Abstract
Objective
Vasa vasorum (VV) neovascularization is a key feature of early atherosclerosis and adds substantial endothelial exchange-surface to the coronary vessel wall. Thus, it is conceivable that VV neovascularization favors the entry of pro-inflammatory and pro-atherosclerotic blood components into the coronary vessel wall. We sought to investigate the effects of Thalidomide (Th) a potent anti-angiogenic drug on vasa vasorum (VV) neovascularization, vessel wall inflammation and neointima formation in early experimental atherosclerosis.
Methods and Results
Female domestic swine, 3-months old, were fed normal (N, n=12) or high-cholesterol diet (HC, n=12) for 3 months. In each group six pigs were randomized to 200 mg Thalidomide daily for the diet period (N+Th, HC+Th). LADs were scanned with micro-CT (20μm cubic voxel size) to determine VV spatial density (#/mm²). Fresh-frozen coronary tissue was used for Western Blotting (VEGF, TNF-α , LOX-1, IkBα and Gro-α ) and electrophoretic mobility shift assay (EMSA, NFkB). Treatment with Thalidomide preserved VV spatial density (2.7±0.3 (N), 6.4±0.7 (HC), 3.5±0.8 (HC+Th); p=ns HC+Th vs. N) and inhibited the expression of VEGF, TNF-alpha and LOX-1 but not NFkB activity in the coronary vessel wall. Immunofluorescense analyses revealed co-localization of vWF but not SMA and NFkB, TNF-α as well as VEGF in HC and HC+Th coronaries. Intima-media thickness was significantly inhibited in HC+Th compared to HC. Serum levels of hs-CRP and TNF-α did not differ among the groups.
Conclusions
Our study supports a role of VV neovascularization in the development of and a therapeutic potential for anti-angiogenic intervention in early atherosclerosis.
Keywords: Vasa vasorum, early atherosclerosis, micro-CT, neovascularization, inflammation
Introduction
It is widely recognized that atherosclerosis is an inflammatory disease [36, 43]. The immediate proximity of vasa vasorum to inflammatory infiltrates and the expression of adhesion molecules strongly suggests that vasa vasorum serve as conduits for the influx of cellular and non-cellular, pro-inflammatory and thus pro-atherogenic blood components into the vessel wall [28, 33]. The clinical relevance of vasa vasorum neovascularization is underscored by recent findings from human pathohistological studies showing that vasa vasorum rupture and intraplaque hemorrhage may lead to plaque destabilization by accumulating macrophages and enlarging the lipid/necrotic core [23]. Thus, there is a growing body of evidence that vasa vasorum neovascularization plays a significant role in the progression and complications of atherosclerosis.
In the porcine model of experimental hypercholesterolemia we have previously shown that coronary vasa vasorum neovascularization occurs even prior to the development of histologically manifested coronary atherosclerosis and is associated with an increase in the local and systemic angiogenic cytokines like the vascular endothelial growth factor (VEGF) [18, 24]. This observation suggests that vasa vasorum neovascularization plays an important pathophysiological role also in the initiating stages of atherogenesis likely by serving as a conduit for the influx of pro-atherogenic and pro-inflammatory blood components into the coronary vessel wall.
Thalidomide is an orally available drug with potent anti-angiogenic properties independent from immunological effects as demonstrated by D’Amato et al. [5] However, it has been demonstrated that Thalidomide may also exhibit anti-inflammatory effects, particularly by its anti-tumor necrosis factor-alpha (TNF-alpha) activity [1]. TNF-alpha, an inflammatory cytokine, has been suggested to be involved in the development of early atherosclerosis by up-regulating vessel wall chemokine and adhesion molecules expression as well as augmenting medial smooth muscle cell proliferation and migration [4, 34, 39, 44].
With the current study we sought to test the hypothesis that the prevention of coronary vasa vasorum neovascularization and vessel wall inflammation through chronic administration of Thalidomide results in inhibition of early neointima formation in the porcine model of hypercholesterolemia-induced early atherosclerosis. In contrast to prior studies, evaluating the role of neovascularization in advanced atherosclerotic lesions, the current study, thus, particularly sought to elucidate the role of neovascularization in the earliest stages of atherogenesis, prior to significant histological plaque development.
Methods
Animal Experiments and Specimen Acquisition
All animal studies were approved by the Mayo Foundation’s Institutional Animal Care and Use Committee. A control group (N) of twelve female domestic crossbred swine (3 months old) were fed normal laboratory chow for three months. Twelve other female pigs (3 months old) were fed a high cholesterol diet (15% lard, 2% Cholesterol; TD 93296, Harlan Teklad, Madison, WI) for three months (HC group) [15, 20, 24]. Six animals of each group were randomized to receive Thalidomide (4 mg/kg/day, Celgene Co.; NJ, gift from Celgene) orally for the duration of the diet (HC+Th and N+Th). This dose showed no major side effects in previously studied animals [26] and has been shown to cause a decrease in bone marrow microvascular density as the recommended dose for treatment of multiple myeloma [6]. All harvested hearts were prepared for micro-CT scanning as described previously [13, 14]. This involved injection of radiopaque Microfil® polymer into the coronary arteries prior to dissecting out five to ten cm long, intact, left anterior descending coronary artery segments.
Micro-Computed Tomography 3D Reconstruction
The arterial segments were scanned by a micro-CT system consisting of a spectroscopy x-ray tube, a fluorescent crystal plate (which converted the x-ray image to a light image), a microscope objective lens and a charge coupled device (CCD) camera [16, 22]. To preserve the connectivity of the vasa vasorum, the arteries were scanned in contiguous 2-cm increments along the entire coronary artery luminal axis without physically cutting the coronary artery into pieces. For this study the micro-CT scanner was configured so that the dimension of the cubic voxels was 21 μm (16-bit gray scale). The resulting 3D images were displayed using image analysis software (Analyze® 7.0™; Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). Computer-generated displays of these 3D images were generated to provide different angles of view.
Western Blot Analysis
Samples of epicardial coronary artery were freshly frozen in liquid nitrogen and homogenized in lysis buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1% Triton-X, 10% glycerol, 2 μg/ml aprotinin, 1 mM PMSF) using a tissue homogenizer[40]. The lysate protein content was analyzed by Bradford assay (Bio-Rad, CA). Equal amounts of protein were diluted in 4x reducing loading buffer and boiled. Samples were then resolved in 8% SDS–polyacrylamide gels. Immunoblotting was performed using a monoclonal antibody anti-VEGF (1:250, Novus Biologicals, MO), polyclonal antibodies anti-TNF-α (1:100, Sigma-Aldrich, MO), anti-p-Iκ B-α (Ser 32, Santa Cruz, CA, 1:200), GRO-α (C-15, Santa Cruz, CA, 1:200) and anti-LOX-1 (1:100, Santa Cruz, CA) in a non-fat milk/Tris buffer; membranes were exposed to secondary antibodies, anti-mouse (1:200 – 1:2500; Amersham Pharmacia Biotech, NJ), donkey anti-goat IgG-HRP (1:2000, Santa Cruz, CA) or anti-rabbit (1:1000 – 1:5000; BD Biosciences, CA) conjugated to horseradish peroxidase, as appropriate. After developing with chemiluminescence (Pierce, IL)[40], membranes were exposed to X-ray film (Kodak, NY). Signals were evaluated for integrated density using ImageJ (National Institutes of Health); β-Actin (1:5000, Sigma-Aldrich, MO) was used as the loading control.
Electrophoretic mobility shift assay (EMSA)
EMSA was performed as previously described [3]. Coronary artery tissue, carefully cleaned from blood and the adventitia, was homogenized in a buffer of the following composition: N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES) 10 mmol/L (pH 7.8), KCl 15 mmol/L, MgCl2 2 mmol/L, ethylenediaminetetraacetic acid (EDTA) 0.1 mmol/L, dithioreitol (DTT) 1 mmol/L, and phenylmethylsulfonyl fluoride (PMSF) 1 mmol/L. The homogenate was centrifuged at 4000x g at 4° C for 10 min. The pellet was resuspended in a homogenization buffer (similar to the above for a KCl concentration of 0.39 mol/L) at 4°C for 1 hour. The probe was subsequently ultracentrifuged at 100,000x g for at 4° C 30 minutes. The supernatant was dialyzed at 4° C overnight in a buffer of the following composition: HEPES 50 mmol/L (p.H. 7.8), KCl 50 mmol/L, EDTA 0.1 mmol/L, DTT 1 mmol/L, PMSF 1 mmol/L, and 10% glycerol. The dialyzed supernatant was analyzed for protein concentration by a Coomassie assay and stored at −80°C for gel shift assays. These were performed as two competition assays [unlabeled specific (NF B) and nonspecific (AP1) consensus competitor oligos] with a commercially available kit (Promega, Madison, WI, USA). Nuclear extracts from HeLa cells (Promega) served as positive control samples to assure technical quality of the assay. Probes of samples of coronary arteries (5 μg) and HeLa cells (6 μg) were loaded to 4% Acrylamide, 60:1 Acrylamide:Bisacrylamide gels in 0.5x TBE buffer and run for 10 minutes at 350 V at 4°C. Following electrophoresis, gels were placed on filter paper, dried, and exposed to an x-ray film (Kodak) with an intensifying screen at −70°C overnight. X-ray films were developed using an automated film-developing machine. The optical density of the NFkB gel shift band was measured using NIH Image and expressed as the difference between non-specific and specific competitors. The setup for each assay was the same, i.e. one probe from each group was examined per gel. The average radioactive incorporation rate for NFkB labeling was 50%.
Lawson’s Elastic Van Gieson and Hematoxylin and Eosin Staining
After micro-CT scanning, the scanned tissue sections were dehydrated and embedded in paraffin for sectioning. Cross-sections were then deparaffinized and rehydrated in graded concentrations of ethanol, and subsequently submerged in hematoxylin-ferric chloride solution for 24 hours [14]. Following exposure, the tissue sections were washed for 5 minutes in H2O and submerged in 0.08% acid fuchsin (Sigma, St Louis, MO) in saturated aqueous picric acid (Sigma, St Louis, MO) for 4 minutes. The tissue sections were differentiated in methanol for 10 seconds, dehydrated in acetone for 10 seconds, and cleared in xylene prior to mounting. H&E stains were performed using a standard histology protocol. Histological analyses were performed at the proximal left anterior descending artery of all animals.
Immunofluorecence
To specify the locations of NFKβ p65, VEGF and TNFα expressions, we carried out double immunofluorescence staining of these markers with smooth muscle actin (SMA) and von Willebrand factor (vWF). Four to five μm thick pig coronary artery paraffin sections were used in the study. Sections were deparaffinized in xylene and alcohol gradient, followed by incubating sections in boiling Dako target retrieval buffer (S1700, Dako, Carpinteria, CA) for 20 min. After cooling down, sections were rinsed in phosphate buffered saline (PBS) for 3x for 5 min, incubated with 10% normal goat serum for 1 hour at room temperature and primary antibodies at 4°C overnight. The primary antibodies were diluted in 5% normal goat serum, the dilutions of the primary antibodies were: NFKB p65 1:50 (ab31481, abcam, Cambridge, MA), VEGF 1:50 (07-1420, Millipore), TNFα 1:100 (ab6671, abcam, Cambridge, MA), SMA 1:400 (M0851, Dako Cytomation) and vWF 1:100 (ab68545, abcam, Cambridge, MA). Sections were then rinsed in PBS 3x for 5min each and incubated with goat anti-rabbit Cy3 (AP132C, Millipore) or goat anti-mouse Alexa (A11001, Invitrogen, Eugene, Oregon) secondary antibodies at 1:200 dilutions for 3 hours at room temperature. After rinsing, sections were mounted with UltraCruz mounting medium (SC-24941, Santa Cruz Biotechnology), viewed and photographed under a LSM 510 confocal microscope.
Systemic TNF-alpha and high-sensitive CRP concentrations
Serum levels of TNF-alpha were measured using a quantitative sandwich enzyme immunoassay technique (Quantikine®, R&D, Minneapolis, MN). High-sensitive CRP concentrations (hs-CRP) were quantified by the Mayo Clinic Clinical Laboratory using a latex particle-enhanced immunoturbidimetric assay.
Data Analysis
Micro-CT
In each micro-CT cross-section, vessel wall area (defined by the outer border of the adventitia) was determined as described in detail before [10, 14, 18, 19]. In brief, distinctive density differences between the adventitia and the periadventitial lipid tissue create a “halo” in the micro-CT cross-sections which closely correlates with the outer adventitial border in conventional histology [14]. Vasa vasorum were manually identified and counted in this vessel wall area to calculate vasa vasorum density (i.e., vasa vasorum per mm² vessel wall area). By scanning through the 3D data set (1000 reconstructed, contiguous, cross-sections per 2-cm of scanned coronary artery) we were able to differentiate vasa vasorum from other vessels that may have a comparable dimension but do not stay within the vascular wall (e.g., intra myocardial branches) and were able to follow each vessel from origin to destination. Moreover, the vasa vasorum vascular area fraction (i.e., the sum of cross-sectional areas of all vasa vasorum per cross-section, divided by the vessel wall area), which represents the vascular area available to flow through vasa vasorum, as well as the sum of vasa vasorum endothelial surface area per mm³ vessel wall area (vasa vasorum endothelial surface fraction) were calculated [16]. Branching points were excluded from analysis.
Histology
Elastic van Gieson stained slides were analyzed for intima-media thickness as described previously [14].
Statistical Analysis
Quantitative data are presented as mean ± SEM for all arteries. Data were analyzed using one-way ANOVA followed by a Bonferroni post-hoc test to establish differences among groups (SPSS 12.0.1 software). A value of P < 0.05 was considered significant in all analyses.
Results
Animals
Body weights and systemic hemodynamics were not different between the groups (see Table 1). Animals on high-cholesterol diet with or without Thalidomide treatment had significant higher lipid levels than the control animals (Table 1).
Table 1.
Hemodynamic data, lipid status, and serum-TNF-alpha and hs-CRP levels (measured at the end of the study).
| Normal (n=6) | High- Cholesterol (n=6) | High- Cholesterol + Thalidomide (n=6) | Normal + Thalidomide (n=6) | |
|---|---|---|---|---|
| MAP (mmHg) | 114±4 | 113±6 | 102±10 | 102±6 |
| Body weight (kg) | 59±4 | 61±3 | 53±2 | 57±3 |
| Total Cholesterol (mg/dL) | 86±3 | 356±48 † | 351±47 † | 87±9 |
| LDL (mg/dL) | 47±7 | 261±42 † | 251±38 † | 50±6 |
| HDL (mg/dL) | 35±4 | 87±7 † | 96±12 † | 33±3 |
| Triglycerides (mg/dL) | 21±3 | 38±6 $ | 21±4 | 17±4 |
| Serum TNF- alpha (pg/mL) | 54±4 | 59±7 | 55±11 | 48±7 |
| Serum hs- CRP (mg/dL) | 0.045±0.012 | 0.040±0.005 | 0.032±0.005 | 0.044±0.007 |
Data are presented in Mean±SEM, p values in Table 2 legend.
Micro-CT Analysis – Vasa Vasorum Parameters
Vasa vasorum density was significantly highest in the group of animals on high-cholesterol diet alone. Chronic administration of Thalidomide during high-cholesterol diet prevented vasa vasorum neovascularization (Figure 1, Table 2). Likewise, hypercholesterolemic animals also had significantly higher values of vascular volume fraction and endothelial surface fraction as well as higher vasa vasorum/luminal endothelial surface ratios than control animals. In comparison, animals on high-cholesterol diet plus administration of Thalidomide showed marked attenuation of these vasa vasorum parameters (Table 2).
Figure 1.
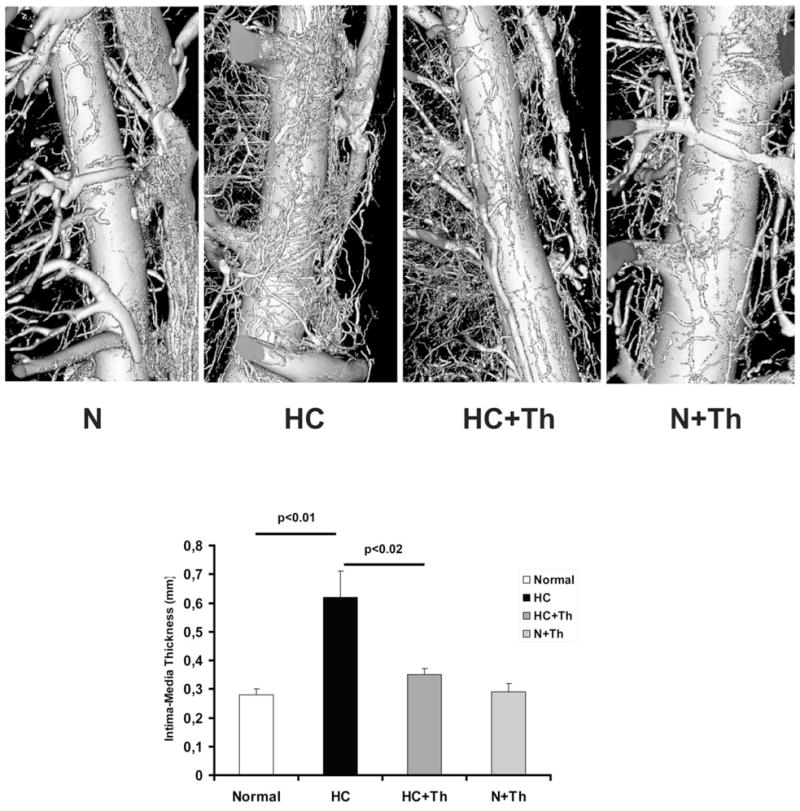
Upper panel: Volume rendered micro-CT images of coronary arteries and their vasa vasorum. Coronaries from hypercholesterolemic (HC) animals exhibit significant vasa vasorum neovascularization in comparison to coronaries from normal (N) and Thalidomide treated animals (HC+Th and N+Th). The lower panel shows a bar graph of the histologically quantified intima-media thickening in all four groups. Chronic administration of Thalidomide prevented intima-media thickening in the hypercholesterolemic animals.
Table 2.
Vasa Vasorum parameters (normalized to coronary lumen radius) and histological measurements.
| Normal (n=6) | High- Cholesterol (n=6) | High- Cholesterol +Thalidomide (n=6) | Normal + Thalidomide (n=6) | |
|---|---|---|---|---|
| VV Density (n/mm²) | 2.7±0.3 | 6.4±0.7 *,# | 3.5±0.8 | 3.8±0.9 |
| VAF (%) | 0.7±0.2 | 1.6±0.2 § | 1.0±0.3 | 1.2±0.2 |
| ESF (mm²/mm³) | 43±5 | 104±9 § | 62±18 | 64±13 |
| Ratio VV Endothelial Surface/Luminal Endothelial Surface | 24±3% | 61±7% § | 40±12% | 41±7% |
| Intima-Media Thickness (mm) | 0.28±0.02 | 0.62±0.09 †,# | 0.35±0.02 | 0.29±0.03 |
Data are presented in Mean±SEM.
VAF=vascular area fraction, ESF=endothelial surface fraction
p<0.01 vs. Normal
p<0.05 vs. High-Cholesterol + Thalidomide
p<0.05 vs. Normal
p ≤ 0.001 vs. Normal and Normal + Thalidomide
p<0.05 vs. all other groups
Histology — Intima-Media Thickness
Changes in intima-media thickness are reported in Table 2. There was a significant reduction in intima-media thickness in the hypercholesterolemic pigs treated with Thalidomide (Figure 2).
Figure 2.
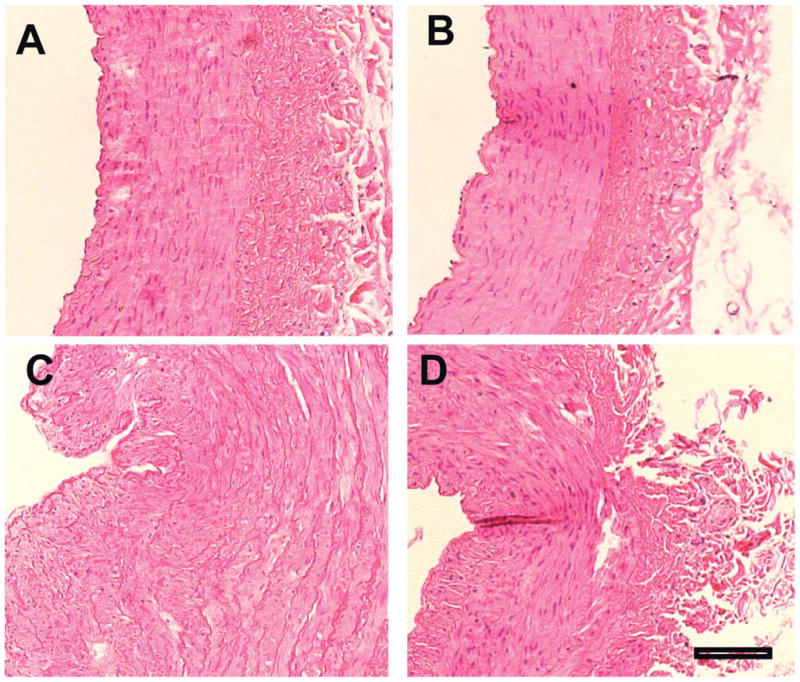
Histology (H&E stains, representative examples from each study group). A. Normal, B. Normal + Thalidomide, C. Hypercholesterolemia, D. Hypercholesterolemia plus Thalidomide. Treatment with Thalidomide significantly inhibited intima-media thickening in hypercholesterolemic coronary arteries (D) compared to animals on hypercholesterolemic diet alone (C). Scale: 100μm.
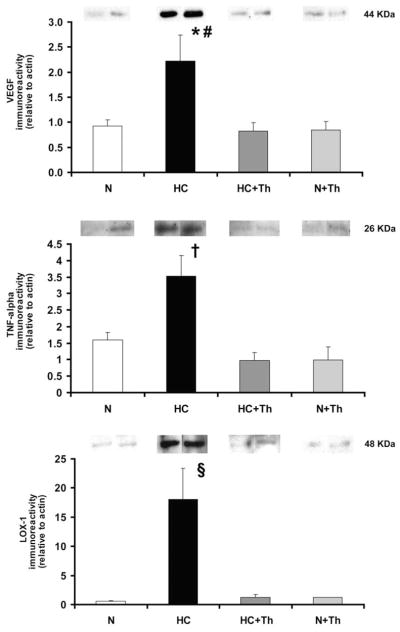
Western Blot Analysis – VEGF, TNF-α , Gro-α , p-IkBα and LOX-1
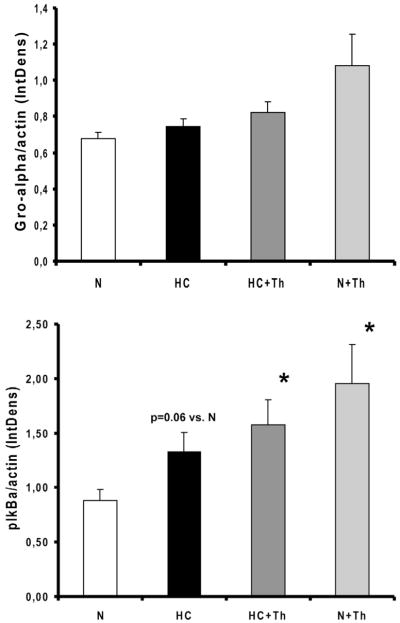
In hypercholesterolemic animals the protein expression of VEGF in the coronary vascular wall was significantly increased compared to the normal groups. The chronic administration of Thalidomide normalized the expression of VEGF in the HC group (Figure 3). Thalidomide administration also normalized the protein expression of the inflammatory marker TNF-α in the coronary vascular wall (Figure 3), which was significantly highest in the high-cholesterol diet only group. The expression of LOX-1, the oxidized LDL receptor, was significantly higher in the high-cholesterol group alone and was also normalized in the high-cholesterol group that received Thalidomide (Figure 3). The results for the phosphorylated nuclear factor of kappa light chain gene enhancer in B cells inhibitor alpha (p-IkBα ) and the growth related oncogene-alpha (Gro-α ) are shown in Figure 4. The expression of p-IkBα was significantly higher in the HC, HC+Th and N+Th groups compared to the control animals (p=0.06 N vs. HC, p<0.05 N vs. HC+Th and N+TH, Figure 4, upper panel). There was no statistical significant difference in Gro-α expression between the groups (Figure 4, lower panel).
Figure 3.
Western blot analysis of the local expression of VEGF, TNF- α , and the receptor of oxidized LDL (LOX-1) in coronary artery tissue.
Thalidomide treatment significantly decreased the expression of VEGF (top panel) and TNF-α (mid panel) in the coronary vessel wall. In addition, the expression of the LOX-1 receptor was significantly blunted (bottom panel). * p<0.05 vs. N, # p< 0.02 vs. HC+Th/N+Th (in upper panel), † p<0.001 vs. N and HC+Th/N+Th (in mid panel), § p<0.01 vs. N and HC+Th/N+Th (in lower panel).
Figure 4.
Upper panel. Western Blot analysis of phosphorylated inhibitory kappB kinase-alpha (IkB-alpha, n=4 in all groups) showed increased expression in HC, HC+Th and N+Th animals (* p<0.05).
Lower panel. Western Blot analysis of growth related oncogene protein-alpha (Gro-alpha, n=4 in N, HC and N+Th and n=5 in HC+Th) demonstrated no significant differences between all groups (N vs. N+Th, p=0.06).
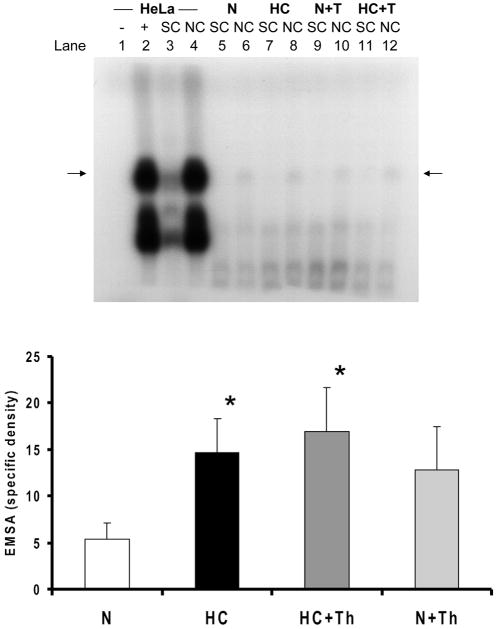
Electrophoretic mobility shift assay (EMSA)
EMSA for NFkB showed increased nuclear translocation of this transcription factor in coronary arteries of HC animals compared with N animals (Figure 5). Similarly, NFkB EMSA bands were more intense in Thalidomide-treated (Th) animals than in N animals (Figure 5).
Figure 5.
Upper panel shows a representative electrophoretic mobility shift assay (EMSA) for NFkB on HeLa cells (first 3 lanes, lane 1 without tissue) and coronary artery samples (lanes 5–12, SC = specific competitor, NS = non-specific competitor). Lower panel highlights the quantitative results as the difference between non-specific and specific competitor. Compared with normal controls (N), nuclear translocation of NFkB is increased in coronary arteries from animals on a high-cholesterol diet without (HC) or with thalidomide treatment (HC+T) for 12 weeks as well as in animals on a normal diet with thalidomide treatment (N+T) for 12 weeks (*p<0.05 vs. N).
Immunofluorescense – VEGF, NFkB, TNF-α , SMA and vWF
To identify cells that express VEGF, NFkB, and TNF-α as detected with Western Blotting we performed immunofluorescense analyses. Co-staining with SMA (identifying vascular smooth muscle cells) showed no co-localization of SMA with VEGF, NFkB, or TNF-α . However, immunofluorescense analysis demonstrated co-localization of vWF (identifying vasa vasorum) with VEGF, NFkB, and TNF-α (Figure 6). Looking at all four study groups separately revealed that in N and N+Th we were unable to detect staining for TNF-α or NFkB and consequently no co-localization with vWF. We found mild staining for VEGF and co-localization of VEGF with vWF in the normal plus Thalidomide group. In the HC group we found expression of NFkB, VEGF and TNF-α and co-localization with vWF. Interestingly, we found the same results in the HC+Th group (Figure 7).
Figure 6.
Co-localization of NFkB p65, VEGF and TNF-α with vWF. A, D, G are NFkB p65, VEGF, and TNF-α, respectively. B, E, and H are vWF. C, F, I are overlays of A and B, D and E, and G and H. Scale: 50μm.
Figure 7.
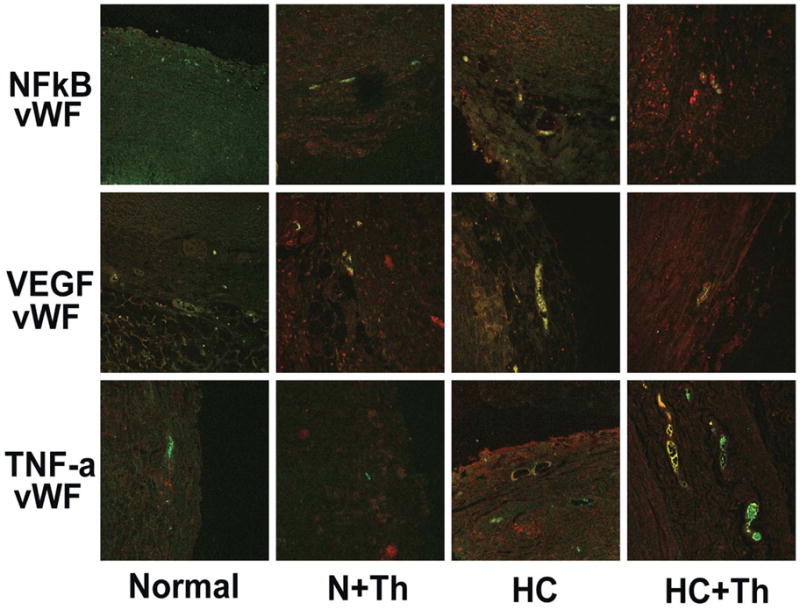
Co-localization of each NFkB, VEGF and TNF-α with vWF. Except for VEGF-vWF in the N+Th group there was no further co-localization found in the N and N+Th groups. Both HC and HC+Th groups showed co-localization of all four markers.
Serum Levels of TNF-alpha and high-sensitivity CRP
Serum levels of TNF-alpha and high-sensitivity CRP (hs-CRP) did not differ significantly among the four groups (Table 1).
Discussion
The current study shows that chronic treatment with Thalidomide inhibits both vasa vasorum neovascularization and neointima formation in the porcine model of hypercholesterolemia-induced early atherosclerosis. Although our study design does not allow showing direct causality, our results support a seminal role of coronary vasa vasorum neovascularization during the early initiating phases of atherogenesis. Vasa vasorum neovascularization provides entry ports and increased endothelial exchange surface for delivery of pro-inflammatory and pro-atherosclerotic blood components into the coronary vessel wall [8, 15, 32]. It is conceivable that Thalidomide’s anti-angiogenic effect on vasa vasorum neovascularization contributed to the reduction of the endothelial exchange surface for pro-inflammatory substances within the vessel wall. We observed an expected, concomitant inhibition of local TNF-alpha expression in the coronary vessel wall via the direct anti-TNF alpha effect of Thalidomide. These findings corroborate previous reports indicating a role of TNF-alpha in the development of early atherosclerosis [4, 35].
Treatment with Thalidomide led to a significant reduction of VEGF expression in the coronary vessel wall. Moreover, Thalidomide treated animals showed significantly higher nuclear translocation of NFkB than control animals, comparable to that of hypercholesterolemic animals alone. Similarly, the growth regulated oncogene-alpha (Gro-alpha) which has been identified as an angiogenesis factor of the NFkB pathway[2] was not inhibited by chronic Thalidomide treatment. Taken together these data indicate that in our model the anti-angiogenic effect of Thalidomide on vasa vasorum angiogenesis was likely mediated through inhibition of VEGF production, corroborating results from earlier in-vitro studies [25, 37]. Moreover, inhibition of local TNF-alpha production and not inhibition of NFkB activity is the predominant anti-inflammatory effect of Thalidomide in this animal model, which is supported by previous studies [1, 4, 42]. Thus, both, prevention of vasa vasorum neovascularization and reduction of local TNF-alpha production led to the observed blunting of neointima formation; which of the two had the main impact cannot be fully elucidated by the current study design. The fact, however, that Thalidomide treatment did not change systemic levels of TNF-alpha indicates that the reduction of local TNF-alpha expression within the coronary vessel wall cannot be explained by Thalidomide action alone but may be in part a result of the prevention of vasa vasorum neovascularization.
Using immunofluorescense analyses we found that in hypercholesterolemia the inflammatory markers NFkB and TNF-alpha are expressed by the endothelial cell lining of the vasa vasorum, not by vascular smooth muscle cells. Interestingly, although Western Blotting showed a significant reduction of TNF-alpha expression in HC+Th animals, TNF-alpha and NFkB were still detectable in the endothelial cell lining of their coronary vasa vasorum. Thus, Thalidomide treatment prevented vasa vasorum neovascularization but not early inflammatory changes of the vasa vasorum endothelial cells. This important finding further supports the crucial role of vasa vasorum as entry ports and possible starting points of early atherosclerosis. A significant reduction of the number of entry ports (i.e. vasa vasorum) leads to a delay of early atherosclerosis despite the presence of a pro-inflammatory and -atherosclerotic risk factor like hypercholesterolemia.
Histopathological studies have shown that vasa vasorum neovascularization is a key feature of advanced human atherosclerotic lesions [7, 29]. Indeed, a recent study suggests that vasa vasorum rupture/hemorrhage may play a role in the growth of the lipid-core and thus the progression of an atherosclerotic lesion towards plaque rupture [23]. Others have shown in an animal model of ApoE−/− knock-out mice that vasa vasorum neovascularization is a key feature of advanced atherosclerotic lesions. There the inhibition of vasa vasorum neovascularization leads to a reduction of advanced aortic plaque progression and the number of infiltrating macrophages [31, 32]. Therefore, the role of vasa vasorum in advanced atherosclerosis continues to emerge.
The current study extends those previous observations made in advanced atherosclerotic lesions to the initiating phase of the atherosclerotic process and provides a potential mechanism in that the inhibition of early coronary vasa vasorum neovascularization may lead to the attenuation of early atherogenesis, i.e. neointimal proliferation. Vasa vasorum are anatomically connected to the coronary artery lumen (vasa vasorum externa and interna [14]) or to concomitant veins (venous vasa vasorum [14]). It is, hence, conceivable that vasa vasorum serve as conduits for the influx of pro-inflammatory and pro-atherosclerotic cellular and non-cellular blood components into the outer two thirds of the coronary vessel wall (also comprehensively discussed in [30]). Indeed, we have shown recently, using cryogenic micro-CT, that vasa vasorum play a significant role in the perfusion and drainage of the porcine coronary vessel wall [12]. With that study we also demonstrated that hypercholesterolemia leads to a significant loss of integrity of the endothelial cell coverage at the main lumen’s as well as the vasa vasorum’s endothelium. In addition, studies on ApoE−/− knock-out mice have demonstrated that the adventitia is the major site of immune cell accumulation [28] and in animals with existing advanced lesions the inhibition of periaortic neovascularization reduced aortic plaque progression and the accumulation of macrophages [32]. Our group has previously demonstrated in a similar animal model that in coronaries vasa vasorum neovascularization is present before the development of the early functional abnormalities of early atherosclerotic endothelial dysfunction and early atherosclerotic lesions [19, 24]. Furthermore, the preservation of coronary endothelial dysfunction was associated with attenuation of the vasa vasorum neovascularization [18, 41]. Hence, previous studies in this animal model underscore the role of vasa vasorum neovascularization particularly in early atherogenesis.
The Lectin-like Oxidized low-density lipoprotein scavenger receptor (LOX-1) whose expression is upregulated upon recognition of oxidized low-density lipoprotein (oxLDL, comprehensively reviewed in [38]) was significantly reduced in the vessel wall of our hypercholesterolemic animals treated with Thalidomide. This may indicate that the inhibition of vasa vasorum neovascularization successfully prevented the uptake of oxidized LDL into the vessel wall, an important mechanism for vessel wall inflammation, since the binding of oxLDL to its receptor LOX-1 has been identified as a potent pro-inflammatory pathway [27]. Hence, we may speculate that vasa vasorum neovascularization serves as a conduit for the influx of blood components (like oxLDL) that promote and nourish vessel wall inflammation, and thus early atherogenesis. It is conceivable that the inhibition of this influx leads to a significant delay of atherogenesis. The inhibition of vasa vasorum neovascularization in our current study led to a significant reduction of vascular area fraction (as an indicator of flow capacity [16]) and endothelial exchange surface within the coronary vessel wall [15]. Thus, there is less interaction between potential pro-atherogenic factors and the vessel wall layers of adventitia and media which both depend on vasa vasorum perfusion. Moreover, the lack of the formation of fragile blood vessels secondary to reduction of VEGF production and the anti-TNF-alpha effect of Thalidomide may also contribute to an attenuation of leakage of substances from the vasa vasorum and thus contribute to the observed inhibition of neointima proliferation [9, 17, 21].
Glagov et al. have determined that the critical vessel wall depth above which nourishment of cells within the vessel wall is no longer accomplished by diffusion from the main lumen only is approximately 300μm [11]. For this reason larger arteries, like normal porcine and human coronary arteries, have vasa vasorum while they are largely missing in arteries from smaller animal species like mice, rats and rabbits. This anatomical similarity to human blood vessels underscores the rational and necessity to study the role of vasa vasorum neovascularization in atherosclerosis in relatively large animal models, since the results from these studies may be transferable to human pathology.
Limitations
Our current study design does not allow complete differentiation of the anti-angiogenic and local anti-TNF-alpha effects of Thalidomide on neointima formation in early atherosclerosis. Previous investigations, have demonstrated the anti-angiogenic impact of Thalidomide independent of its immunologic effects [5]. Hence, we may conclude that the preservation of the normal vasa vasorum density is of vital importance for the here observed inhibition of early neointima formation. Even accepting that direct local anti-inflammatory effects may have contributed does not diminish the conclusion of this study, namely the fact that prevention of vasa vasorum neovascularization and inhibition of vessel wall inflammation may delay or even prevent early atherogenesis. The study was not designed to assess the underlying molecular mechanism of Thalidomide’s inhibition of vasa vasorum neovascularization.
Our group has shown in several previous publications that the assessment of vasa vasorum density with micro-CT is accurate in one-to-one comparison with immunostaining for endothelial cell markers. The clear advantage of 3D micro-CT over histological section analysis, however, is that we include only functional vessels in our calculations, i.e. vessels that show continuity/connectivity in the 3D analysis, and thus, are still perfused. We therefore did not assess the microvasculature with conventional histological analyses.
Conclusions and Perspectives
The current study shows that the chronic treatment with Thalidomide prevents coronary vasa vasorum neovascularization, reduces local TNF-alpha production and inhibits neointima formation in the porcine model of hypercholesterolemic early atherosclerosis. These findings expand previous findings in advanced atherosclerosis to the early initiating phases of coronary atherosclerosis and underline the seminal role of coronary vasa vasorum neovascularization by providing entry ports for the influx of pro-inflammatory and pro-atherosclerotic substances into the coronary vessel wall. The early inhibition of vasa vasorum neovascularization with systemic or local anti-inflammatory treatment may thus be a potential therapeutic target for the inhibition of the development of early coronary atherogenesis.
Acknowledgments
This study was in part funded by the NIH grant numbers HL77131 and DK73608. The authors want to thank Darrell L. Loeffler, Monica L. Olson and James D. Krier, MS, for their help with the animal experiments.
References
- 1.Aydogan S, Celiker U, Turkcuoglu P, Ilhan N, Akpolat N. The effect of thalidomide on vascular endothelial growth factor and tumor necrosis factor-alpha levels in retinal ischemia/reperfusion injury. Graefes Arch Clin Exp Ophthalmol. 2008;246:363–368. doi: 10.1007/s00417-007-0663-9. [DOI] [PubMed] [Google Scholar]
- 2.Caunt M, Hu L, Tang T, Brooks PC, Ibrahim S, Karpatkin S. Growth-regulated oncogene is pivotal in thrombin-induced angiogenesis. Cancer Res. 2006;66:4125–4132. doi: 10.1158/0008-5472.CAN-05-2570. [DOI] [PubMed] [Google Scholar]
- 3.Chade AR, Best PJ, Rodriguez-Porcel M, Herrmann J, Zhu X, Sawamura T, Napoli C, Lerman A, Lerman LO. Endothelin-1 receptor blockade prevents renal injury in experimental hypercholesterolemia. Kidney Int. 2003;64:962–969. doi: 10.1046/j.1523-1755.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- 4.Chew M, Zhou J, Daugherty A, Eriksson T, Ellermann-Eriksen S, Hansen PR, Falk E. Thalidomide inhibits early atherogenesis in apoE-deficient mice. APMIS. 2003;(Suppl):113–116. [PubMed] [Google Scholar]
- 5.D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dmoszynska A, Podhorecka M, Manko J, Bojarska-Junak A, Rolinski J, Skomra D. The influence of thalidomide therapy on cytokine secretion, immunophenotype, BCL-2 expression and microvessel density in patients with resistant or relapsed multiple myeloma. Neoplasma. 2005;52:175–181. [PubMed] [Google Scholar]
- 7.Fleiner M, Kummer M, Mirlacher M, Sauter G, Cathomas G, Krapf R, Biedermann BC. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation. 2004;110:2843–2850. doi: 10.1161/01.CIR.0000146787.16297.E8. [DOI] [PubMed] [Google Scholar]
- 8.Foell D, Hernandez-Rodriguez J, Sanchez M, Vogl T, Cid MC, Roth J. Early recruitment of phagocytes contributes to the vascular inflammation of giant cell arteritis. J Pathol. 2004;204:311–316. doi: 10.1002/path.1660. [DOI] [PubMed] [Google Scholar]
- 9.Fu BM, Shen S. Acute VEGF effect on solute permeability of mammalian microvessels in vivo. Microvasc Res. 2004;68:51–62. doi: 10.1016/j.mvr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Galili O, Herrmann J, Woodrum J, Sattler KJ, Lerman LO, Lerman A. Adventitial vasa vasorum heterogeneity among different vascular beds. J Vasc Surg. 2004;40:529–535. doi: 10.1016/j.jvs.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 12.Gossl M, Beighley PE, Malyar NM, Ritman EL. Transendothelial Solute Transport in the Coronary Vessel Wall - Role of Vasa Vasorum - A Study with Cryostatic Micro-CT. Am J Physiol Heart Circ Physiol. 2004;287:H2346–2351. doi: 10.1152/ajpheart.00066.2004. [DOI] [PubMed] [Google Scholar]
- 13.Gossl M, Malyar NM, Rosol M, Beighley PE, Ritman EL. Impact of coronary vasa vasorum functional structure on coronary vessel wall perfusion distribution. Am J Physiol Heart Circ Physiol. 2003;285:H2019–2026. doi: 10.1152/ajpheart.00399.2003. [DOI] [PubMed] [Google Scholar]
- 14.Gossl M, Rosol M, Malyar NM, Fitzpatrick LA, Beighley PE, Zamir M, Ritman EL. Functional anatomy and hemodynamic characteristics of vasa vasorum in the walls of porcine coronary arteries. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:526–537. doi: 10.1002/ar.a.10060. [DOI] [PubMed] [Google Scholar]
- 15.Gossl M, Versari D, Mannheim D, Ritman EL, Lerman LO, Lerman A. Increased spatial vasa vasorum density in the proximal LAD in hypercholesterolemia--implications for vulnerable plaque-development. Atherosclerosis. 2007;192:246–252. doi: 10.1016/j.atherosclerosis.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Gossl M, Zamir M, Ritman EL. Vasa vasorum growth in the coronary arteries of newborn pigs. Anat Embryol. 2004;208:351–357. doi: 10.1007/s00429-004-0400-7. [DOI] [PubMed] [Google Scholar]
- 17.Hansen PR, Svendsen JH, Hoyer S, Kharazmi A, Bendtzen K, Haunso S. Tumor necrosis factor-alpha increases myocardial microvascular transport in vivo. Am J Physiol. 1994;266:H60–67. doi: 10.1152/ajpheart.1994.266.1.H60. [DOI] [PubMed] [Google Scholar]
- 18.Herrmann J, Best PJ, Ritman EL, Holmes DR, Lerman LO, Lerman A. Chronic endothelin receptor antagonism prevents coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Am Coll Cardiol. 2002;39:1555–1561. doi: 10.1016/s0735-1097(02)01798-9. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann J, Lerman LO, Rodriguez-Porcel M, Holmes DR, Richardson DM, Ritman EL, Lerman A. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res. 2001;51:762–766. doi: 10.1016/s0008-6363(01)00347-9. [DOI] [PubMed] [Google Scholar]
- 20.Herrmann J, Samee S, Chade A, Porcel MR, Lerman LO, Lerman A. Differential effect of experimental hypertension and hypercholesterolemia on adventitial remodeling. Arterioscler Thromb Vasc Biol. 2005;25:447–453. doi: 10.1161/01.ATV.0000152606.34120.97. [DOI] [PubMed] [Google Scholar]
- 21.Isner JM. Vasa vasorum: therapeutic implications. Cathet Cardiovasc Diagn. 1996;39:221–223. doi: 10.1002/(SICI)1097-0304(199611)39:3<221::AID-CCD2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Jorgensen SM, Demirkaya O, Ritman EL. Three-dimensional imaging of vasculature and parenchyma in intact rodent organs with X-ray micro-CT. Am J Physiol. 1998;275:H1103–1114. doi: 10.1152/ajpheart.1998.275.3.H1103. [DOI] [PubMed] [Google Scholar]
- 23.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 24.Kwon HM, Sangiorgi G, Ritman EL, McKenna C, Holmes DR, Jr, Schwartz RS, Lerman A. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest. 1998;101:1551–1556. doi: 10.1172/JCI1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Liu X, Wang J, Wang Z, Jiang W, Reed E, Zhang Y, Liu Y, Li QQ. Effects of thalidomide on the expression of angiogenesis growth factors in human A549 lung adenocarcinoma cells. Int J Mol Med. 2003;11:785–790. [PubMed] [Google Scholar]
- 26.Mannheim D, Versari D, Daghini E, Gossl M, Galili O, Chade A, Rajkumar VS, Ritman EL, Lerman LO, Lerman A. Impaired myocardial perfusion reserve in experimental hypercholesterolemia is independent of myocardial neovascularization. Am J Physiol Heart Circ Physiol. 2007;292:H2449–2458. doi: 10.1152/ajpheart.01215.2006. [DOI] [PubMed] [Google Scholar]
- 27.Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006;69:36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Moos MP, John N, Grabner R, Nossmann S, Gunther B, Vollandt R, Funk CD, Kaiser B, Habenicht AJ. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:2386–2391. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 29.Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, Badimon JJ, O'Connor WN. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–2038. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- 30.Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006;113:2245–2252. doi: 10.1161/CIRCULATIONAHA.105.578955. [DOI] [PubMed] [Google Scholar]
- 31.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 32.Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo KM, Gillies S, Javaherian K, Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation. 1996;93:672–682. doi: 10.1161/01.cir.93.4.672. [DOI] [PubMed] [Google Scholar]
- 34.Ohta H, Wada H, Niwa T, Kirii H, Iwamoto N, Fujii H, Saito K, Sekikawa K, Seishima M. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis. 2005;180:11–17. doi: 10.1016/j.atherosclerosis.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Park SJ, Kim HS, Yang HM, Park KW, Youn SW, Jeon SI, Kim DH, Koo BK, Chae IH, Choi DJ, Oh BH, Lee MM, Park YB. Thalidomide as a potent inhibitor of neointimal hyperplasia after balloon injury in rat carotid artery. Arterioscler Thromb Vasc Biol. 2004;24:885–891. doi: 10.1161/01.ATV.0000124924.21961.c3. [DOI] [PubMed] [Google Scholar]
- 36.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 37.Vacca A, Scavelli C, Montefusco V, Di Pietro G, Neri A, Mattioli M, Bicciato S, Nico B, Ribatti D, Dammacco F, Corradini P. Thalidomide downregulates angiogenic genes in bone marrow endothelial cells of patients with active multiple myeloma. J Clin Oncol. 2005;23:5334–5346. doi: 10.1200/JCO.2005.03.723. [DOI] [PubMed] [Google Scholar]
- 38.Vohra RS, Murphy JE, Walker JH, Ponnambalam S, Homer-Vanniasinkam S. Atherosclerosis and the Lectin-like OXidized low–density lipoprotein scavenger receptor. Trends Cardiovasc Med. 2006;16:60–64. doi: 10.1016/j.tcm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Westermann D, Van Linthout S, Dhayat S, Dhayat N, Schmidt A, Noutsias M, Song XY, Spillmann F, Riad A, Schultheiss HP, Tschope C. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2007;102:500–507. doi: 10.1007/s00395-007-0673-0. [DOI] [PubMed] [Google Scholar]
- 40.Wilson SH, Caplice NM, Simari RD, Holmes DR, Jr, Carlson PJ, Lerman A. Activated nuclear factor-kappaB is present in the coronary vasculature in experimental hypercholesterolemia. Atherosclerosis. 2000;148:23–30. doi: 10.1016/s0021-9150(99)00211-7. [DOI] [PubMed] [Google Scholar]
- 41.Wilson SH, Herrmann J, Lerman LO, Holmes DR, Jr, Napoli C, Ritman EL, Lerman A. Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation. 2002;105:415–418. doi: 10.1161/hc0402.104119. [DOI] [PubMed] [Google Scholar]
- 42.Ye Q, Chen B, Tong Z, Nakamura S, Sarria R, Costabel U, Guzman J. Thalidomide reduces IL-18, IL-8 and TNF-alpha release from alveolar macrophages in interstitial lung disease. Eur Respir J. 2006;28:824–831. doi: 10.1183/09031936.06.00131505. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol. 2008;103:398–406. doi: 10.1007/s00395-008-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Peppel K, Sivashanmugam P, Orman ES, Brian L, Exum ST, Freedman NJ. Expression of tumor necrosis factor receptor-1 in arterial wall cells promotes atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:1087–1094. doi: 10.1161/ATVBAHA.0000261548.49790.63. [DOI] [PMC free article] [PubMed] [Google Scholar]