Abstract
Glycosaminoglycans (GAG) are known to participate in central nervous system processes such as development, cell migration, and neurite outgrowth. In this paper, we report an initial glycomics study on GAGs from porcine central nervous system. GAGs of the porcine central nervous system, brain and spinal cord, were isolated and purified by defating, proteolysis, anion-exchange chromatography and methanol precipitation. The isolated GAG content in brain was 5-times higher than in spinal cord (0.35 mg/g, compared to 0.07 mg/g dry sample). In both tissues, chondroitin sulfate (CS) and heparan sulfate (HS) were the major and the minor GAG. The average molecular weight of CS from brain and spinal cord was 35.5 and 47.1 kDa, respectively, and HS from brain and spinal cord was 56.9 and 34 kDa, respectively. The disaccharide analysis showed that the composition of CS from brain and spinal cords are similar with uronic acid (1→3) 4-O-sulfo-N-acetylgalactosamine residue corresponding to the major disaccharide unit (CS type-A) along with five minor disaccharide units. The major disaccharides of both brain and spinal cord HS were uronic acid (1→4) N-acetylglucosamine and uronic acid (1→4) 6-O-sulfo-N-sulfoglucosamine but their composition of minor disaccharides differed. Analysis by 1H- and two-dimensional-NMR spectroscopy confirmed these disaccharide analyses and provided the glucuronic/iduronic acid ratio. Finally, both purified CS and HS were biotinylated and immobilized on BIAcore SA biochips. Interactions between these GAGs and fibroblast growth factors (FGF1 and FGF2) and sonic hedgehog (Shh) were investigated by surface plasmon resonance.
Brain and spinal cord are the two main componenets of the central nervous system (CNS)1. The extracellular matrix of the CNS serves as both a supporting structure for cells and a rich source of signaling molecules that can influence cell proliferation, survival, migration and differentiation (1)
Chondroitin sulfate proteoglycans (CSPGs), known to be diffusely present in the CNS matrix and the condensed matrix of perineuronal nets (PNNs) (2), are involved in the regulation of neuronal plasticity (3,4), in neuroprotection (5,6), and in support of ion homeostasis around highly active neurons (7–9). The polysaccharide component of CSPGs, the CS-GAGs are key to the binding and biological activities of CSPGs, as the result of the positioning of sulfo groups by specific saccharide sequences. There is increasing evidence that CS is a uniquely important GAG in morphogenesis, cell division and cartilage development. CSPGs are spatiotemporally regulated during brain development and upregulated after injury in the CNS. CS is a sulfated GAG composed of repeating disaccharide backbone of →4) β-D-glucuronic acid (GlcA) (1→3) β-D-N-acetylgalactosamine (GalNAc) (1→ potentially containing some L-iduronic acid (IdoA) residues and O-sufo group substitution. GlcA containing CS can belong to the class CS-A (chondroitin 4-sulfate), CS-C (chondroitin 6-sulfate), CS-D (chondroitin 2,4-disulfate) and CS-E (chondroitin 4,6-disulfate). CS-B (dermatan sulfate) is comprised of a 4-O-sulfo-GalNAc 1→4 linked to IdoA. Nonsulfated (chondroitin) disaccharide can also be found in the CS structure. Studies relying on synthetic approaches using carbohydrate microarrays have demonstrated that CS-E is the principal motif involved in CS interaction with midkine a heparin-binding growth factor involved in neural development (10). Chondroitin-4-sulfate (CS-A) also has been shown to negatively regulate axonal guidance and growth (11–15).
HS, consisting of →4) β-D-GlcA (or IdoA) (1→4) β-D-N-acetylglucosamine (GlcNAc) (1→ with various N-sulfo and O-sufo subtitution (16). HS-PGs are ubiquitous in all animal tissues and are also found in brain and nervous tissue. HS-PG glypican-2 (cerebroglycan), for example, is uniquely important in the developing nervous system and is expressed predominantly during neuronal differentiation (17). HS can mediate repulsion and collapse of olfactory axons (18) and is essential for the binding of various growth factors (19) and Semaphorin 5A (20).
Glycomics research is currently undergoing rapid development as a result of recent advances in technologies for glycan structural analysis that is beginning to unravel the structure-activity relationships of glycan-protein interactions (21). In this paper, we report an initial glycomics study on GAGs from porcine CNS. CS and HS were isolated from porcine brain and spinal cords, purified, quantified and their average molecular weight (MWavg) determined. Disaccharide composition, determined using liquid chromotography and mass spectrometry (LC-MS), and structural analysis relying on 1H and two-dimensional NMR spectroscopy. These purified CS and HS GAGs were biotinylated, immobilized on BIAcore SA biochips, and their interactions with fibroblast growth factors (FGF1 and FGF2) and sonic hedgehog (Shh) were investigated using surface plasmon resonance (SPR).
EXPERIMENTAL PROCEDURES
Materials
Adult healthy porcine brains (two brains) and spinal cords (four cords) were purchased from Pel-freez Biological In. (Rogers, AR). Actinase E was from Kaken Biochemicals (Tokyo, Japan). CS-A (bovine tracheal cartilage) and CS-E (squid cartilage), chondroitin lyases (ABC and ACII) were from Seikagaku (Tokyo, Japan). HS (porcine intestine) was from Celsus Laboratories (Cincinnati, OH). Flavobacterial heparin lyases I, II, III were expressed in E. coli and purified in our laboratory. Polyacrylamide, urea, CHAPS, Alcian blue dye, 2-cyanoacetamide, tetra-n-butylamonium hydrogen sulfate, were from Sigma (St. Louis, MO).
Unsaturated CS disaccharides standards (Di-0S, ΔUA-GalNAc (where ΔUA is Δ-deoxy-L-threo-hex-4-enopyranosyl uronic acid); Di-4S, ΔUA-GalNAc4S; Di-6S, ΔUA-GalNAc6S; Di-UA2S, ΔUA2S-GalNAc; Di-diSB, ΔUA2S-GalNAc4S; Di-diSD ΔUA 2S-GalNAc6S; Di-diSE, ΔUA-GalNAc4S6S; Di-triS, ΔUA2S-GalNAc4S6S); and unsaturated heparin/HS disaccharides standards (Di-0S, ΔUA-GlcNAc; Di-NS, ΔUA-GlcNS; Di-6S, ΔUA-GlcNAc6S; Di-UA2S, ΔUA2S-GlcNAc; Di-UA2SNS, ΔUA2S-GlcNS; Di-NS6S, ΔUA-GlcNS6S; Di-UA2S6S, ΔUA2S-GlcNAc6S; and Di-triS, ΔUA2S-GlcNS6S) were obtained from Seikagaku Corporation (Japan).
Fibroblast growth factor 1 (FGF1) and fibroblast growth factor 2 (FGF2) were gifts from Amgen (Thousands Oaks, CA). Sonic hedgehog (Shh) was generous provided by Dr. Dan Leahy from Johns Hopkins University.
Methods
Isolation and purification of GAGs
Porcine brain and spinal cord samples were crushed with dry ice into very fine homogenized powder using a blender (from Fisher Scientific). Fat was removed by washing the tissues with chloroform/methanol mixture of (2:1, 1:1, 1:2 (v/v)) each left overnight. Defatted samples were proteolyzed at 55° C with 10 % of actinase E (20 mg/ml) for 18 h. After the proteolysis, dry urea and dry CHAPS were added to each sample (2 wt % in CHAPS and 8 M in urea). The resulting cloudy solutions were clarified by passing through a syringe filter containing a 0.2 μm membrane from Millipore (Billerica, MA). A Vivapure MAXI Q H spin column was equilibrated with 3 ml of 8 M urea containing 2% CHAPS (pH 8.3). The clarified filtered samples were loaded onto and run through the Vivapure MAXI Q H spin columns (Sartoriou Stedim Biotech, Bohemia, NY) under centrifugal force (500 × g). The columns were first washed with 3 ml of 8 M urea containing 2% CHAPS at pH 8.3. The columns were then washed five times with 5 ml of 200 mM NaCl. GAG was recovered from the spin column by washing 3-times with 1 ml of 16% NaCl and these washes were collected and combined and methanol (12 ml) was added to afford an 80 vol% methanol solution that was equilibrated at 4°C for 18 h resulting in a precipitate that was recovered by 30 min centrifugation (2500 × g). The precipitate was dissolved in 0.5 ml of water and the recovered total GAGs were stored frozen for further analysis.
Isolation of CS and HS from total GAG mixture
GAG samples were digested with a mixture of heparin lyases I, II and III (10 m-units each) at 35 °C for 38 h to isolate CS. CS was purified by Vivapure MINI Q H spin column and centrifugal filtration using 3000 molecular weight cut-off (MWCO) membrane (Millipore, Bedford, MA). HS was recovered by digestion total GAGs with chondroitinase ABC and chondroitinase ACII (5 m-units each) at 37 °C for 38 h. The HS products were recovered with the same process as CS. HS was purified by Vivapure MINI Q H spin column and centrifugal filtration using 3000 MWCO membrane. The total GAGs, CS, and HS were quantified by carbazole assay (22) using CS-A as standard.
Polyacrylamide gel electrophoresis analysis
Polyacrylamide gel electrophoresis (PAGE) was applied to determine the MWavg and polydispersity of each GAG sample. To each lane ~5 μg of total GAG, CS or HS was subjected to electrophoresis against a standard composed of a mixture of oligosaccharides of known molecular weight that had been prepared enzymatically from bovine lung heparin. The gel was visualized with alcian blue staining and then digitized with UN-Scan-it software (Silk Scientific, Utah) and MWavg and polydispersity was calculated (23).
Disaccharide analysis using LC-MS
GAG substrate (20 μg/μl) was treated with 5 μl of a mixture of 0.1 U each of chondroitin lyase ABC and chondroitin lyase ACII dissolved in 500 μl of 0.1% BSA and incubated at 37°C overnight. The products were filtered using 3000 MWCO centrifugal filters and the CS disaccharides were recovered in the filtrate. The disaccharides were freeze-dried and exactly 100 μl H2O was added prior to their analysis. Next, heparin lyase I, II, III (3 m-unit each in 10 μl of sodium phosphate (5 mM, pH 7.1) buffer) were added to the retentate and incubated at 37°C overnight. The products were again filtered using 3000 MWCO centrifugal filters and the HS disaccharides were recovered in the filtrate. The disaccharides were freeze-dried and exactly 100 μl H2O was added prior to their analysis.
Disaccharide analysis was performed on a LC-MS system (Agilent LC/MSD trap MS). Solution A and B for the HPLC separation contained 37.5 mM NH4HCO3 and 11.25 mM tributylamine in 15% and 70% acetonitrile, respectively. The pH values of these solutions were adjusted to 6.5 with acetic acid. Separation was performed on a C-18 column (2.1 × 150 mm, Waters, Milford, MA) at a flow rate was 10 μl/min using solution A for 20 min, followed by a linear gradient from 20 to 45 min of 0% to 50% solution B. The column effluent entered the source of the ESI-MS for continuous detection by MS. The electrospray interface was set in the negative ionization mode with the skimmer potential of −40.0 V, capillary exit at −40.0 V, and a source of temperature at 325 °C to obtain the maximum abundance of the ions in full scan spectra (150–1500 Da, 10 full scans/s). Nitrogen was used as a drying (5 liters/min) and nebulizing gas (20 p.s.i.). The CS disaccharide separation was performed on an Acquity UPLC BEH C18 column (2.1 ×150 mm, 1.7 μm, Waters, Milford, MA, USA) using solution A for 10 min, followed by a linear gradient from 10 to 40 min of 0 to 50% solution B. The column temperature was maintained at 45 °C. The flow rate was 100 μl/min. Solutions A and B for UPLC were 0 and 75% acetonitrile, respectively, containing the same concentration of 15 mM hexylamine (HXA) as an ion-pairing reagent and 100 mM 1,1,1,3,3,3 hexafluoroisopropanol (HFIP) as an organic modifier. We extracted the ions based on their theoretical mass unit, ionization mode, and addition of ion-pairing reagent.
NMR analysis
Total GAG, CS, and HS isolated from porcine brain and porcine spinal cord were analyzed by 1H-NMR and two-dimensional NMR (HSQC and HHCOSY) spectroscopy to characterize their structures. All NMR experiments were performed on Bruker Advance II 600 MHz spectrometer with Topsin 2.0 software. Commercial HS and CS-A and samples were each dissolved in 0.5 ml D2O (99.996%, Sigma, Co.) and freeze-dried repeatedly to remove the exchangeable protons. The samples were re-dissolved in 0.3 ml D2O and transferred to NMR microtubes (OD 5 mm, Shigemi). The conditions for one-dimensional 1H spectra were as follows: wobble sweep width of 12.3 kHz, acquisition time of 2.66 s, and relaxation delay of 8.00 s. Temperature was 298 K and 323 K. The conditions for two-dimensional HMQC spectra were as follows: 32 scans, sweep width of 6.15 kHz, acquisition time of 0.33 s, and relaxation delay of 0.90 s. The conditions for two-dimensional COSY spectra were as follows: 16 scans, sweep width of 7.40 kHz, acquisition time of 0.28 s, and relaxation delay of 1.50 s.
Biotinylation of GAG
CS and HS (300 μg) were incubated in 500 mM NaOH, solution at 4°C for 16 h and then neutralized by gradual addition of glacial acetic acid to cleave xylose-serine linkage. The products were desalted using YM-3 spin column. The resulting CS and HS GAG chains (200 μg) and amine-PEO3-biotin (200 μg) were dissolved in 100 μl H2O, then 10 μg NaCNBH3 was added. The reaction mixture was heated at 70°C for 24 h, and a further 10 mg NaCNBH3 was added and the reaction was heated at 70°C for another 24 h. After cooling to room temperature, the mixture was desalted with a spin column (3000 MWCO). Biotinylated GAGs were collected, freeze-dried and used for SA chip preparation.
Preparation of SPR biochip
SPR was performed on a BIAcore3000 (GE Healthcare, Uppsala, Sweden). Buffers were filtered (0.22 μM) and degassed. The biotinylated GAGs were immobilized to flow cells in a streptavidin chip. A flow cell was treated with biotin alone and served as a control. The successful immobilization of GAG was confirmed by the observation of a ~300 resonance unit (RU) increase in the sensor chip. Two chips were prepared: HS chip immobilized with HS from brain, HS from spinal cord and commercial HS as a positive control; CS chip immobilized with CS from brain, CS from spinal cord and commercial CS-E as a positive control.
Kinetic measurements of protein-GAGs interaction using SPR
The protein sample (FGF1, FGF2, and Shh) was diluted in HBS-EP buffer (0.01 M Hepes at pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.005% surfactant P20) (GE Healthcare, Uppsala, Sweden). Different dilutions of protein samples in buffer were injected at a flow rate of 30 μL/min. At the end of the sample injection (180 sec), the same running buffer was passed over the sensor surface to facilitate dissociation for 180 sec. After dissociation, the sensor surface was regenerated by injecting 2 M NaCl. The response was monitored as a function of time (sensorgram) at 25 °C. SPR experiments were run in duplicated or triplicate at each concentration to confirm the bindings were repeatable. Multi-concentration data were globally fit and residuals were calculated and used to assess the goodness of fit.
RESULTS
GAGs purification and quantification
A simple four-step procedure involving defatting, protease digestion, strong-anion-exchange chromatography on a spin column and methanol precipitation was used to isolate GAGs from the porcine brain and spinal cord. This method had been previously established in our laboratory for the quantitative isolation of heparin from human plasma (24) (Table 1). After freeze-drying and weighing the samples were defatted. As expected, both spinal cord and brain showed a high fat content of 85 % and 69 % (g fat/g dry wt) × 100), respectively. The total GAG isolated from each sample was next determined using carbazole assay for uronic acid. The GAG content of the dry, defatted sample from brain (0.35 mg/g) was 5-times higher than the GAG content of spinal cord (0.07 mg/g). CS and HS were each purified from the total GAG by selective polysaccharide digestion using heparin and chondritin lyases, respectively. The CS/HS ratio in porcine spinal cord was 5.9 and in porcine brain was 3.4.
Table 1.
Quantification and average MW characterization of isolated GAGs from porcine brain and spinal cords.
| Porcine brain | Porcine spinal cords | |
|---|---|---|
| Wet weight (g) | 75.60 | 313.51 |
| Dry weight (g) | 15.15 | 87.54 |
| Defatted weight (g) | 4.74 | 12.79 |
| Total GAGs isolated (mg) | 5.3 | 6.0 |
| GAGs (mg)/g dry tissue | 0.35 | 0.069 |
| CS/HS ratio | 3.4 | 5.9 |
| Average CS MWavg (kDa) | 35.5 | 47.1 |
| Average HS MWavg (kDa) | 56.9 | 34 |
Polyacrylamide gel electrophoresis (PAGE) analysis (see Supporting Information Fig. S1 for gel pictures)
Total GAG, CS and HS, isolated from the brain and spinal cord were analyzed by using PAGE with alcian blue staining. PAGE analysis established that CS and HS were both present in spinal cord and brain and showed a broad band of expected polydispersity and the MWavg (23). The MWavg of CS was 35.5 kDa of brain and 47.1 kDa of spinal cord and the MWavg of HS was 56.9 kDa of brain and 34 kDa of spinal cord.
GAG disaccharide compositional analysis
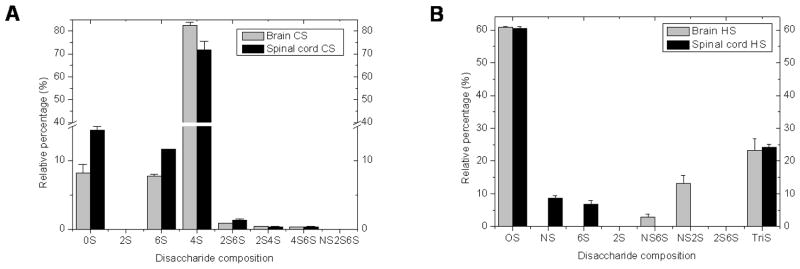
Compositional analysis of disaccharides gives important structural information and is an efficient method to measure the variation of GAG structures. HS can contain different disaccharide sequences including those corresponding to the eight heparin/HS disaccharide standards (see Materials section for details). Similarly, CS/DS GAGs also can contain different disaccharide sequences including those corresponding to the eight CS/DS disaccharide standards. An LC/MS analysis method that relies on ion-pairing reversed-phase capillary HPLC was used to determine the GAG disaccharide composition (25, 26). This method affords good resolution in the separation of eight heparin/HS or eight CS/DS disaccharides (see Supporting Information Fig. S2–S5 for MS spectra). CS and HS from porcine brain and spinal cords were first digested with chondroitin lyase ABC and chondroitin lyase AC II or heparin lyases I, II and III, followed by disaccharide composition analysis using LC-ESI-MS. All peaks are conclusively identified by retention time and through their mass spectra (see Supporting Information Fig. S2–S5 for MS spectra). The results (Fig. 2A) showed that the major disaccharide of CS was ΔUA-GalNAc4S (over 83% and 73% in brain and in spinal cord, respectively). In addition, ΔUA-GalNAc, ΔUA-GalNAc6S, small amounts of ΔUA2S-GalNAc6S, ΔUA2S-GalNAc4S, and ΔUA4S-GalNAc6S were also observed in CS from both tissues. The major disaccharide of HS (Fig. 2B) from both brain and spinal cord were ΔUA-GlcNAc (~ 60%) and ΔUA2S-GlcNS6S (~ 25%). There were some differences in the minor HS disaccharide composition of porcine brain and porcine spinal cord. The ΔUA-GlcNS6S and ΔUA2S-GlcNS were found in brain HS, while ΔUA-GlcNS and ΔUA-GlcNAc6S were found in spinal cord HS.
Fig. 2.
Disaccharide compositional analysis of HS and CS from different tissues by LC-ESI-MS. A. Disaccharide composition of CS from porcine brain and spinal cord; B. Disaccharide composition of HS from porcine brain and spinal cord. Data represent the average values with SE bar of triplicate experiments.
NMR spectra
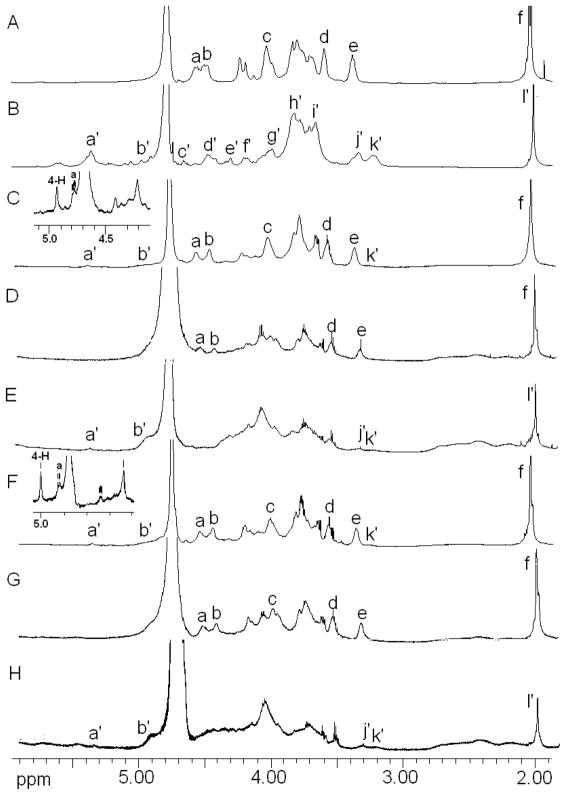
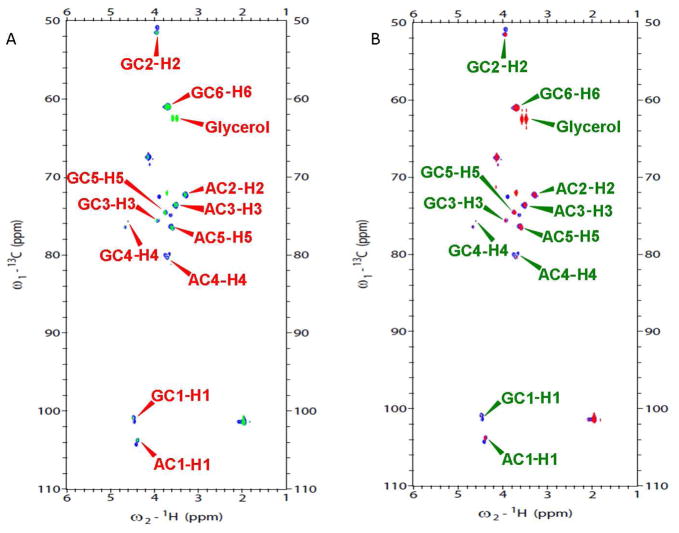
Since there are no detailed reports on the structural characterization of the GAGs from porcine brain and spinal cord, one-dimensional and two-dimensional NMR analysis of the GAGs were performed. 1H NMR spectra and 2D NMR (HMQC, COSY) of total GAGs from porcine brain and spinal cord along with 1H NMR spectra of CS/HS were obtained (Table 2 and Figs. 3 and 4). The majority of the signals of present in the 1H NMR spectra of the total GAGs isolated from both the brain and spinal cord were present in the 1H NMR of a standard commercial CS-A obtained from bovine tracheal cartilage. The prominant signals include the anomeric protons of GalNAc at 4.478 ppm and of GlcA at 4.393 ppm, H-2 and H-3 of GalNAc at 3.9 ppm, H-3 of GlcA at 3.498 ppm, H-2 of GlcA at 3.285 ppm and CH3 of acetyl group of GalNAc at 2.02 ppm. In addition, the 1H NMR spectra of total GAGs showed weak signals at 3.2 ppm and the signals after 5.0 ppm, which are not found in the 1H NMR spectra of CS-A standard. 1H NMR of standard commercial HS shows prominant signals at 3.19 ppm and 5.00 ppm are the signals of H2 of GlcNS6S and H1 of IdoA of HS, respectively. The ratio of GlcA to IdoA in the total GAG samples from brain and spinal cord is > 10 based on the integration of the intense 4.393 ppm peak for GlcA (in both CS and HS) and the 5.00 ppm peak for IdoA. The ratio of the 4.478 ppm signal for GalNAc, the hexoamine unit in CS, and the 5.38 ppm signal GlcNAc, the hexoamine unit in HS, was 9 to 1 corresponding closely to ratio of CS to HS in both tissues that was obtained using carbazole assay (Table 1). The 1H NMR spectra of HS recovered from both the brain and spinal cord was typical of an HS showing a characteristic ratio of D-GlcA to L-IdoA ratio of > 2.0 (16). The 1H NMR spectra of CS recovered from both the brain and spinal cord contained only GlcA and were typical of a CS. 1H NMR spectra of total GAG from the two tissues were performed at elevated temperature (323 K) to confirm that chondroitin-4-sulfate (CS-A) was the main GAG component in porcine brain and porcine spinal cord (Fig. 3 insert). At 323 K, all the signals of GAGs shift downfield by ~0.3 ppm. The strong peak at 5.00 ppm was identified as H-4 of GalNAc4S, this peak overlapped with the HOD signal in the 1H NMR spectra at 298 K. The HMQC spectra of total acidic GAGs from brain and spinal cord were also overlaid onto the HMQC spectrum of CS-A (Fig. 4). The correlation signals of the CS-A covered all the major peaks present in the HMQC spectra of GAGs from both brain and spinal cord.
Table 2.
Proton and carbon chemical shifts of major GlcUA and GalNAc residues present in chondroitin sulfate (CS-A)
| Proton or carbon of CS-A | Chemical shift (δ, ppm) |
|
|---|---|---|
| GlcA | GalNAc | |
| H-1 | 4.393 | 4.478 |
| H-2 | 3.285 | 3.946 |
| H-3 | 3.498 | 3.924 |
| H-4 | 3.693 | 4.670 |
| H-5 | 3.627 | 3.754 |
| H-6a | - | 3.711 |
| H-6b | - | 3.711 |
| CH3 | - | 2.02 |
| C-1 | 104.30 | 101.10 |
| C-2 | 72.30 | 51.33 |
| C-3 | 73.83 | 75.79 |
| C-4 | 80.14 | 75.55 |
| C-5 | 76.34 | 74.51 |
| C-6 | - | 61.05 |
| CH3 | - | 22.14 |
Fig. 3.
600 MHz 1H-NMR spectra of standard CS-A from bovine tracheal cartilage (A), standard heparan sulfate obtained from porcine intestine (B), total GAG from brain (C), CS from brain (D), HS from brain (E), total GAG from spinal cord (F), CS from spinal cord (G), and HS from spinal cord (H) recorded in D2O at 298 K. Signals, a, H-1 of GalNAc; b, H-1 of GlcA; c, H-2 and H-3 of GalNAc; d, H-3 of GlcA; e, H-2 of GlcA; f, CH3 of acetyl group of GalNAc; a′, H-1 GlcNAc or IdoA2S; b′, H-1 IdoA; c′, H-5 IdoA2S; d′, H-1 GlcA; e′, H-2 IdoA2S; f′, H-3 IdoA2S and H-6 GlcNS6S or GlcNAc6S; g′, and h′, H-2 and H-3 GlcNAc, H-6 and H-5 GlcNS or GlcNAc; i′, H-3 and H-4 GlcNS, GlcNAc, GlcNS6S or GlcNAc6S; j′, H-2 GlcA; k′, H-2 GlcNS or GlcNS6S; l ′ CH3 of acetyl group of GlcNAc.
Fig. 4.
600 MHz 1H-13C HMQC spectrum for total GAG from brain (green) overlaid onto CS-A (blue) (A), and total GAG from spinal cord (red) overlaid onto CS-A (blue) (B) at 298 K. A, GlcA; G, GalNAc.
SPR measurements of the interaction of proteins with CS/HS from porcine brain and spinal cord
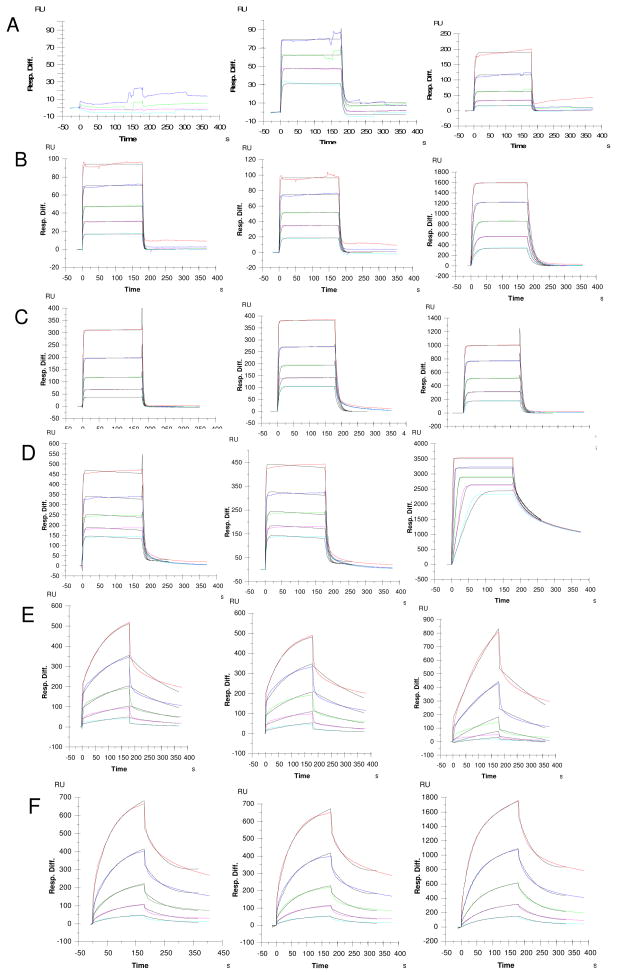
The interactions between the GAGs and proteins from fibroblast growth factor signaling system (FGF1 and FGF2) and hedgehog signaling pathway (Shh) were investigated using surface plasmon resonance (SPR) to determine the bioactivities of GAGs from two tissues of porcine CNS. The results (Table 3, and Figure 5) showed that brain CS had negligible (> 1 μM KD) binding for all proteins except for Shh. Spinal cord HS bound tighter than brain HS to all proteins tested. Brain and spinal cord CS bound Shh (< 1 μM KD) even while the more highly charged CS-E failed to interact. Shh showed very slow off-rates compared to those determined for FGF1 and FGF2. Spinal cord HS and CS showed very strong binding to FGF1 and FGF2, while brain CS only weakly bound these growth factors.
Table 3.
Summary of kinetic data of protein-GAGs interactions
| Proteins | GAGs | kon(1/MS) | koff(1/S) | KD (μM) |
|---|---|---|---|---|
| FGF1 | Brain CS | N.B | N.B | N.B. |
| Spinal cord CS | 1.3 ×106 | 0.22 | 0.17 | |
| CS-E control | 4.1 ×104 | 0.28 | 6.9 | |
| Brain HS | 4.9 ×105 | 0.42 | 0.88 | |
| Spinal cord HS | 5.0 ×105 | 0.21 | 0.43 | |
| HS control | 2.0 ×105 | 0.073 | 0.37 | |
| FGF2 | Brain CS | 2.0 ×105 | 0.39 | 2.0 |
| Spinal cord CS | 5.1 ×105 | 0.13 | 0.24 | |
| CS-E control | 2.2 ×105 | 0.16 | 0.70 | |
| Brain HS | 3.8 ×105 | 0.23 | 0.62 | |
| Spinal cord HS | 5.2 ×105 | 0.17 | 0.32 | |
| HS control | 9.0×106 | 0.12 | 0.013 | |
| Shh | Brain CS | 7.2 ×103 | 4.8×10−3 | 0.67 |
| Spinal cord CS | 1.1 ×104 | 4.6×10−3 | 0.43 | |
| CS-E control | 851 | 2.8×10−3 | 3.3 | |
| Brain HS | 1.1 ×104 | 0.012 | 1.1 | |
| Spinal cord HS | 1.5×104 | 0.014 | 0.95 | |
| HS control | 1.8 ×104 | 0.015 | 0.84 | |
Fig. 5.
Sensorgrams of GAGs-protein interactions. Concentrations of proteins (from top to bottom): 1000, 500, 250, 125, and 63 nM, respectively. The black curves in all sensorgrams are the fitting curves using models from BIAevaluate 4.0.1. A. SPR sensorgrams of CS-FGF1 interaction. left: Brain CS, middle: spinal cord CS, right: CS-E control; B. SPR sensorgrams of HS-FGF1 interaction. left: Brain HS, middle: spinal cord HS, right: HS control; C. SPR sensorgrams of CS-FGF2 interaction. left: Brain CS, middle: spinal cord CS, right: CSE control; D. SPR sensorgrams of HS-FGF2 interaction. left: Brain HS, middle: spinal cord HS, right: HS control; E. SPR sensorgrams of CS-Shh interaction. left: Brain CS, middle: spinal cord CS, right: CS-E control; F. SPR sensorgrams of HS-Shh interaction. left: Brain HS, middle: spinal cord HS, right: HS control.
DISCUSSION
Over the past decades of genomics and proteomics have led to high throughput measurements of expression of several thousand genes and the of hundreds of protein-protein interactions required for understanding comprehensive biochemical pathways and interaction networks within cells and organisms. Applying the same “omics” concept in glycobiology, or glycomics, requires a multi-dimensional approach involving isolation, structural characterization and functional studies of glycans that eventually can lead to important structure-function relationships (27). Glycomics necessarily relies on a diverse range of analytical technologies including MS, NMR, HPLC, CE, SPR, arrays, natural and synthetic glycan libraries, microfliudic system, bioinformatics and molecular modeling of glycans. The most extensively studied complex glycans are the GAGs, which involved in many critical biological processes including development, angiogenesis, anticoagulation, axonal growth, cancer, microbial/viral pathogenesis.
As part of our interest in the treatment of spinal cord injury with chondroitin lyases (28), a class of enzymes extensively studied in our laboratory (29) we became interested in the GAGs present in the CNS. Despite research activity in this area, it was surprising to discover how little was known about the GAG content, structure and protein binding affinities of CNS PGs in adult mammals. Approximately 20% of the volume of the adult CNS is occupied by extracellular matrix (ECM), which is composed primarily of PGs (30). The major proteoglycans found in the CNS are members of the lectican family, which have a protein core whose N-terminus binds to hyaluronan (HA) and a middle portion that contains attachment sites for CS GAG chains. It is known that CSPGs are widely expressed in the developing and adult central nervous system (CNS). They have been implicated in regulating cell proliferation, survival, migration and differentiation. Furthermore, CSPGs are the principal inhibitory component of glial scars, which form after damage to the adult central nervous system and act as a barrier to regenerating axons (31). CSPG-mediated inhibition of growth seems to be linked to the activation of several signaling pathways. Thus, the importance of these critical functions of the CSPGs in CNS suggested that we undertake a glycomics study of porcine CNS for which brain and spinal cord tissues are readily available as a model the human CNS.
The current study reveals that the brain has a 5-fold higher GAG content than the spinal cord. CS is the prominent GAG in CNS PGs (both brain and spinal cord), corresponding to 77–86 %, with HS being a minor component (14–23 %) in total GAG (Table 1). While brain CS had a 30% lower MWavg than spinal cord CS, both showed comparable disaccharide analyses (Table 1 and Figure 2). CNS CS is primarily (>70%) chondrotin 4-sulfate (CS-A) sequences, followed by nearly equal (~10–15% each) of chondroitin and chondrotin 6-sulfate CS-C sequences, and minor amounts (<1% each) of disulfated sequences (chondroitin 2,6-sulfate (CS-D), chondroitin 4,6-sulfate (CS-E) and chondroitin 2,4-sulfate. NMR analysis fails to detect the presence of significant amounts of IdoA, suggesting little if any DS is present in the porcine CNS. The placement of these disaccharides into larger sequence motifs, however, appears to be different resulting in selective binding to critical in CNS growth and development. Brain CS shows no measurable binding to FGF1 and weak binding to FGF2 while spinal cord CS binds tightly to both growth factors. Interestingly, spinal cord CS binds these FGFs with greater affinity than the more highly sulfated CS-E. Thus, it is clear that while similar in disaccharide composition, brain and spinal cord CS exhibit different protein binding affinities suggesting that the sequence or arrangement of these disaccharides is critical to their biological function.
We next turned our attention to the HS present in the CNS. Brain HS was ~30% larger than spinal cord HS. While both contained nearly identical amounts of two major disaccharides, the expected unsulfated disaccharide (~60%) and the unusual trisulfated disaccharide (~24%), possibly corresponding to low and high sulfate domains associated with HS-based signaling (32). In contrast the content of monosulfated and disulfated disaccharides in the brain and spinal cord HS were very different with the brain HS being richer in disulfated disaccharides and the spinal cord HS being richer in monosulfated disaccharides. A comparison of our results, on the HS disaccharide composition of mammalian brain, with those from other investigators, demonstrates a relative abundance of the TriS disaccharide (20–25% in porcine brain). This value is considerably higher than the results obtained on bovine brain in our laboratory (33) and on rat brain by others (34, 35). In these prior publications, the percent TriS was <5%. This unexpectedly high abundance of TriS in the HS from porcine brain and spinal cord tissues might result from the higher recovery of the highly sulfated HS chains compared to the under sulfated HS chains causing an enrichment of HS chains containing TriS during the extraction process. It is noteworthy that the structural differences have been reported for GAGs isolated from different species, different organ systems within given single species, and even even within the same organ system of a single species at different stages in development.
Differences in either composition or sequences result in a consistent higher binding affinity of spinal cord HS for FGF1, FGF2 and Shh.
Extensive studies on the FGF family show their multiple critical roles in the formation of the CNS from the early stage of neural induction through to the late stage of terminal differentiation (36) FGF-1 and FGF-2 have been found to be potent modulators of proliferation in the developing nervous system (37). Recently, it was reported that FGF-1 was a potent neurotrophic factor that affects neuronal survival in the injured spinal cord (38). FGF signaling begins with the formation of a ternary complex of FGF, FGF receptor (FGFR), and HS. HS serves primarily as a template for the assembly of the FGF2-FGFR2-HS2 signal transduction complex (39). It noteworthy that most HS used to study FGF signaling have been obtained from porcine intestine used in the commercial preparation of heparin, few if any reports have examined the FGF-binding of tissue specific GAGs.
Shh is a member of the hedgehog family of signaling molecules identified by homology to Drosophila hedgehog. Shh was identified as a morphogen that is directly responsible for dorso–ventral patterning of the CNS. Additional multiple actions of Shh during CNS development have been well established, including the specification of oligodendrocytes, proliferation of neural precursors and control of axon growth (40). Recently, Lowry et al reported that the transplantation of endothelial-expanded neural stem cells treated with Shh and retinoic acid, into an adult mouse spinal cord injury model resulted in significant recovery of sensory and motor function (41). In hedgehog signaling pathway, HSPGs are essential for the proper distribution and signaling activity of signaling proteins (42). It is well established that Shh interacts with heparin/HS, and these interactions are important for normal hedgehog signaling (38, 43). Thus, it is interesting that Shh binds both porcine brain and porcine spinal cord CS with higher affinity than HS from the same tissues.
In conclusion, glycosaminoglycans (GAG) were successfully isolated and purified central nervous system (brain and spinal cord). Their molecular structure (i.e. average molecular weight, and disaccharide composition) was characterized by PAGE and LC-MS disaccharide analysis and more detailed structural features were revealed by 1H- and two-dimensional-NMR spectroscopy. Finally, interactions between these GAGs and proteins (including fibroblast growth factors (FGF1 and FGF2) and sonic hedgehogs (Shh)) were investigated by SPR providing important structural/activity information. The methodology described will be useful in tissue specific glycomic research to discover novel glycotherapeutics that target disease related protein-GAGs interactions.
Supplementary Material
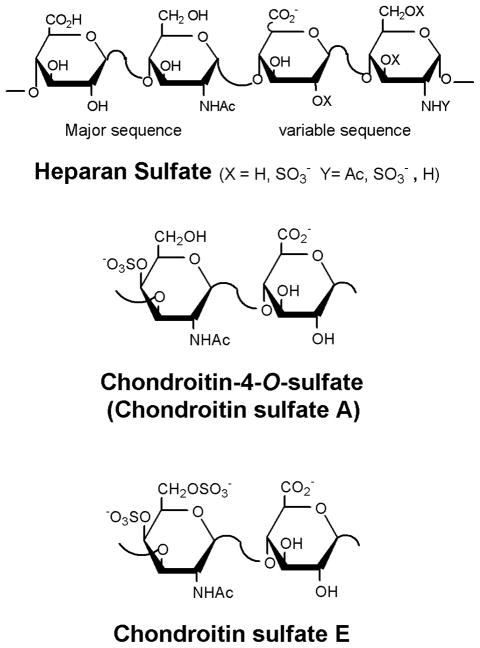
Fig. 1.
Chemical structures of HS, CS-A and CS-E
Abbreviations
- CNS
central nervous system
- CSPGs
chondroitin sulfate proteoglycans
- HS
heparan sulfate
- PGs
proteoglycans
- CS
chondroitin sulfate
- SPR
surface plasmon resonance
- FGF
fibroblast growth factor
- Shh
sonic hedgehog
- GAG
glycosaminoglycan
- ECM
extracellular matrix
- SA
streptavidin
- FC
flow cell
- PAGE
polyacrylamide gel electrophoresis
- MW
molecular weight
- CO
cutoff
- GlcA
glucuronic acid
- GalNAc
N-acetylgalactosamine
- IdoA
iduronic acid
- GlcNAc
N-acetylglucosamine
- LC-MS
liquid chromotography and mass spectrometry
- NMR
nuclear magnetic resonance
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- RU
resonance unit
Footnotes
This work was supported by grants from the funded by the National Institutes of Health GM38060 and New York State Spinal Cord Injury Program C022061 (to RJL), ZL was supported by China Scholarship Council.
SUPPORTING INFORMATION AVAILABLE
Fig. S1 PAGE analysis of GAGs, Fig. S2-S5 MS spectrum. These materials are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Sherman LS, Back SA. A ‘GAG’ reflex prevents repair of the damaged CNS. TRENDS in Neurosciences. 2008;31:44–52. doi: 10.1016/j.tins.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Sarama SD, Daniela C, Clare G, Kate R, Junko F, Tadahisa M, Kazuyuki S, James WF. Composition of Perineuronal Net Extracellular Matrix in Rat Brain, A different disaccharide composition for the net-associated proteoglycans. J Biol Chem. 2006;281:17789–17800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- 3.Hockfield S, Kalb RG, Zaremba S, Fryer H. Expression of Neural Proteoglycans Correlates with the Acquisition of Mature Neuronal Properties in the Mammalian Brain. Cold Spring Harb Symp Quant Biol. 1990;55:505–514. doi: 10.1101/sqb.1990.055.01.049. [DOI] [PubMed] [Google Scholar]
- 4.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 5.Bruckner G, Hausen D, Hartig W, Drlicek M, Arendt T, Brauer K. Cortical areas abundant in extracellular matrix chondroitin sulphate proteoglycans are less affected by cytoskeletal changes in Alzheimer’s disease. Neuroscience. 1999;92:791–805. doi: 10.1016/s0306-4522(99)00071-8. [DOI] [PubMed] [Google Scholar]
- 6.Morawski M, Bruckner MK, Riederer P, Bruckner G, Arendt T. Perineuronal nets potentially protect against oxidative stress. Exp Neurol. 2004;188:309–315. doi: 10.1016/j.expneurol.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Bruckner G, Brauer K, Hartig W, Wolff JR, Rickmann MJ, Derouiche A, Delpech B, Girard N, Oertel WH, Reichenbach A. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia. 1993;8:183–200. doi: 10.1002/glia.440080306. [DOI] [PubMed] [Google Scholar]
- 8.Bruckner G, Hartig W, Kacza J, Seeger J, Welt K, Brauer K. Extracellular matrix organization in various regions of rat brain grey matter. J Neurocytol. 1996;25:333–346. doi: 10.1007/BF02284806. [DOI] [PubMed] [Google Scholar]
- 9.Hartig W, Derouiche A, Welt K, Brauer K, Grosche J, Mader M, Reichenbach A, Bruckner G. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 1999;842:15–29. doi: 10.1016/s0006-8993(99)01784-9. [DOI] [PubMed] [Google Scholar]
- 10.Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M, Vaidehi N, Goddard WA, Nishi A, III, Hsieh-Wilson LC. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol. 2006;2:467–473. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- 11.Hwang HY, Olson SK, Esko JD, Horvitz HR. Caenorhabditis elegans early embryogenesis and vulval morphogenesis require chondroitin biosynthesis. Nature. 2003;423:439–443. doi: 10.1038/nature01634. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZXT, Fei, Santiago L, Mills EM, Wang Y, Symes AJ, Geller HM. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci. 2008;121:3083–3091. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laabs T, Carulli D, Geller HM, Fawcett JW. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol. 2005;15:116–120. doi: 10.1016/j.conb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Sirko S, von Holst A, Wizenmann A, Gotz M, Faissner A. Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development. 2007;134:2727–2738. doi: 10.1242/dev.02871. [DOI] [PubMed] [Google Scholar]
- 15.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev eurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 16.Toida T, Yoshida H, Toyoda H, Koshiishi I, Imanari T, Hileman RE, Fromm JR, Linhardt RJ. Structural differences and the presence of unsubstituted amino groups in heparan sulphates from different tissues and species. Biochem J. 1997;322:499–506. doi: 10.1042/bj3220499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stipp CS, Litwack ED, Lander AD. Cerebroglycan: an integral membrane heparan sulfate proteoglycan that is unique to the developing nervous system and expressed specifically during neuronal differentiation. J Cell Boil. 1994;124:149–160. doi: 10.1083/jcb.124.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu H. Cell-surface heparan sulfate is involved in the repulsive guidance activities of Slit2 protein. Nat Neurosci. 2001;4:695–701. doi: 10.1038/89482. [DOI] [PubMed] [Google Scholar]
- 19.Kreuger J, Jemth P, Sanders-Lindberg E, Eliahu L, Ron D, Basilico C, Salmivirta M, Lindahl U. Fibroblast growth factors share binding sites in heparan sulphate. Biochem J. 2005;389:145–150. doi: 10.1042/BJ20042129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, Kolodkin AL. Semaphorin 5A is a Bifunctional Axon Guidance Cue Regulated by Heparan and Chondroitin Sulfate Proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Turnbull JE, Field RA. Emerging glycomics technologies. Nat Chem Biol. 2007;3:74–77. doi: 10.1038/nchembio0207-74. [DOI] [PubMed] [Google Scholar]
- 22.Bitter T, Muir HM. A modiWed uronic acid carbazole reaction. Anal Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 23.Edens RE, Al-Hakim A, Weiler JM, Rethwisch DG, Fareed J, Linhardt RJ. Gradient polyacrylamide gel electrophoresis for determination of the molecular weights of heparin preparations and low-molecular-weight heparin derivatives. J Pharm Sci. 1992;81:823–827. doi: 10.1002/jps.2600810821. [DOI] [PubMed] [Google Scholar]
- 24.Zhang F, Sun P, Munoz E, Chi L, Sakai S, Toida T, Zhang H, Mousa S, Linhardt RJ. Microscale Isolation and Analysis of Heparin from Plasma using an Anion Exchange Spin Column. Anal Biochem. 2006;353:284–286. doi: 10.1016/j.ab.2006.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rice TC, Toida KGT, Linhardt RJ. LC/MS sequencing of highly sulfated heparin-derived oligosaccharides. J Biol Chem. 2004;279:2608–2615. doi: 10.1074/jbc.M304772200. [DOI] [PubMed] [Google Scholar]
- 26.Solakyildirim K, Zhang ZQ, Linhardt RJ. Ultraperformance liquid chromatography with electrospray ion trap mass spectrometry for chondroitin disaccharide analysis. Anal Chem. 2010;397:24–28. doi: 10.1016/j.ab.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasisekharan R, Raman R, Prabhakar V. Glycomics Approach to Structure-Function Relationships of Glycosaminoglycans. Annu Rev Biomed Eng. 2006;8:181–231. doi: 10.1146/annurev.bioeng.8.061505.095745. [DOI] [PubMed] [Google Scholar]
- 28.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Park Y, Kemp M, Zhao W, Im A, Shaya D, Cygler M, Kim Y, Linhardt RJ. Liquid chromatography-mass spectrometry to study chondroitin lyase action pattern. Anal Biochem. 2009;385:57–64. doi: 10.1016/j.ab.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholson C, Sykova E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998;21:207–221. doi: 10.1016/s0166-2236(98)01261-2. [DOI] [PubMed] [Google Scholar]
- 31.Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Gallagher JT, Turnbull JE, Lyon M. Patterns of sulphation in heparan sulphate: polymorphism based on a common structural theme. Int J Biochem. 1992;24:553–560. doi: 10.1016/0020-711x(92)90326-v. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Xie J, Liu H, Liu J, Linhardt RJ. Quantification of heparan sulfate disaccharides using ion-pairing reversed-phase microflow high-performance liquid chromatography with electrospray ionization trap mass spectrometry. Anal Chem. 2009;81:4349–4355. doi: 10.1021/ac9001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi X, Zaia J. Organ-specific heparan sulfate structural phenotypes. J Biol Chem. 2009;284:11806–11814. doi: 10.1074/jbc.M809637200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW. Composition of Perineuronal Net Extracellular Matrix in Rat Brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem. 2006;281:17789–17800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- 36.Vaccarino FM, Schwartz ML, Raballo R, Rhee J, Lyn-Cook R. Fibroblast growth factor signaling regulates growth and morphogenesis at multiple steps during brain development. Curr Top Dev Biol. 1999;46:179–200. doi: 10.1016/s0070-2153(08)60329-4. [DOI] [PubMed] [Google Scholar]
- 37.Ford-Perriss M, Abud H, Murphy M. fibroblast growth factors in the developing central nervous system. Clin Exp Pharmacol Physiol. 2001;28:493–503. doi: 10.1046/j.1440-1681.2001.03477.x. [DOI] [PubMed] [Google Scholar]
- 38.Tsai Ming-Chu, Shen Li-Fen, Kuo Huai-Sheng, Cheng Henrich, Chak Kin-Fu. Involvement of Acidic Fibroblast Growth Factor in Spinal Cord Injury Repair Processes Revealed by a Proteomics Approach. Molec Cell Proteom. 2008;7:1668–1687. doi: 10.1074/mcp.M800076-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang F, McLellan JS, Ayala AM, Leahy DJ, Linhardt RJ. Kinetic and structural studies on interactions between heparin or heparan sulfate and proteins of the hedgehog signaling pathway. Biochemistry. 2007;46:3933–3941. doi: 10.1021/bi6025424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martí Elisa, Bovolenta Paola. Sonic hedgehog in CNS development: one signal, multiple outputs. Trends Neurosci. 2002;25:89–96. doi: 10.1016/s0166-2236(02)02062-3. [DOI] [PubMed] [Google Scholar]
- 41.Lowry N, Goderie SK, Adamo M, Lederman P, Charniga C, Gill J, Silver J, Temple S. Multipotent embryonic spinal cord stem cells expanded by endothelial factors and Shh/RA promote functional recovery after spinal cord injury. Exp Neurol. 2008;209:510–522. doi: 10.1016/j.expneurol.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 42.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 43.McLellan JS, Yao S, Zheng X, Geisbrecht BV, Ghirlando R, Beachy PA, Leahy DJ. Structure of a heparin-dependent complex of hedgehog and Ihog. Proc Natl Acad Sci U S A. 2006:17208–17213. doi: 10.1073/pnas.0606738103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.