by the reexpression of miR-146a either by miR-146a transfection or by the treatment of pancreatic cancer cells with B-DIM or G2535 (Fig. 5A and B). However, anti–miR-146 increased the invasion of pancreatic cancer cells and restored invasive capacity of pancreatic cancer cells treated with B-DIM and G2535 (Fig. 5A and B). We further treated pancreatic cancer cells with erlotinib, one of the EGFR inhibitors, after miR-146a or anti–miR-146 transfection and found that the level of miR-146 influenced the inhibitory effect of erlotinib, B-DIM, and G2535 on cell growth (Fig. 5C), suggesting the relationship between miR-146a and EGFR. These results clearly suggest that B-DIM and G2535 could inhibit invasion and metastasis of pancreatic cancer cells, which is in part due to the upregulation of miR-146a, and subsequent downregulation of EGFR, NF-κB, and MTA-2 signaling.
Figure 5.
Invasion assay showed that the reexpression of miR-146a by transfection studies or by the treatment of pancreatic cancer cells with 25 μmol/L B-DIM or 25 μmol/L isoflavone significantly inhibited the invasion of pancreatic cancer cells through the Matrigel matrix membrane. However, inhibition of miR-146 increased the invasion of pancreatic cancer cells and restored the invasive capacity of cells treated with B-DIM and G2535. A, Panc-1 cells; B, Colo357 cells; a, negative control miRNA transfection; b, transfected with anti–miR-146; c, transfected with miR-146a; d, treated with 25 μmol/L B-DIM; e, transfected with anti–miR-146 followed by B-DIM treatment; f, treated with 25 μmol/L isoflavone; g, transfected with anti–miR-146 followed by G2535 treatment (×200). The graphs showed the value of fluorescence from the invaded pancreatic cancer cells. The values indicated the comparative number of invaded pancreatic cancer cells. Columns, mean from two independent experiments; bars, SEM; P < 0.05 for each group compared with control. C, the level of miR-146 influenced the inhibitory effect of erlotinib, B-DIM, and G2535 on cell proliferation. Columns, mean from three independent experiments; bars, SEM. D, diagram showing the hypothetical cross-regulation between miR-146a, EGFR, MTA-2, IRAK-1, IκBα, and NF-κB in the inhibition of cancer cell invasion and metastasis by B-DIM and isoflavone.
Discussion
Although recent years have witnessed the deregulated expression of miR-146, which has been implicated both in the development of various cancers and in the negative regulation of inflammation induced through the innate immune response (22, 23), controversies exist regarding the effects of miR-146a on cancer development and progression. The elevated miR-146a levels has been found in papillary thyroid carcinoma (23) and cervical cancer (24), whereas reduced miR-146a expression was associated with hormone-refractory prostate cancer (12). In our study, we found lower levels of miR-146a in pancreatic cancer cells compared with normal HPDE cells, although we recognize that HPDE cells may not be a perfect control for comparing miRNA expression with pancreatic glandular cancer cells. Most importantly, we found that the reexpression of miR-146a could reduce the invasive capacity of both Colo357 and Panc-1 pancreatic cancer cells, suggesting that the regulated expression of miR-146a and its targeted genes could be exploited for designing novel strategies for the treatment of pancreatic cancer in the future. Our finding is consistent with recent reports showing that upregulation of miR-146a inhibited cancer cell metastasis in breast (13, 14, 25) and prostate (12) cancers. These results collectively suggest that miR-146a could exert its critical effects in the later stages of cancer rather than the initial stage, and thus it is tempting to speculate that novel strategies by which one could induce higher levels of miR-146a in cancer cells could be useful for suppressing tumor progression by inhibiting invasion and metastasis.
The molecular mechanism(s) involved in the miR-146a–mediated inhibition of metastasis are still unclear. From our results, we believe that miR-146a inhibits invasion and metastasis partly through the regulation of EGFR and NF-κB signaling (Fig. 5D). From the Sanger database, it has been found that EGFR and MTA-2 are predicted targets of miR-146a. Hurst and colleagues recently reported that miR-146a and miR-146b downregulated the expression of EGFR and inhibited breast cancer metastasis (14); however, how EGFR regulates cancer metastasis is unknown. In our study, we found that the reexpression of miR-146a by transfection suppressed the expression of EGFR and MTA-2 in pancreatic cancer cells. Furthermore, downregulation of EGFR by siRNA could also decrease MTA-2 expression, suggesting their cross-talks. MTA (metastasis-associated gene or protein) is a newly discovered family of cancer gene that is associated with tumor progression and metastasis (26). MTA-2 could deacetylate and inactivate the tumor suppressor p53 protein (27). High expression of MTA-2 was associated with more aggressive behaviors of ovarian epithelial cancer (28). Therefore, miR-146a could directly inhibit the expression of MTA-2 or indirectly downregulate MTA-2 through the inhibition of EGFR, leading to the suppression of cancer cell invasion and metastasis as partly supported by our results (Fig. 5D). Moreover, it has been reported that EGFR could regulate NF-κB signaling (29); thus, miR-146a could indirectly downregulate NF-κB signaling by suppressing EGFR, resulting in the inhibition of cancer cell invasion and metastasis. However, further in-depth mechanistic studies are warranted based on our exciting results.
NF-κB is activated in a wide array of human cancers, and it is thought to be involved in promoting invasion, angiogenesis, and metastasis in various cancers (30, 31). It has been reported that NF-κB could be activated by IRAK-1 (32, 33), which is a target of miR-146a (13). Therefore, miR-146a–mediated inhibition of invasion could be through the down-regulation of IRAK-1 and subsequent inactivation of NF-κB. Indeed, we found that the expression of IRAK-1 was significantly inhibited by miR-146a transfection. We also observed increased levels of cytoplasmic IκBα and decreased levels of cytoplasmic phosphorylated IκBα and nuclear NF-κB after reexpression of miR-146a or EGFR siRNA transfection, suggesting that miR-146a could regulate IκB/NF-κB signaling through the inhibition of both IRAK-1 and EGFR (Fig. 5D). Our results are also consistent with the findings reported by other investigators showing that IRAK-1 could inhibit IκBα, leading to the activation of NF-κB (32, 33), and that EGFR or Her-2/neu are known activators of NF-κB through the phosphorylation of IκBα mediated through Akt (34) and the degradation of IκBα by calpain (35). Therefore, the inactivation of NF-κB could be another molecular mechanism by which miR-146a inhibits cancer cell invasion and metastasis.
The inhibition of cancer cell invasion and metastasis should be an important strategy for the successful treatment of pancreatic cancer, and thus any novel strategies by which the invasive and metastatic ability of pancreatic cancer cells could be inhibited should be useful for improving the devastating outcome of patients diagnosed with pancreatic cancer. We and others previously reported that isoflavone and DIM (non-toxic natural agents) could inhibit invasion and metastasis of prostate, breast, ovarian, and bladder cancer cells (36–41); however, the molecular mechanism(s) involved has not been fully elucidated. The data presented in this article showed, for the first time, that the nontoxic natural agents such as B-DIM and isoflavone could upregulate the expression of miR-146a, which in turn downregulate the expression of EGFR, MTA-2, IRAK-1, and NF-κB, resulting in the inhibition of invasion of pancreatic cancer cells (Fig. 5D). These results clearly suggest that instead of reexpression of miR-146a by transfection studies, one could simply treat pancreatic cancer cells with nontoxic natural agents that will result in the induction of miR-146a expression. Thus, we believe that such a strategy could be useful for the inactivation of multiple genes downstream of miR-146a toward pancreatic cancer therapy, especially because EGFR-targeted therapy (gefitinib, erlotinib, or antibodies to EGFR) has essentially failed in clinical trials.
In conclusion, our results clearly show that B-DIM and isoflavone could upregulate miR-146a and inhibit cancer cell invasion, which was in part due to downregulation of EGFR and NF-κB, the molecular targets that are commonly activated in pancreatic cancer. These results suggest that further in vivo studies and clinical trials are needed to establish whether these agents could also be useful in combination with conventional chemotherapeutics or conventional targeted agents for the treatment of pancreatic cancer for which there is no effective and curative therapy.
Acknowledgments
We thank the Guido and Puschelberg Foundations for their generous contribution for the completion of this study.
Grant Support
National Cancer Institute, NIH [5R01CA131151, 3R01-CA131151-02S109, 1R01CA132794, 5R01CA083695, and 5R01CA101870 (F.H. Sarkar) and a subcontract award to F.H. Sarkar from the University of Texas M.D. Anderson Cancer Center through a Specialized Programs of Research Excellence grant (5P20CA101936-05, 3P20CA101936-05S109) on pancreatic cancer awarded to James Abbruzzese].
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Xiong HQ, Abbruzzese JL. Epidermal growth factor receptor-targeted therapy for pancreatic cancer. Semin Oncol. 2002;29:31–7. doi: 10.1053/sonc.2002.35645. [DOI] [PubMed] [Google Scholar]
- 3.Fujioka S, Sclabas GM, Schmidt C, et al. Function of nuclear factor κB in pancreatic cancer metastasis. Clin Cancer Res. 2003;9:346–54. [PubMed] [Google Scholar]
- 4.Yamanaka Y, Friess H, Kobrin MS, Buchler M, Beger HG, Korc M. Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res. 1993;13:565–9. [PubMed] [Google Scholar]
- 5.Sclabas GM, Fujioka S, Schmidt C, Evans DB, Chiao PJ. NF-κB in pancreatic cancer. Int J Gastrointest Cancer. 2003;33:15–26. doi: 10.1385/IJGC:33:1:15. [DOI] [PubMed] [Google Scholar]
- 6.Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA. MicroRNAs—the micro steering wheel of tumour metastases. Nat Rev Cancer. 2009;9:293–302. doi: 10.1038/nrc2619. [DOI] [PubMed] [Google Scholar]
- 7.Saxena S, Jonsson ZO, Dutta A. Small RNAs with imperfect match to endogenous mRNA repress translation. Implications for off-target activity of small inhibitory RNA in mammalian cells. J Biol Chem. 2003;278:44312–9. doi: 10.1074/jbc.M307089200. [DOI] [PubMed] [Google Scholar]
- 8.Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–6. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 9.Gironella M, Seux M, Xie MJ, et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci U S A. 2007;104:16170–5. doi: 10.1073/pnas.0703942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–70. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 11.Takamizawa J, Konishi H, Yanagisawa K, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–6. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 12.Lin SL, Chiang A, Chang D, Ying SY. Loss of mir-146a function in hormone-refractory prostate cancer. RNA. 2008;14:417–24. doi: 10.1261/rna.874808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of microRNA-146 suppresses NF-κB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27:5643–7. doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurst DR, Edmonds MD, Scott GK, Benz CC, Vaidya KS, Welch DR. Breast cancer metastasis suppressor 1 up-regulates miR-146, which suppresses breast cancer metastasis. Cancer Res. 2009;69:1279–83. doi: 10.1158/0008-5472.CAN-08-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths-Jones S, Saini HK, van DS, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–58. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali S, Banerjee S, Ahmad A, El-Rayes BF, Philip PA, Sarkar FH. Apoptosis-inducing effect of erlotinib is potentiated by 3,3′-diindolylmethane in vitro and in vivo using an orthotopic model of pancreatic cancer. Mol Cancer Ther. 2008;7:1708–19. doi: 10.1158/1535-7163.MCT-08-0354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Inactivation of nuclear factor κB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65:6934–42. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa T, Duguid WP, Rosenberg L, Viallet J, Galloway DA, Tsao MS. Long-term culture and immortalization of epithelial cells from normal adult human pancreatic ducts transfected by the E6E7 gene of human papilloma virus 16. Am J Pathol. 1996;148:1763–70. [PMC free article] [PubMed] [Google Scholar]
- 19.Anderton MJ, Manson MM, Verschoyle R, et al. Physiological modeling of formulated and crystalline 3,3′-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab Dispos. 2004;32:632–8. doi: 10.1124/dmd.32.6.632. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, VandenBoom TG, Kong D, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–12. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agazie YM, Hayman MJ. Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol Cell Biol. 2003;23:7875–86. doi: 10.1128/MCB.23.21.7875-7886.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–6. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He H, Jazdzewski K, Li W, et al. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci U S A. 2005;102:19075–80. doi: 10.1073/pnas.0509603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Tang S, Le SY, et al. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS One. 2008;3:e2557. doi: 10.1371/journal.pone.0002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edmonds MD, Hurst DR, Vaidya KS, Stafford LJ, Chen D, Welch DR. Breast cancer metastasis suppressor 1 coordinately regulates metastasis-associated microRNA expression. Int J Cancer. 2009;125:1778–85. doi: 10.1002/ijc.24616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toh Y, Nicolson GL. The role of the MTA family and their encoded proteins in human cancers: molecular functions and clinical implications. Clin Exp Metastasis. 2009;26:215–27. doi: 10.1007/s10585-008-9233-8. [DOI] [PubMed] [Google Scholar]
- 27.Luo J, Su F, Chen D, Shiloh A, Gu W. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature. 2000;408:377–81. doi: 10.1038/35042612. [DOI] [PubMed] [Google Scholar]
- 28.Ji Y, Zhang P, Lu Y, Ma D. Expression of MTA2 gene in ovarian epithelial cancer and its clinical implication. J Huazhong Univ Sci Technolog Med Sci. 2006;26:359–62. doi: 10.1007/BF02829576. [DOI] [PubMed] [Google Scholar]
- 29.Huang L, Verstrepen L, Heyninck K, et al. ABINs inhibit EGF receptor-mediated NF-κB activation and growth of EGF receptor-overexpressing tumour cells. Oncogene. 2008;27:6131–40. doi: 10.1038/onc.2008.208. [DOI] [PubMed] [Google Scholar]
- 30.Park BK, Zhang H, Zeng Q, et al. NF-κB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med. 2007;13:62–9. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- 31.Huber MA, Azoitei N, Baumann B, et al. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–81. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu G, Park YJ, Abraham E. Interleukin-1 receptor-associated kinase (IRAK)-1-mediated NF-κB activation requires cytosolic and nuclear activity. FASEB J. 2008;22:2285–96. doi: 10.1096/fj.07-101816. [DOI] [PubMed] [Google Scholar]
- 33.Luftig M, Prinarakis E, Yasui T, et al. Epstein-Barr virus latent membrane protein 1 activation of NF-κB through IRAK1 and TRAF6. Proc Natl Acad Sci U S A. 2003;100:15595–600. doi: 10.1073/pnas.2136756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le PC, Koumakpayi IH, Lessard L, Saad F, Mes-Masson AM. Independent role of phosphoinositol-3-kinase (PI3K) and casein kinase II (CK-2) in EGFR and Her-2-mediated constitutive NF-κB activation in prostate cancer cells. Prostate. 2005;65:306–15. doi: 10.1002/pros.20291. [DOI] [PubMed] [Google Scholar]
- 35.Pianetti S, Arsura M, Romieu-Mourez R, Coffey RJ, Sonenshein GE. Her-2/neu overexpression induces NF-κB via a PI3-kinase/Akt pathway involving calpain-mediated degradation of IκB-α that can be inhibited by the tumor suppressor PTEN. Oncogene. 2001;20:1287–99. doi: 10.1038/sj.onc.1204257. [DOI] [PubMed] [Google Scholar]
- 36.Lakshman M, Xu L, Ananthanarayanan V, et al. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res. 2008;68:2024–32. doi: 10.1158/0008-5472.CAN-07-1246. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Kucuk O, Hussain M, Abrams J, Cher ML, Sarkar FH. Antitumor and antimetastatic activities of docetaxel are enhanced by genistein through regulation of osteoprotegerin/receptor activator of nuclear factor-κB (RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res. 2006;66:4816–25. doi: 10.1158/0008-5472.CAN-05-3752. [DOI] [PubMed] [Google Scholar]
- 38.Singh AV, Franke AA, Blackburn GL, Zhou JR. Soy phytochemicals prevent orthotopic growth and metastasis of bladder cancer in mice by alterations of cancer cell proliferation and apoptosis and tumor angiogenesis. Cancer Res. 2006;66:1851–8. doi: 10.1158/0008-5472.CAN-05-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vantyghem SA, Wilson SM, Postenka CO, Al-Katib W, Tuck AB, Chambers AF. Dietary genistein reduces metastasis in a postsurgical orthotopic breast cancer model. Cancer Res. 2005;65:3396–403. doi: 10.1158/0008-5472.CAN-04-4109. [DOI] [PubMed] [Google Scholar]
- 40.Kong D, Li Y, Wang Z, Banerjee S, Sarkar FH. Inhibition of angiogenesis and invasion by 3,3′-diindolylmethane is mediated by the nuclear factor-κB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res. 2007;67:3310–9. doi: 10.1158/0008-5472.CAN-06-4277. [DOI] [PubMed] [Google Scholar]
- 41.Hsu EL, Chen N, Westbrook A, et al. Modulation of CXCR4, CXCL12, and tumor cell invasion potential in vitro by phytochemicals. J Oncol. 2009;2009:491985. doi: 10.1155/2009/491985. [DOI] [PMC free article] [PubMed] [Google Scholar]