Abstract
The transcription factor Pitx3 is critical for lens formation. Deletions in the promoter of this gene cause abnormal lens development in the aphakia (ak) mouse mutant, which has only rudimentary lenses. In this study, we investigated the role of Pitx3 in lens development and differentiation. We found that reduced expression of Pitx3 leads to changes in the proliferation, differentiation and survival of lens cells. The genetic interactions between Pitx3 and Foxe3 were investigated, as these two transcription factors are expressed at the same time in lens development and their absence has similar consequences for lens development. We found no evidence that these two genes genetically interact.
In general, our study shows that the abnormal phenotype of the ak lenses is not due to just one molecular pathway, rather in the absence of Pitx3 expression multiple aspects of lens development are disrupted.
Keywords: aphakia, Foxe3, Pitx3, lens
Introduction
During vertebrate lens development, the lens placode undergoes a carefully programmed morphogenetic process that leads to the formation of a lens with relatively undifferentiated, proliferative lens epithelial cells in the anterior of the lens, and highly differentiated, mitotically-inactive fiber cells in the posterior of the lens (for review see (McAvoy et al., 1999; Chow and Lang, 2001; Donner et al., 2006; Cvekl and Duncan, 2007; Medina-Martinez and Jamrich, 2007)). The proliferation and differentiation of the lens cells are controlled by several transcription factors. While the transcription factor Pax6 appears to be the key regulator of lens development, as mutations in this gene lead to eye malformations in humans (Ton et al., 1991; Jordan et al., 1992; Glaser et al., 1994; Hanson et al., 1994), mice and rats (Hill et al., 1991; Fujiwara et al., 1994), several other transcriptional regulators are also critical for lens formation. One of them is the homeodomain-containing factor Pitx3. Pitx3 is expressed during early vertebrate lens development. A double deletion that eliminates the promoter region and a part of the coding region of this gene causes the abnormal lens phenotype in the mouse line aphakia (ak) (Semina et al., 2000; Rieger et al., 2001) and mutations in PITX3 lead to the development of autosomal-dominant cataract in humans (Semina et al., 1998). In ak mice the lens begins to form, but its development is abnormal. Eventually, lens development arrests and the lens disappears (Varnum and Stevens, 1968; Zwaan, 1975; Zwaan and Kirkland, 1975; Grimm et al., 1998). Some aspects of lens development in the aphakia mutant are similar to the lens development in Foxe3 null mice (Medina-Martinez et al., 2005). In both mutants the invaginating lens placode does not separate properly from the head surface ectoderm and the lens remains connected to the surface ectoderm with a stalk (Varnum and Stevens, 1968; Zwaan, 1975; Zwaan and Kirkland, 1975; Grimm et al., 1998; Blixt et al., 2000; Brownell et al., 2000; Medina-Martinez et al., 2005; Blixt et al., 2006; Medina-Martinez and Jamrich, 2007). Foxe3 is a conserved forkhead transcription factor that is critical for lens development in several vertebrate species (for review see (Medina-Martinez and Jamrich, 2007)). Mutations in this gene, or its altered expression, cause abnormal lens development in mouse, humans and zebrafish (Blixt et al., 2000; Brownell et al., 2000; Semina et al., 2001; Ormestad et al., 2002; Medina-Martinez et al., 2005; Shi et al., 2006; Valleix et al., 2006; Medina-Martinez and Jamrich, 2007; Swindell et al., 2008).
Since the expression of these genes is very similar during early stages of lens development and mutations in these genes result in a similar lens phenotype, we investigated whether these two transcription factors are a part of the same regulatory cascade during mouse lens development. As these experiments provided no evidence for genetic interaction of these two genes, we have compared the expression of several diagnostic markers of lens development and differentiation in the ak and wild type lenses. Results of these experiments have provided a better understanding of the requirement for the Pitx3 function in the mouse lens.
Results
Expression of Foxe3 and Pitx3 in wild type and ak embryos
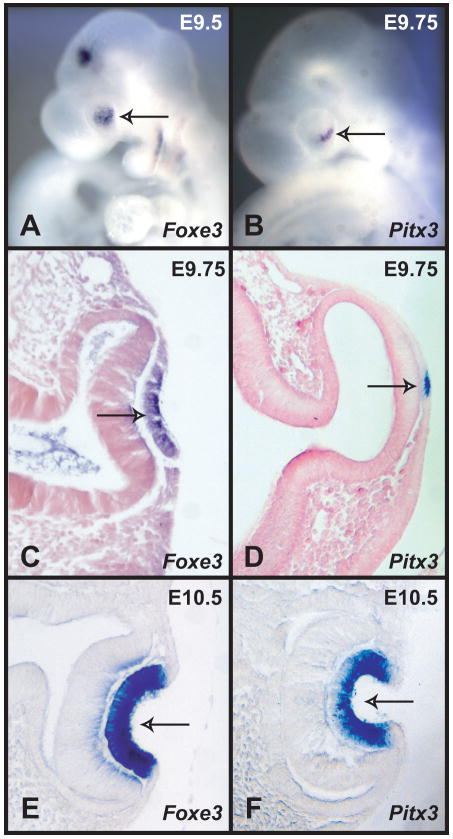
Since previous reports indicated that the expression of Pitx3 and Foxe3 is similar during early lens development (Semina et al., 1997; Semina et al., 1998; Blixt et al., 2000; Brownell et al., 2000), and since mouse embryos with mutations in these two genes display similar lens phenotypes (Varnum and Stevens, 1968; Blixt et al., 2000; Brownell et al., 2000; Medina-Martinez et al., 2005; Medina-Martinez and Jamrich, 2007), we investigated the expression of Pitx3 and Foxe3 in more detail. To obtain better information about the temporo-spatial expression pattern of Foxe3 and Pitx3 during early lens development, we analyzed expression of these two genes by whole mount in situ hybridization. Using this method, Foxe3 expression can be easily detected in the developing lens placode at E9.5 (Fig. 1A) (Blixt et al., 2000; Brownell et al., 2000). Pitx3 expression can be detected by whole mount in situ hybridization few hours later, around E9.75 (Fig. 1B). Sections of these embryos show that at E9.75 Pitx3 is expressed only in a small portion of the lens placode, while Foxe3 is expressed in the entire lens placode (Fig. 1C, D). However, by the time the lens placode begins to invaginate, both Foxe3 and Pitx3 are expressed in the entire lens pit (Fig. 1E,F). Thus, Foxe3 expression precedes Pitx3 during the formation of the lens placode, but at later stages their expression appears to be identical.
Figure 1.
In situ hybridizations of Foxe3 and Pitx3 probes to wild type mouse embryos. (A) Whole mount in situ hybridization of a Foxe3 probe to an E9.5 embryo showing expression in the lens placode (arrow). (B) Whole mount in situ hybridization of Pitx3 probe to an E9.75 embryo showing expression in a part of the lens placode (arrow). (C) A section showing the expression of Foxe3 in the lens placode (arrow). (D) A section through the embryo in B showing expression of Pitx3 in a few cells of the lens placode (arrow). (E) A section of an E10.5 embryo hybridized with Foxe3 probe showing strong expression in the lens pit (arrow). (E) A section of an E10.5 embryo hybridized with Pitx3 probe showing strong expression in most of the cells of the lens pit (arrow).
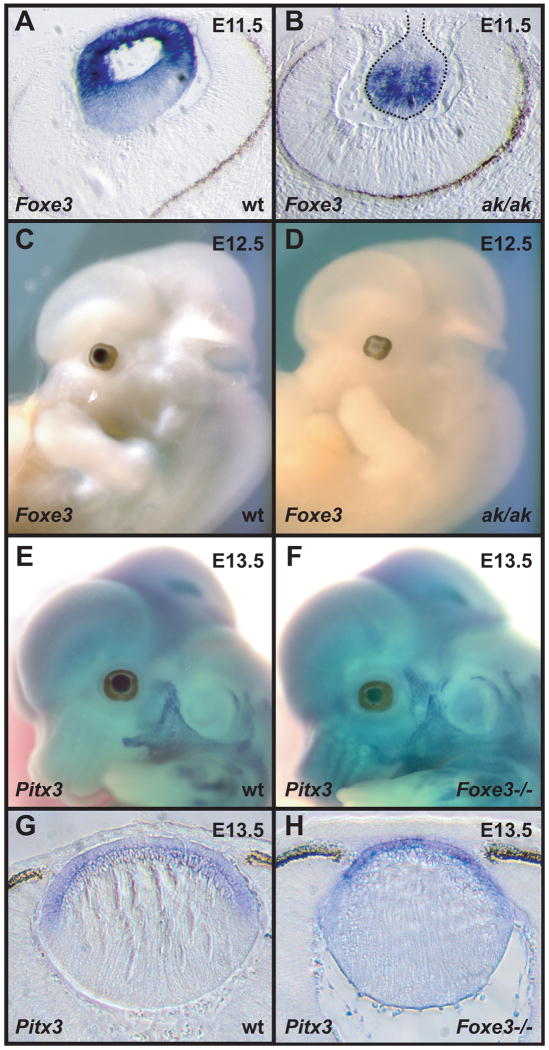
Since Foxe3 and Pitx3 expression is similar in the developing lens, we evaluated whether the expression of these two genes depends on each other. For this purpose, we analyzed the expression of Foxe3 in the ak mutant and the expression of Pitx3 in the Foxe3 null mutant by in situ hybridization. During normal lens development Foxe3 is initially expressed in the entire lens vesicle. As lens development proceeds, Foxe3 remains active in the anterior of the lens, in cells that will form the anterior lens epithelium (Blixt et al., 2000; Brownell et al., 2000). The differentiating lens fiber cells at the posterior of the lens cease to express Foxe3. In situ hybridizations on E11.5 wild type and ak embryos show an altered spatial distribution of Foxe3 transcripts within the ak lens. While in the wild type lens the highest levels of Foxe3 expression are found in the anterior of the lens (Fig. 2A), which develops into the lens epithelium, in the ak lens most of the Foxe3 transcripts are found in the posterior of the lens (Fig. 2B). Expression of Foxe3 in the posterior of the ak lens indicates that the primary lens fiber cells in the ak embryo do not undergo proper differentiation. Lack of expression of Foxe3 in the anterior compartment indicates that Pitx3 activity is necessary for the maintenance of Foxe3 expression in the anterior lens epithelium. Indeed, the significant downregulation of Foxe3 expression in the ak mutant by E12.5 (Fig. 2C,D) suggests that Pitx3 is necessary for the maintenance of the Foxe3 expression. In contrast, Pitx3 expression is relatively unaffected in the Foxe3 null embryos (Fig. 2E-H), suggesting that Foxe3 does not have an important role in the regulation of Pitx3 transcription during early lens development.
Figure 2.
In situ hybridizations of Foxe3 and Pitx3 to wild type, ak and Foxe3-deficient embryos. (A) A section of an E11.5 wild type embryo hybridized with Foxe3 probe showing strong labeling in the developing lens. (B) A section of an E11.5 ak embryo hybridized with a Foxe3 probe showing expression of this gene in the abnormal lens. (C) Whole mount in situ hybridization of Foxe3 probe to an E12.5 wild type embryo showing expression in the lens. (D) Whole mount in situ hybridization of Foxe3 probe to an E12.5 ak embryo showing dramatically reduced expression of this gene in the lens. (E) Whole mount in situ hybridization of Pix3 probe to an E13.5 embryo wild type embryo showing expression of this gene in the lens. (F) Whole mount in situ hybridization of Pitx3 probe to an E13.5 Foxe3-deficient embryo showing only slightly reduced levels of expression of this gene in the lens. (G) A section through the embryo in (E) showing the expression of Pitx3 in wild type embryo. (H) A section through the embryo in (F) showing the expression of Pitx3 in Foxe3-/- embryo.
Analysis of genetic interactions between Foxe3 and Pitx3
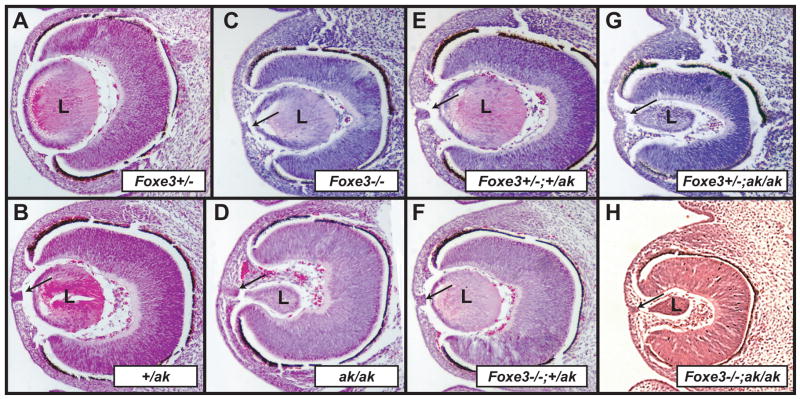
Since some of the defects in the Foxe3 and Pitx3 mutants appear superficially similar, there was a possibility that these two genes genetically interact. For this reason, we compared lens development in Foxe3 and Pitx3 compound heterozygous and homozygous animals by interbreeding Foxe3+/- heterozygous mice with +/ak heterozygous mice. As previously reported (Medina-Martinez et al., 2005), the Foxe3+/- heterozygous mice display no abnormal phenotype (Fig. 3A), whereas the lenses in +/ak heterozygous animals are almost normal, occasionally displaying a stalk connecting the lens and the surface ectoderm (Fig. 3B). Lenses in Foxe3-deficient animals are smaller than normal and display a persistent connection with the surface ectoderm (Fig. 3C). Lenses in ak/ak animals are typically club-shaped and smaller than lenses in Foxe3-deficient animals. They also display a persistent connection to the surface ectoderm (Fig. 3D). Analysis of lens development in mice with different genotypic combinations of Foxe3 and Pitx3 revealed that in Foxe3+/-;+/ak double heterozygous embryos (Fig. 3E), the morphology of the lens is similar to the lens in ak heterozygous animals (Fig. 3C). Thus, superposition of Foxe3+/- heterozygosity does not exacerbate the +/ak phenotype suggesting that there is no synergistic genetic interaction between Foxe3 and Pitx3. We next evaluated the lens development in Foxe3+/-;ak/ak, Foxe3-/-;+/ak, and Foxe3-/-;ak/ak compound mutants. As expected, the lens in the Foxe3-/-;+/ak had the same morphology (Fig. 3F) as in the Foxe3 null allele (Fig. 3C). The Foxe3+/-;ak/ak lenses (Fig. 3G) resemble those of the ak mutants (Fig. 3D). In the Foxe3-/-;ak/ak compound mutants (Fig. 3H), the lenses are similar to lenses in ak/ak animals (Fig. 3D). These experiments suggest that there is no synergistic interaction between Foxe3 and Pitx3 during lens development.
Figure 3.
Morphological analysis of genetic interactions between Foxe3 and Pitxe3. (A-H) Hematoxylin and eosin (H&E) stained sections of a Foxe3+/- eye (A), an eye from +/ak embryo (B), an eye from a Foxe3-/- embryo (C), an eye from ak/ak embryo (D), an eye of an embryo heterozygous for Foxe3 and Pitx3 (E), an eye from an embryo deficient for Foxe3-/- and heterozygous for Pitx3 (F), an embryo heterozygous for Foxe3 and deficient for Pitx3 (G) and an eye of an embryo deficient for Foxe3 and Pitx3 (H). Notice the club-shaped lens in the ak/ak embryos. Arrows in sections point to the connections between the lens and surface ectoderm that broke off during the preparations of sections. L- lens
Expression of crystallins in ak embryos
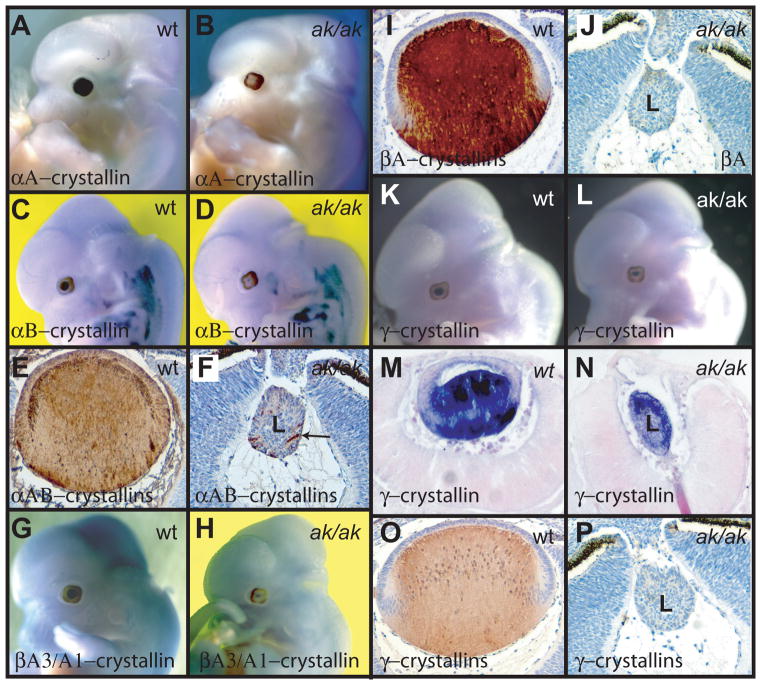
To better understand the developmental state of lens cells in the ak mutant, we investigated the expression of crystallins. Crystallins are a family of chaperone proteins that are expressed at very high levels in the vertebrate lens (Wistow and Piatigorsky, 1987; Piatigorsky, 1998; Cvekl and Duncan, 2007). Expression of individual crystallin genes is characteristic of the state of differentiation of individual lens cells. During mouse lens development, αB-crystallin is the first crystallin gene activated. Its transcription starts already in the lens placode (Robinson and Overbeek, 1996; Cvekl and Duncan, 2007). It is followed by αA-crystallin, which is activated at the transition from the lens pit to lens vesicle (Robinson and Overbeek, 1996). The β and γ-crystallins are highly expressed in differentiating fiber cells. We have examined the expression of crystallins in the ak lenses using both in situ hybridizations and antibodies against different crystallins. In situ hybridization shows that at E12.5 αA-crystallin (Crya1) and αB-crystallin (Crya2) are transcribed in the wild type embryos at high levels (Fig. 4A and C), but their transcription is strongly reduced in the ak mutant (Fig. 4B and D). Using antibodies that recognize αA and αB-crystallins, we found that only a few cells are positive for these two proteins in the aphakia lens (Fig.4F), while the entire lens labels with these antibodies in the wild type embryo (Fig. 4E). Expression of βA3/A1-crystallin (Cryba1) is also dramatically altered. In the wild type lens, this gene is strongly transcribed already at E12.5 (Fig. 4G). In the ak lens, the βA3/A1-crystallin is only weakly transcribed at E12.5 (Fig. 4H). Immunostaining using antibodies against βA-crystallins shows that in the wild type lens the distribution of βA-crystallins proteins is not uniform. Most of the βA-crystallins can be found in mature fibers (Fig. 4I). In the ak mutant, the βA-crystallins are almost undetectable (Fig. 4J). Finally, γ-crystallin transcripts, as well as the γ-crystallin proteins, are present primarily in the mature lens fiber cells of the wild type lens (Fig. 1K, M and O). Interestingly, in the ak mutant, relatively high levels of γ-crystallin transcripts can be detected (Fig. 4L, N), but the γ-crystallins proteins are practically undetectable when using antibodies against γ-crystallins (Fig. 4P). Furthermore, the γ-crystallin transcripts show an abnormal distribution within the ak lens. In contrast to the wild type lens, where the transcripts are present mostly in the lens fiber cells (Fig. 4M), in the ak lens, the γ-crystallin transcripts are more abundant in the anterior of the lens (Fig. 4N). This indicates that the differentiation process of lens cells is abnormal in the ak mutant, since all lens cells transcribe γ-crystallin mRNAs at relatively high levels and that the temporo-spatial regulation of γ-crystallin expression is abnormal. In addition, our results suggest that in ak lenses, the expression of γ-crystallins and maybe also βA-crystallins, is controlled at a post-transcriptional level.
Figure 4.
Analysis of crystallin expression in E12.5 wild type and ak embryos. (A, B) Whole mount in situ hybridizations of an αA-crystallin (Crya1) probe to a wild type embryo (A) and to an ak embryo (B). (C, D) Whole mount in situ hybridizations of an αB-crystallin (Crya2) probe to a wild type embryo (C) and to an ak embryo (D). (E, F) Immunostaining of lens sections from a wild type embryo (E) and an ak embryo (F) with antibodies directed against αA and αB-crystallins. Only few cells show expression in the ak lens (arrow). (G, H) Whole mount in situ hybridizations of βA3/A1-crystallin (Cryba1) probe to a wild type embryo (G) and an ak embryo (H). (I, J) Immunostaining of lens sections from a wild type embryo (I) and an ak embryo (J) with antibodies directed against βA-crystallins. (K, L) Whole mount in situ hybridizations of γ-crystallin probe to a wild type embryo (K) and to an ak embryo (L). (M) A section of the embryo in (K) showing high levels of γ-crystallin expression in the posterior of the lens. (N) A section of the embryo in (L) showing γ-crystallin expression in the anterior part of the lens. (O, P) Immunostaining of lens sections from a wild type embryo (O) and an ak embryo (P) with antibodies direct against γ-crystallins.
Expression of key transcriptional regulators in ak embryos
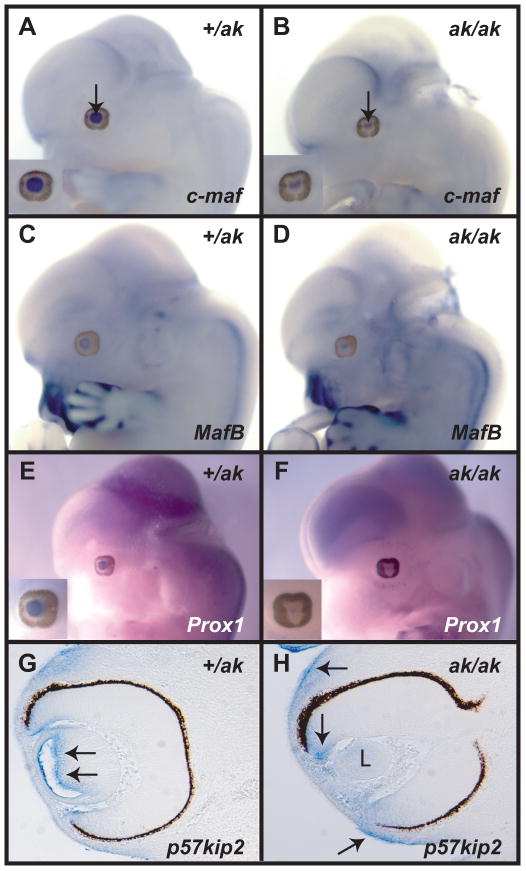
Since the altered expression of crystallins was diagnostic of abnormal differentiation of lens cells, we investigated in the ak mutant the expression of transcription factors that were previously implicated in the regulation of lens development and differentiation. We evaluated expression of several regulatory genes including c-maf, Maf-B, Sox1, Sox2, Six3, Prox1 and Hsf-4 by in situ hybridization. At E12.5, we have observed only slight reduction in the intensity of expression of c-maf in the ak mutant when compared to the +/ak embryos (Fig. 5A, B). Expression of Maf-B (Fig.5C, D), Hsf4, Sox1 and Sox2 (data not shown) in the ak lenses was not significantly altered with the caveat that the area of expression was smaller than in +/ak lenses. The most dramatic reduction was observed in the expression of Prox1 (Fig. 5E, F). Prox1 is involved in the transition of lens epithelium to the lens fiber cells. Prox1-deficient lenses do not develop fiber cells and remain arrested at the lens vesicle stage (Wigle et al., 1999). Prox1 promotes exit of cells from the cell cycle and Prox1-deficient cells have been shown to be less likely to stop dividing (Wigle et al., 1999; Dyer, 2003; Dyer et al., 2003).
Figure 5.
Analysis of expression of c-maf, MafB, Prox1 and p57KIP2 in Pitx3 heterozygous and deficient embryos. (A, B) Whole mount in situ hybridizations of a c-maf probe to a +/ak embryo (A) and an ak/ak embryo (B). Arrows point to the lens. Insets provide a high magnification of the eye region. (C, D) Whole mount in situ hybridization of a MafB probe to a +/ak embryo (C) and an ak embryo (D). (E, F) Whole mount in situ hybridizations of a Prox1 probe to a +/ak embryo (E) and an ak embryo (F). Insets provide a high magnification of the eye region. Notice the virtual absence of Prox1 expression in F. (G) A section of a +/ak eye hybridized with p57KIP2 probe. Arrows point to the expression of this gene in the lens. (H) A section of an ak eye hybridized with the p57KIP2 probe. There is no expression detected in the lens (L), but the expression in the retina and surface ectoderm remains (arrows).
Since expression of Prox-1 was affected in ak mutants, we investigated whether the expression of the cell cycle regulator p57KIP2 is also affected. Expression of p57KIP2 is known to be controlled by Prox-1 (Wigle et al., 1999) and reduction in p57KIP2 expression would indicate that cell cycle control in this mutant is affected. We found that p57KIP2 is not expressed in the ak lens (Fig. 5H), an observation that is consistent with the absence of Prox1 expression in the ak lens.
Apoptosis and proliferation in ak embryos
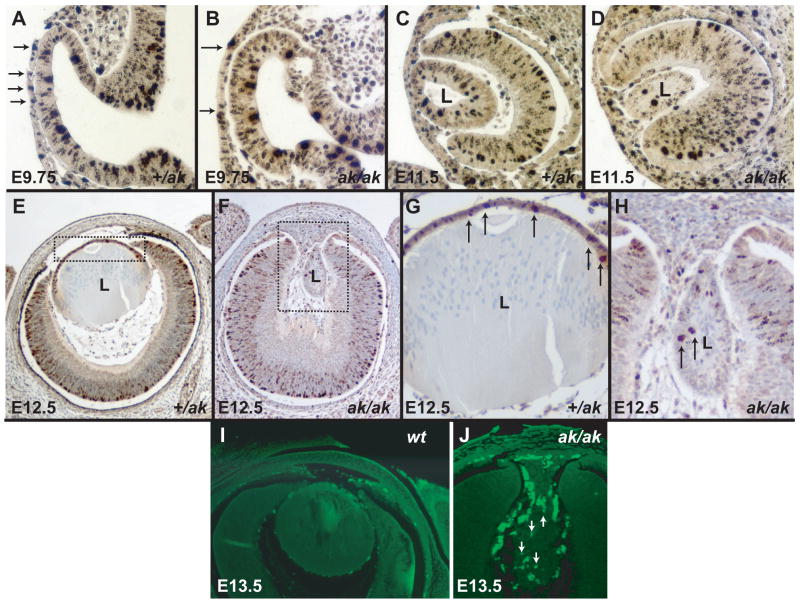
To examine whether proliferation and apoptosis is affected in ak lenses, we analyzed cell proliferation by using antibodies against phosphohistone H3 (Wei et al., 1999). Phosphohistone H3 (phH3) is an indicator of cell proliferation, as histone H3 is phosphorylated during the M-phase of the cell cycle. We have examined the distribution of phosphohistone H3 in +/ak and ak embryos ranging from E9.75 to E12.5. We found that at all stages there are fewer lens cells labeling in the ak mutant than in the +/ak lenses. Already at E9.75 and E11.5 the presumptive lens placode and the lens vesicle shows fewer phH3 positive cells than the +/ak counterparts (Fig. 6A-D). At E12.5, in +/ak embryos the antibodies against phH3 label many proliferating cells in the anterior lens epithelium (Fig. 6E, G). In the ak lenses, only occasional cells can be found displaying a positive reaction using these antibodies (Fig.6F, H). These cells are typically not in the anterior of the lens, but rather in the posterior compartment. This indicates that in ak embryos cells in the anterior of the lens stopped proliferation. Only aberrant proliferation of cells in the posterior of the lens is present. A TUNEL assay demonstrates that at the same time as the proliferation of lens cells is reduced in the ak mutant, the cell death is increased when compared to the wild type lens (6I, J).
Figure 6.
Proliferation and apoptosis in Pitx3 heterozygous and Pitx3-deficient embryos. (A) A section of an E9.75 +/ak eye reacted with antibody against phosphohistone H3. Arrows point to the phH3 positive cells. (B) A section of an E9.75 ak/ak eye showing reaction with an antibody against phosphohistone H3. Arrows bracket the presumptive lens placode that shows a reduced labeling with antibodies against phH3. (C) A section of an E11.5 +/ak eye reacted with antibody against phosphohistone H3. (D) A section of an E11.5 ak/ak eye showing reaction with the antibodies against phosphohistone H3. (E) A section of an E12.5 +/ak eye reacted with antibody against phosphohistone H3. The boxed region is magnified in G. (F) A section of an E12.5 ak/ak eye showing reaction with the antibody against phosphohistone H3. The boxed region is magnified in H. Arrows indicate phosphohistone H3 positive cells. (I, J) TUNEL assay on the wild type eye (I) and on the ak eye (J). Apoptosis is not observed in the wild type lens, while TUNEL positive cells (fluorescent nuclei) are seen in the ak lens (arrows). Fluorescent cells surrounding the lens are likely to be of neural crest origin.
Discussion
Lack of Pitx3 activity causes reduced proliferation and increased apoptosis in the ak lenses
In this paper, we report studies of gene expression, proliferation and differentiation of lens cells in the aphakia mouse mutant (Semina et al., 2000). In this mutant, the activity of Pitx3 is reduced by about 90% (Semina et al., 2000; Rieger et al., 2001). As a result of this reduced Pitx3 activity, in the ak animals the lens is abnormally small and club-shaped. It remains connected to the surface ectoderm and eventually it disappears. In order to understand better the causes for the abnormal lens phenotype, we investigated apoptosis, proliferation and gene expression in the ak lenses. We found that the proliferation in the developing lens is reduced in the ak mutant as early as E9.75. The reduced proliferation is concomitant with the abnormal expression of the transcription factor Foxe3. Since the proliferation of lens cells is also affected in Foxe3 mutants (Blixt et al., 2000; Brownell et al., 2000; Medina-Martinez et al., 2005), we investigated whether these two transcription factors cooperate in the regulation of proliferation and whether the reduced proliferation in the ak lens can be explained by the abnormal expression of Foxe3. Genetic analysis showed no evidence of synergistic interaction between these two genes, as the lenses in the compound heterozygous animals did not have a more severe phenotype than the lenses in ak heterozygous animals. A comparison of expression of Pitx3 and Foxe3 in wild type, ak and Foxe3 mutants showed that Foxe3 is activated in the lens placode before Pitx3 and therefore Pitx3 is unlikely to have a role in the activation of Foxe3. However, Foxe3 expression in the ak mutant is not maintained past E12.5, suggesting that Pitx3 is directly or indirectly required for the maintenance of Foxe3 transcription. Since Foxe3 was shown to play an role in the proliferation of the anterior epithelium (Blixt et al., 2000; Brownell et al., 2000; Medina-Martinez et al., 2005; Medina-Martinez and Jamrich, 2007), it is possible that the decreased proliferation of anterior lens cells in the ak mutant is due to the reduced activity of Foxe3. In contrast to the wild type lens, in the ak lens the Foxe3 is no longer expressed in the anterior lens cells. Reduction in Foxe3 expression is known to reduce the proliferation of anterior lens cells. Interestingly, Foxe3 transcripts can be found in the posterior lens cells. This must be detrimental to the differentiating lens fiber cells since a persistent expression of Foxe3 blocks cytoskeletal remodeling during the differentiation of lens fiber cells (Landgren et al., 2008). This block in cytoskeletal remodeling might explain while we never see true fiber cells in the ak lens. However, the reduction in Foxe3 expression cannot account entirely for the lack of proliferation in ak lenses. This is because in Foxe3-deficient embryos, the proliferation of the lens epithelium is less affected than in the ak mutants, which have at least some Foxe3 activity. However the reduction in proliferation in conjunction with the observed apoptosis of the lens cells is likely to be the cause of smaller lenses in the ak mutant.
Lack of Pitx3 activity causes abnormal differentiation in the ak lenses
While some differentiation of lens cells takes place in the ak mutant, expression of crystallins, markers of lens differentiation, is abnormal. In contrast to the wild type lens, the expression of α and β-crystallins is rare in the posterior lens cells. γ-crystallins are transcribed, but they are not efficiently translated. Furthermore, their transcription is spatially disregulated. While typically γ-crystallins transcripts are found in the differentiating lens fiber cells of the wild type lens, but not in the cells of the anterior lens epithelium, in the ak lens, high levels of γ-crystallins transcripts can be found in the anterior lens cells indicating that γ-crystallins are transcribed inappropriately early during lens cell differentiation. Our observations taken together with the reports that the lens cells that move posteriorly do not assume typical fiber shape (Varnum and Stevens, 1968; Zwaan, 1975; Malinina and Koniukhov, 1981; Semina et al., 1997; Grimm et al., 1998; Semina et al., 2000; Rieger et al., 2001) show that the differentiation of lens fiber cells is grossly abnormal in the ak mutants. The deregulation of crystallin expression might be important for the survival of lens cells. The reduction of expression of αA-crystallin and αB-crystallin might contribute to the observed apoptosis of lens cells in ak animals. αA/αB-crystallins were shown to inhibit apoptosis, as lens fibers disintegrate in the αA/αB-crystallin double knockout (Morozov and Wawrousek, 2006).
The downregulation of crystallin expression in the ak mutant might be due to the downregulation of Prox1 expression. Prox1 has been shown to regulate expression of crystallin genes (Cui et al., 2004; Chen et al., 2008). On the other hand, almost normal crystallin expression in the Prox1-deficient embryos argues against this possibility (Wigle et al., 1999).
In general, the development and differentiation of the lens in the ak mutants is strongly disrupted. Pitx3 is clearly essential for the normal lens development and differentiation. In the ak mutant, the developing lens does not undergo a typical development in which an anterior lens epithelium is formed. The lens cells do not undergo a proper differentiation and fiber cells are not formed. The proliferation of lens cells is reduced and the apoptosis is increased. The temporo-spatial expression of genes diagnostic of early and late stages of lens development is abnormal. Based on these results we conclude that the abnormal phenotype of the ak lenses is not due to just one molecular pathway or a specific interaction, rather in the absence of Pitx3 expression, the apoptosis, proliferation and differentiation of lens cells is severely disrupted.
Experimental Procedures
Mouse genetics
Foxe3-deficient mice were previously described (Medina-Martinez et al., 2005). The ak mice that were generously provided by Dr. Paul Overbeek (Semina et al., 2000; Varnum and Stevens, 1968) arose as a spontaneous mutation in the C57BL/6 genetic background, but our mutants have a mixed genetic background. In order to generate animals with different genotypic combinations of Foxe3 and Pitx3, Foxe3+/- heterozygous mice were backcrossed at least two generations with +/ak heterozygous mice. The ak mice were crossed to Foxe3-/- (C57BL/6X 129 SvEv) mice. Double heterozygous animals (Foxe3+/-;/+/ak) were interbred to obtain the desired embryos. Three embryos were analyzed for each genotype.
Histology, in situ hybridization and nomenclature
For histological analysis, embryos were fixed in 10% formalin, dehydrated in an ethanol series. They were embedded in paraffin, sectioned and stained with hematoxilin and eosin. Whole mount in situ hybridizations were performed according to standard procedures (Wilkinson, 1992).
In this paper, we refer as αA-crystallin and αB-crystallin to the mouse genes Crya1 and Crya2. βA3/A1-crystallin refers to the mouse gene Cryba1.
Immunohistochemistry
Embryos were fixed in 10% formalin overnight, dehydrated, embedded in paraffin and cut into 8mm serial sections. Sections were treated with different anti-crystallin antibodies (Yoshimoto et al., 2005) or with a phosphohistone H3 antibody (Hendzel et al., 1997) from Upstate Biotechnology.
Acknowledgments
We would like to thank Dr. Kondoh for a generous gift of crystallin antibodies, Wilbur Harrison for technical help and Dr. Paul Overbeek for a critical reading of this manuscript. We would like to thank the anonymous reviewers of this paper for valuable suggestions. This research was sponsored by NIH/NEI grants EY12505 and EY12163 to M.J., NIH/NEI training grant T32 EY07102 to O.M. and RRF research grant to MJ.
References
- Blixt A, Landgren H, Johansson BR, Carlsson P. Foxe3 is required for morphogenesis and differentiation of the anterior segment of the eye and is sensitive to Pax6 gene dosage. Dev Biol. 2006 doi: 10.1016/j.ydbio.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Blixt A, Mahlapuu M, Aitola M, Pelto-Huikko M, Enerback S, Carlsson P. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 2000;14:245–254. [PMC free article] [PubMed] [Google Scholar]
- Brownell I, Dirksen M, Jamrich M. Forkhead Foxe3 maps to the dysgenetic lens locus and is critical in lens development and differentiation. Genesis. 2000;27:81–93. doi: 10.1002/1526-968x(200006)27:2<81::aid-gene50>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Chen X, Taube JR, Simirskii VI, Patel TP, Duncan MK. Dual roles for Prox1 in the regulation of the chicken betaB1-crystallin promoter. Invest Ophthalmol Vis Sci. 2008;49:1542–1552. doi: 10.1167/iovs.07-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Cui W, Tomarev SI, Piatigorsky J, Chepelinsky AB, Duncan MK. Mafs, Prox1, and Pax6 can regulate chicken betaB1-crystallin gene expression. J Biol Chem. 2004;279:11088–11095. doi: 10.1074/jbc.M312414200. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Duncan MK. Genetic and epigenetic mechanisms of gene regulation during lens development. Prog Retin Eye Res. 2007;26:555–597. doi: 10.1016/j.preteyeres.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner AL, Lachke SA, Maas RL. Lens induction in vertebrates: Variations on a conserved theme of signaling events. Semin Cell Dev Biol. 2006 doi: 10.1016/j.semcdb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Dyer MA. Regulation of proliferation, cell fate specification and differentiation by the homeodomain proteins Prox1, Six3, and Chx10 in the developing retina. Cell Cycle. 2003;2:350–357. [PubMed] [Google Scholar]
- Dyer MA, Livesey FJ, Cepko CL, Oliver G. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet. 2003;34:53–58. doi: 10.1038/ng1144. [DOI] [PubMed] [Google Scholar]
- Fujiwara M, Uchida T, Osumi-Yamashita N, Eto K. Uchida rat (rSey): a new mutant rat with craniofacial abnormalities resembling those of the mouse Sey mutant. Differentiation. 1994;57:31–38. doi: 10.1046/j.1432-0436.1994.5710031.x. [DOI] [PubMed] [Google Scholar]
- Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet. 1994;7:463–471. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- Grimm C, Chatterjee B, Favor J, Immervoll T, Loster J, Klopp N, Sandulache R, Graw J. Aphakia (ak), a mouse mutation affecting early eye development: fine mapping, consideration of candidate genes and altered Pax6 and Six3 gene expression pattern. Dev Genet. 1998;23:299–316. doi: 10.1002/(SICI)1520-6408(1998)23:4<299::AID-DVG5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Hanson IM, Fletcher JM, Jordan T, Brown A, Taylor D, Adams RJ, Punnett HH, van Heyningen V. Mutations at the PAX6 locus are found in heterogeneous anterior segment malformations including Peters' anomaly. Nat Genet. 1994;6:168–173. doi: 10.1038/ng0294-168. [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox- containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Jordan T, Hanson I, Zaletayev D, Hodgson S, Prosser J, Seawright A, Hastie N, van Heyningen V. The human PAX6 gene is mutated in two patients with aniridia. Nat Genet. 1992;1:328–332. doi: 10.1038/ng0892-328. [DOI] [PubMed] [Google Scholar]
- Landgren H, Blixt A, Carlsson P. Persistent FoxE3 expression blocks cytoskeletal remodeling and organelle degradation during lens fiber differentiation. Invest Ophthalmol Vis Sci. 2008 doi: 10.1167/iovs.08-2243. [DOI] [PubMed] [Google Scholar]
- Malinina NA, Koniukhov BV. Action of mutant genes on crystallin synthesis in the developing mouse lens. III. The aphakia gene. Ontogenez. 1981;12:589–595. [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG, de Iongh RU, Hales AM, Lovicu FJ. Lens development. Eye. 1999;13(Pt 3b):425–437. doi: 10.1038/eye.1999.117. [DOI] [PubMed] [Google Scholar]
- Medina-Martinez O, Brownell I, Amaya-Manzanares F, Hu Q, Behringer RR, Jamrich M. Severe defects in proliferation and differentiation of lens cells in Foxe3 null mice. Mol Cell Biol. 2005;25:8854–8863. doi: 10.1128/MCB.25.20.8854-8863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Martinez O, Jamrich M. Foxe view of lens development and disease. Development. 2007;134:1455–1463. doi: 10.1242/dev.000117. [DOI] [PubMed] [Google Scholar]
- Morozov V, Wawrousek EF. Caspase-dependent secondary lens fiber cell disintegration in alphaA-/alphaB-crystallin double-knockout mice. Development. 2006;133:813–821. doi: 10.1242/dev.02262. [DOI] [PubMed] [Google Scholar]
- Ormestad M, Blixt A, Churchill A, Martinsson T, Enerback S, Carlsson P. Foxe3 haploinsufficiency in mice: a model for Peters' anomaly. Investigative Ophthalmology & Visual Science. 2002;43:1350–1357. [PubMed] [Google Scholar]
- Piatigorsky J. Gene sharing in lens and cornea: facts and implications. Prog Retin Eye Res. 1998;17:145–174. doi: 10.1016/s1350-9462(97)00004-9. [DOI] [PubMed] [Google Scholar]
- Rieger DK, Reichenberger E, McLean W, Sidow A, Olsen BR. A double-deletion mutation in the Pitx3 gene causes arrested lens development in aphakia mice. Genomics. 2001;72:61–72. doi: 10.1006/geno.2000.6464. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Overbeek PA. Differential expression of alpha A- and alpha B-crystallin during murine ocular development. Invest Ophthalmol Vis Sci. 1996;37:2276–2284. [PubMed] [Google Scholar]
- Semina EV, Brownell I, Mintz-Hittner HA, Murray JC, Jamrich M. Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum Mol Genet. 2001;10:231–236. doi: 10.1093/hmg/10.3.231. [DOI] [PubMed] [Google Scholar]
- Semina EV, Ferrell RE, Mintz-Hittner HA, Bitoun P, Alward WL, Reiter RS, Funkhauser C, Daack-Hirsch S, Murray JC. A novel homeobox gene PITX3 is mutated in families with autosomal- dominant cataracts and ASMD. Nat Genet. 1998;19:167–170. doi: 10.1038/527. [DOI] [PubMed] [Google Scholar]
- Semina EV, Murray JC, Reiter R, Hrstka RF, Graw J. Deletion in the promoter region and altered expression of Pitx3 homeobox gene in aphakia mice. Hum Mol Genet. 2000;9:1575–1585. doi: 10.1093/hmg/9.11.1575. [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter RS, Murray JC. Isolation of a new homeobox gene belonging to the Pitx/Rieg family: expression during lens development and mapping to the aphakia region on mouse chromosome 19. Hum Mol Genet. 1997;6:2109–2116. doi: 10.1093/hmg/6.12.2109. [DOI] [PubMed] [Google Scholar]
- Shi X, Luo Y, Howley S, Dzialo A, Foley S, Hyde DR, Vihtelic TS. Zebrafish foxe3: Roles in ocular lens morphogenesis through interaction with pitx3. Mech Dev. 2006;123:761–782. doi: 10.1016/j.mod.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Swindell EC, Zilinski CA, Hashimoto R, Shah R, Lane ME, Jamrich M. Regulation and function of foxe3 during early zebrafish development. Genesis. 2008;46:177–183. doi: 10.1002/dvg.20380. [DOI] [PubMed] [Google Scholar]
- Ton CC, Hirvonen H, Miwa H, Weil MM, Monaghan P, Jordan T, van Heyningen V, Hastie ND, Meijers-Heijboer H, Drechsler M, et al. Positional cloning and characterization of a paired box- and homeobox- containing gene from the aniridia region. Cell. 1991;67:1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- Valleix S, Niel F, Nedelec B, Algros MP, Schwartz C, Delbosc B, Delpech M, Kantelip B. Homozygous Nonsense Mutation in the FOXE3 Gene as a Cause of Congenital Primary Aphakia in Humans. Am J Hum Genet. 2006;79:358–364. doi: 10.1086/505654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum DS, Stevens LC. Aphakia, a new mutation in the mouse. J Hered. 1968;59:147–150. doi: 10.1093/oxfordjournals.jhered.a107667. [DOI] [PubMed] [Google Scholar]
- Wei Y, Yu L, Bowen J, Gorovsky MA, Allis CD. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell. 1999;97:99–109. doi: 10.1016/s0092-8674(00)80718-7. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Whole mount in situ hybridization of vertebrate embryos. Oxford: Oxford University Press; 1992. pp. 75–83. [Google Scholar]
- Wistow G, Piatigorsky J. Recruitment of enzymes as lens structural proteins. Science. 1987;236:1554–1556. doi: 10.1126/science.3589669. [DOI] [PubMed] [Google Scholar]
- Yoshimoto A, Saigou Y, Higashi Y, Kondoh H. Regulation of ocular lens development by Smad-interacting protein 1 involving Foxe3 activation. Development. 2005;132:4437–4448. doi: 10.1242/dev.02022. [DOI] [PubMed] [Google Scholar]
- Zwaan J. Immunofluorescent studies on aphakia, a mutation of a gene involved in the control of lens differentiation in the mouse embryo. Dev Biol. 1975;44:306–312. doi: 10.1016/0012-1606(75)90401-7. [DOI] [PubMed] [Google Scholar]
- Zwaan J, Kirkland BM. Malorientation of mitotic figures in the early lens rudiment of aphakia mouse embryos. Anat Rec. 1975;182:345–354. doi: 10.1002/ar.1091820308. [DOI] [PubMed] [Google Scholar]