Abstract
We conducted a study to determine the effect of container size and location on oviposition site selection by Ae. aegypti in large outdoor field enclosures (10 × 10 × 4 m high). There was a strong positive relationship between increasing container diameter, container volume, and water surface area with egg numbers over both high (rainy, July) and low (cool-dry, January) dengue transmission seasons. Location of containers (indoors versus immediately outdoors and underneath houses) did not influence the number of eggs deposited for containers 5–32 cm in diameter in either season. No trends based on container color (black, brown, or grey) were observed. A slight trend with a greater numbers of eggs laid outdoors in the largest containers (42 cm diameter) during the dry season was observed. Three separate models were run using the mixed model procedure in SAS for each container attribute. Controlling for season, time, and date, the most important container attribute predicting total egg numbers was container volume (total capacity) explaining 88% of the variation, followed by water surface area (85%), and container diameter opening (83%). Oviposition peaked in the afternoon at 1600 hrs and 2000 hrs in the dry and rainy seasons, respectively. Few eggs were laid overnight (2000 hrs–0600 hrs). Our results indicate that physical attributes of oviposition sites, such as size, light-dark contrasts, and specular reflectance from water surfaces, play a significant role in oviposition site selection. Key Words: Aedes aegypti—Oviposition—Container dimensions—Site selection—Thailand.
Introduction
Selection of an oviposition site is one of the most important behavioral components of mosquito survival (Bentley and Day 1989). An improved understanding of oviposition behavior may not only lead to new insights about mosquito life history, but may also lead to more refined dengue surveillance and control practices.
Several studies have been conducted in the laboratory to evaluate components of Ae. aegypti attraction to oviposition sites including chemical and visual cues. Relatively few studies, however, have been conducted in the field under natural settings despite the critical importance of validating laboratory-based observations under more realistic field conditions.
Kennedy (1942) and others documented the attractiveness of water vapor to Ae. aegypti and, to a lesser extent, surface reflection. Different components of water may also be similarly attractive (Reiter et al. 1991, Torres-Estrada et al. 2001) including the presence of Aedes eggs and conspecific larvae or pupae (Allan and Kline 1998, Soman and Reuben 1970). In contrast, components of water may be repellent at certain concentrations or in the presence of con-specific larvae (Allan and Kline 1995, Chadee et al. 1990). Zahiri and Rau (1998) observed a rising and then declining relationship with larval biomass and attraction to gravid Ae. aegypti in the laboratory. Oviposition substrates were especially repellent when conspecifics were stressed from starvation or crowding (Lowen-berger and Rau 1994, Zahiri et al. 1997a, Zahiri et al. 1997b). Kaur et al. (2003) suggested that female Ae. aegypti may be conditioned during larval stages for a particular level of site attraction or repellence as adults.
Surprisingly, the role of container size on oviposition site selection and suitability has been examined in only a few cases. Colton et al. (2003) found that more female Ae. aegypti responded to large water surfaces (177 cm2) as opposed to small surfaces (57 cm2), although the authors felt that container color (black) influenced selection more than water surface area.
Ae. aegypti tends to oviposit with afternoon activity peaks. Oviposition peaks in Trinidad occurred 2 hrs after sunrise and 2 hrs before sunset (Corbet and Chadee 1990, Corbet and Chadee 1992, Corbet and Chadee 1993). In French Polynesia, Russell and Ritchie (2004) reported Ae. aegypti oviposition peaks from noon to midnight.
Location of oviposition sites may also be important in site selection. Ritchie et al. (2003) reported significantly greater Ae. aegypti females captured in sticky ovitraps placed outdoors than they did for ovitraps placed indoors in Northern Queensland, Australia.
Our goal was to determine whether Ae. aegypti make oviposition choices based on the suitability of individual sites and whether there is a relationship between physical container attributes and the presence or absence and quantity of eggs laid. We also determined daily patterns of oviposition in the field. Three experiments were conducted over 2 yrs and 2 seasons (cool-dry, low-dengue transmission and warm-rainy, high-dengue transmission) in large outdoor enclosures in the Mae Sot region of Thailand, where Ae. aegypti and dengue infections are common (Harrington et al. 2005).
Materials and Methods
Field site
Our study was conducted in the village of Pai Lom (16° 45′N, 98° 33′E) located in Mae Pa district, 5 km north of Mae Sot in Tak Province, along the Thailand-Myanmar border (Harrington et al. 2005). Experiments were conducted during the cool dry season (January 2003 and 2004) and warm rainy season (July 2003). These seasons in Thailand correspond to low (dry) and high (rainy) dengue transmission periods (Watts et al. 1987). Average temperature and relative humidity during collection periods in January 2003 was 25°C (range 17–34) and 60% RH (range 38–98), in July–August 2003 was 28°C (range 24–35) and 82% RH (range 51–99), and in January–February 2004 was 24°C (range 16–34) and 62% RH (range 35–78).
Large outdoor field cages
Large enclosures (10 × 10 × 4 m high) were constructed around a vacant house and yard (Fig. 1) representing typical houses within the region. The bamboo cage frame was covered with nylon mesh netting (1.2 mm2 mesh size) (Thai Sawang Enterprise Company Limited, Nakhon Pathom, Thailand). The mesh allowed ambient light and rainfall into the cage. Local clay or glass water storage jars ranging in size from 5–45 cm in diameter were placed in and immediately outside or underneath the house within field cages (Fig. 2A–C). Container colors included black, brown, and grey. Total volume and water surface area was calculated based on the dimensions of each jar (Table 1). Jars were numbered and filled to 75% capacity with rainwater collected in large holding tanks in the village. A strip of seed germination paper was used as a removable oviposition substrate. The paper was placed around the rim of each container extending into the water and was secured tightly to the inside of the container with clips. (Fig. 2B–C). The same jars were used over multiple seasons. Two different cages were built around different vacant Thai houses in the community during each season.
FIG. 1.

One of 2 large outdoor field cages used for oviposition studies (10 × 10 × 4 m high). A house and yard were enclosed in mesh over a bamboo frame.
FIG. 2.

Examples of potential oviposition containers; each container was lined with brown seed germination paper. (A) Different sizes of containers; (B) Container lined with paper and located outside the house; (C) close up of a relatively large, lined container.
Table 1.
Container Size and Location by Season for Containers Placed Inside Large Enclosures in Thailand, January 2003, July 2004, and January 2004
| Date | Season | No. of containers | Color | Location | Diameter (cm) | Water surface area (cm2) | Container volume (L) |
|---|---|---|---|---|---|---|---|
| Jan. 2003 | Dry | 2 | Dk. brown | Out | 42 | 62 | 0.1 |
| Jan. 2003 | Dry | 1 | Lt. brown | In | 42 | 62 | 0.1 |
| Jan. 2003 | Dry | 3 | Lt. brown | Out | 31 | 42 | 0.1 |
| Jan. 2003 | Dry | 3 | Lt. brown | In | 31 | 42 | 0.1 |
| Jan. 2003 | Dry | 2 | Lt. brown | In | 32 | 52 | 0.6 |
| Jan. 2003 | Dry | 1 | Grey | Out | 20 | 45 | 0.6 |
| Jan. 2003 | Dry | 1 | Lt. brown | In | 20 | 45 | 0.6 |
| Jan. 2003 | Dry | 1 | Lt. brown | In | 32 | 52 | 0.6 |
| Jan. 2003 | Dry | 1 | Black | Out | 8 | 8 | 0.6 |
| Jan. 2003 | Dry | 1 | Black | In | 8 | 8 | 0.6 |
| Jul. 2003 | Rainy | 1 | Lt. brown | Out | 20 | 45 | 8.3 |
| Jul. 2003 | Rainy | 1 | Lt. brown | Out | 32 | 52 | 8.3 |
| Jul. 2003 | Rainy | 1 | Dk. brown | Out | 42 | 62 | 8.3 |
| Jul. 2003 | Rainy | 2 | Black | Out | 5 | 5 | 8.3 |
| Jul. 2003 | Rainy | 1 | Black | Out | 8 | 8 | 8.3 |
| Jul. 2003 | Rainy | 1 | Lt. brown | In | 32 | 52 | 25.5 |
| Jul. 2003 | Rainy | 1 | Lt. brown | In | 42 | 62 | 25.5 |
| Jul. 2003 | Rainy | 1 | Black | In | 5 | 5 | 25.5 |
| Jul. 2003 | Rainy | 1 | Black | In | 8 | 8 | 30.3 |
| Jul. 2003 | Rainy | 1 | Lt. brown | In | 32 | 52 | 30.3 |
| Jul. 2003 | Dry | 1 | Lt. brown | Out | 42 | 62 | 30.3 |
| Jan. 2004 | Dry | 2 | Dk. brown | In | 42 | 62 | 30.3 |
| Jan. 2004 | Dry | 1 | Dk. brown | Out | 32 | 52 | 30.3 |
| Jan. 2004 | Dry | 1 | Lt. brown | In | 31 | 42 | 30.3 |
| Jan. 2004 | Dry | 1 | Lt. brown | Out | 20 | 45 | 67.5 |
| Jan. 2004 | Dry | 1 | Grey | In | 20 | 45 | 67.5 |
| Jan. 2004 | Dry | 1 | Black | Out | 8 | 8 | 67.5 |
| Jan. 2004 | Dry | 1 | Black | In | 8 | 8 | 67.5 |
| Jan. 2004 | Dry | 1 | Black | Out | 5 | 5 | 67.5 |
| Jan. 2004 | Dry | 1 | Black | In | 5 | 5 | 67.5 |
| Jan. 2004 | Dry | 1 | Lt. brown | Out | 42 | 62 | 67.5 |
Mosquitoes
Ae. aegypti were reared from pupae collected from containers in Pai Lom and Lao Bao villages (16° 45′N, 98° 34′E). Emerged adults were held under ambient conditions in a field laboratory (local house) approximately 1 km from Pai Lom. Mosquito identification was confirmed as Ae. aegypti adults emerged. Other species present in low frequency (Ae. albopictus in the rainy season and Culex p. quinquefasciatus) were removed from emergence containers. Mated adult females were offered blood from one of the investigators' arms in accordance with Institutional Review Board approvals from Cornell University (FWA00004513), University of California at Davis (200210073), Walter Reed Army Institute of Research (752), and the Thai Ministry of Health Ethical Review Committee for Research in Human Subjects. Blood-fed females were held with males in 4-liter bucket cages until fully gravid (4–6 days). To prevent mosquitoes from ovipositing, no substrate was provided during the holding period.
Mosquito Release and Egg Collection
January–February 2003
Gravid Ae. aegypti females were released at approximately 1800–2000 hrs (after sunset) every evening from 28 January to 1 February 2003 in the center of the experimental house enclosure. Estimates of the total number of females released ranged from 200 to 350 per day. Egg papers were removed, were immediately replaced, and were inspected three times each day (AM: 0800–0930 hrs, noon: 1130–1300, PM: 1730–1900 hrs). Both sides of papers with eggs were examined under a dissecting scope, and egg numbers were recorded by container and time. On 2 February, a second replication using the same methods was conducted in a separate enclosure. All containers were emptied, and eggs were transferred to the second enclosure and were randomly allocated to a new position inside or outside the house. Gravid Ae. aegypti were released into the enclosure at 2100 hrs on 3 and 4 February 2003. On subsequent days, new gravid females were not released, because investigators assumed that other previously released gravid females were present. Study collaborators entered the houses daily and the collaborators allowed females to feed on them so they would continue to develop and lay eggs. Egg papers were removed and were inspected three times daily.
July 2003
Gravid females (approximately 300 per night) were released in one of the enclosures as described above between 2000 and 2100 hrs every evening from 15–23 July 2003. Egg papers were removed and were replaced with new paper every 2 hrs from 0600 to 2000 hrs each day. New egg papers were left in containers overnight from 2000 to 0600 hrs. Each paper was carefully labeled with the container number, time, and date, and the papers were returned to the field lab for inspection. The total number of eggs in each container for each time interval and date were recorded.
January 2004
The experiment was replicated from 19–22 January 2004 following the same methods as described previously. We released gravid females into the field cages and replaced egg papers every 2 hrs and from 2000 to 600 hrs overnight. Egg data for each container were recorded as described previously. We released approximately 150–200 gravid females each night.
Data Analysis
The total number of eggs laid in each container at several time intervals over multiple days in each season was recorded. Data for the preliminary study (January–February 2003) were analyzed for oviposition timing, container location, and the number of eggs laid. The data from July and January 2004 were analyzed in more detail. These data had a multilevel structure, because each container was repeatedly measured over time. Diameter, volume, container color, water surface area, and location were fixed effects at the container level, whereas season, time, and date nested within season were within container fixed effects.
Time at 0600 hrs was removed from the analysis because few eggs were collected between sunset and sunrise. Because of a high frequency of zeroes and the multilevel data structure, the analysis was performed in two steps.
First a binomial response was created differentiating between no eggs laid in any given container at any given time on any given day versus any number of eggs laid. A generalized estimating equation model with a binomial distribution, a logit link, and an exchangeable covariance structure was used to determine whether any of the fixed effects, including their interactions, were significantly related to the presence or absence of eggs. This analysis was performed using Proc Genmod in SAS (SAS Institute 1999).
In a second step only observations with number of eggs larger than zero were included in the analysis. A multilevel linear model was used to regress the log-transformed number of eggs on the fixed effects to determine if any of the fixed effects, including their interactions, were significantly related to the number of eggs being laid (Singer 1988). This analysis was performed using Proc Mixed in SAS.
Data analysis showed a collinear relationship between diameter, volume, and water surface area. As a result, separate models were run with each of these variables appearing as an independent variable one at the time. The p-values were adjusted using a Bonferroni correction for multiple comparisons.
Results
Timing of ovipositions
Peak oviposition occurred from 1200 to 1800 hrs during the cool, dry season in January 2003. During the warm, rainy season (July 2003), oviposition activity gradually increased through the day, reaching a peak at 2000 hrs. A similar trend was observed during the January 2004 cool, dry season, but with an egg-laying peak at 1600 hrs (Fig. 3). Few eggs were laid overnight from 2000 hrs to 0600 hrs during all of the study periods.
FIG. 3.
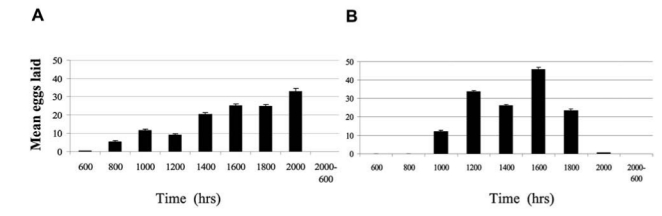
Time of day for oviposition by Ae. aegypti in Thailand per day. (A) Warm-rainy season, July 2003; (B) cool-dry season, January 2004.
Container location
Subsamples of eggs on paper were hatched to confirm identification as Ae. aegypti. No significant differences by location were detected in total eggs deposited inside versus outside or underneath houses across replicates and seasons. In January 2003, a total of 4597 eggs (¯ = 511 mean per day ± 38 SE) were laid inside, and 4584 eggs were laid outside (¯ = 655 ± 18 SE). A grand total of 7292 eggs were laid outside (¯ = 511 ± 38 SE) during any of the study periods, and 6927 eggs (¯ = 172 ± 4.7 SE) were laid inside during July 2003. In January 2004, 2990 eggs were laid inside (¯ = 133 ± 30 SE) and 2394 (¯ = 166 ± 42 SE) were laid outside (Fig. 4). No differences in egg numbers were found when the effect of specific container location (i.e., front of house, underneath north corner, etc.), within indoor versus outdoor locations, was examined. In addition, no significant trends by container color were found.
FIG. 4.
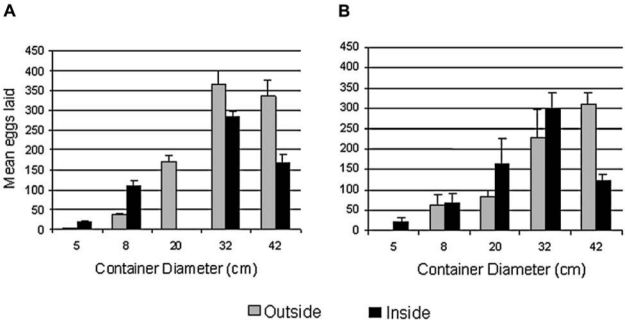
Mean eggs deposited indoors and outdoors by container diameter per container. (A) Warm-rainy season, July 2003; (B) cool-dry season, January 2004.
Presence or absence of eggs
There were no significant main effects or interaction effects for season, date nested within season, or location for any of the 3 models. There was an overall significant effect of time of day. Using multiple comparisons, egg numbers at 0800 hrs, 1000 hrs, and 2000 hrs were not significantly different from each other, as were those at 1200 hrs, 1400 hrs, 1600 hrs, and 1800 hrs, but both sets of times were significantly different from each other in all three models.
Each of three major traits of the oviposition site (container opening diameter, container volume, and water surface area) was compared to determine if they were significantly related to the presence or absence of eggs. The model with diameter as an independent variable had a significant linear effect (coeff. = 0.2221, p < 0.0001) and quadratic effect (coeff. = − 0.0037, p < 0.0009). When volume was set as an independent variable, a significant linear effect (coeff. = 0.0911, p = 0.0003) and quadratic effect (coeff. = −0.0011, p = 0.0003) resulted. In addition, when water surface was set as an independent variable, there was a significant linear effect (coeff. = 0.0977, p = 0.023) but no quadratic effect.
Number of eggs laid
For each of the models, there was a significant day nested within season effect (p < 0.001) as well as a significant interaction effect between season and time (p < 0.01). The season-time interaction effect captured in all 3 models a significant difference between seasons at 0800 hrs and 2000 hrs, with no eggs laid in the dry season at 0800 hrs and significantly more eggs collected in the rainy season at 2000 hrs than in the dry season at the same time of day (p < 0.01). In the dry season, the number of eggs collected was significantly fewer at 2000 hrs than at 1200 hrs, 1400 hrs, and 1600 hrs (p <0.01).
For models with each of the 3 container attributes set individually as independent variables, there was a significant linear effect of container diameter (coeff. = 0.0154, p = 0.002), with an increasing numbers of eggs laid as the diameter of the container increased, a significant linear volume effect (coeff. = 0.0253, p = 0.01), and quadratic volume term (coeff. = −0.0003, p = 0.038) with increasing eggs as volume increased, and increasing numbers of eggs with increasing water surface area (coeff. = 0.0093, p = 0.002).
After controlling for season, time, and date, the amount of explainable variation between containers explained by container diameter was 83%, by container volume was 88%, and by water surface area was 85%.
Discussion
Our results demonstrate the important impact of physical container attributes on oviposition site selection of the yellow fever mosquito. To the best of our knowledge this is the first study of Ae. aegypti oviposition behavior conducted in large outdoor field enclosures, which provide useful settings for manipulative experiments in a contained environment that is as close as possible to field conditions. Container diameter, water surface area, and volume were all positively correlated with number of eggs laid across all replicates and seasons. Container attributes were not mutually exclusive. By controlling for season, time, and date, and by running separate models with each single attribute independently, we determined that container volume was the strongest predictor of the number of egg(s) laid per container, followed by water surface area, and then opening diameter. Container volume was an estimate of the total capacity of the container, although each vessel in our study was filled to 75% capacity. The greater capacity of containers that were not completely full may provide large, protected, humid resting surfaces for females engaged in or preparing for oviposition. A few published studies with other mosquito species have reported varied responses to container size and attraction of ovipositing females (Derraik and Slaney 2005, Lester and Pike 2003, Wynn and Paradise 2001). Aedes aegypti is a day active mosquito that may rely more on optical cues, such as contrast between dark container openings and water surface (specular) reflections, for selection of resting and oviposition sites than night active mosquito species.
Potential limitations to our study include our lack of knowledge about carrying capacity within the enclosure. In nature, Ae. aegypti tends to be a lower density species than other mosquitoes (Scott et al. 2000). No studies on the carrying capacity of this species in its natural habitat have been conducted, but the numbers released in our enclosures may have been artificially high. Other limitations may have been the lack of normal host activity within the enclosure, which would have provided more blood meals. In addition, we may have prevented natural predators from accessing mosquitoes. Furthermore, comparing the eggs per cm surface area available inside containers may have provided us with more precise information on whether surface area on the inner container wall or overall container volume was responsible for the trends we observed. Another factor that may influence the oviposition site choice is the presence of predators. One might expect larger containers with more permanent bodies of water to contain more predators than smaller containers. In our experiments, predators were not included in containers. Despite these limitations, our study showed significant relative differences in oviposition patterns based on oviposition site size for this container breeding species.
Similar oviposition patterns were observed during the dry and rainy seasons with slight shifts in the beginning and ending of diel oviposition activity by season. Oviposition activity continued 2 hrs later during the rainy season in July 2003 than during the dry season (January 2004). This effect is likely a result of differences in the day length and humidity between the two seasons. Sunset occurred at 1920–1922 hrs in the study area during July 2003 and from 1814–1816 hrs during January 2004 (United States Naval Observatory 2005).
No differences were found between the number of eggs laid inside and outside or underneath the house. Because Ae. aegypti tend to rest inside houses (Scott et al. 2000), we would expect decreasing egg numbers as their distance from human dwellings increases. Houses within enclosures were of a typically rural, Thai style; the elevated open wood design allows free movement of mosquitoes in and out through doorways, window openings, and cracks in the floor boards and walls. Our cages encompassed the inside and outside areas that are normally used by this species in its natural habitat. If releasing gravid females had caused the females to lay in the first site they encountered, then we would have detected more egg numbers inside the house near the point of release. Furthermore, we did not perceive differences in water quality in the containers over the short duration of the experiments, as they were all filled from the same rainwater source at the beginning of each experiment.
In addition to providing more information on mosquito behavior, our results have implications for monitoring mosquito activity in nature. Surveillance using small containers, such as a typical CDC ovitrap (7.5 cm) (Reiter et al. 2001) may lack sensitivity and can be expected to underestimate Ae. aegypti oviposition activity, adult female population density, and patterns of egg laying behavior. This lack of sensitivity may be especially pronounced when other competing, natural oviposition sites are available. In our study, we included CDC style surveillance containers with diameters of 5 and 8 cm. Low egg frequencies for both the presence or absence, as well as total eggs laid, were observed for these small containers as compared with larger water storage vessels. In some instances, over the same time period, we collected few or no eggs in the 5–8 cm diameter containers and more than 1000 eggs in large containers.
Oviposition behavior can have a significant impact on arthropod life history. There is selection pressure for females to make choices that maximize survival of their progeny. We have identified one strategy for a container-breeding mosquito where larger containers may increase the chances of larval survival. Larger containers may be more likely to contain adequate food for immature stages and optimally humid resting sites for adults. In addition, large containers may contain a more permanent aquatic habitat than smaller containers. Further studies to investigate how these effects are modulated, and what the role of existing conspecifics, presence of predators, or other species may play in oviposition choices of gravid females, are warranted.
Acknowledgments
We thank the residents of Pai Lom and Lao Bao, Mae Sot, Thailand, for their support and cooperation. We thank health professionals Dr. Swaddigwongporni and Dr. Pradit of the Community Health Program at Mae Sot Hospital and Mr. BoonruangYakeaw at Mae Pa Health Center. Ms. Porapol Meungcheun assisted with the building of enclosures. Ms. Benjamas Pongnapakul, Mr. Somboon Intawong, Mr. Pornchai Harujai, Mr. Aun Manowong, Mr. Chaiwut Lerchai, Mr. Somboon Kantang, Mr. Manit Chaiya, Mr. Chatree Chumpen, and Mr. Boonkuea Malisor assisted with collections. National Institutes of Health Grant AI-22119 supported this research.
References
- Allan SA. Kline DL. Evaluation of organic infusions and synthetic compounds mediating oviposition in Aedes albopictus and Aedes aegypti (Diptera: Culicidae) J Chem Ecol. 1995;21:1847–1860. doi: 10.1007/BF02033681. [DOI] [PubMed] [Google Scholar]
- Allan SA. Kline DL. Larval rearing water and preexisting eggs influence oviposition by Aedes aegypti and Aedes albopictus (Diptera: Culicidae) J Med Entomol. 1998;35:943–947. doi: 10.1093/jmedent/35.6.943. [DOI] [PubMed] [Google Scholar]
- Bentley MD. Day JF. Chemical ecology and behavioral aspects of mosquito oviposition. Ann Rev Entomol. 1989;34:401–421. doi: 10.1146/annurev.en.34.010189.002153. [DOI] [PubMed] [Google Scholar]
- Chadee DD. Corbet PS. Greenwood JJD. Egg-laying yellow fever mosquitoes avoid sites containing eggs laid by themselves or by conspecifics. Entomol Exp Appl. 1990;57:295–298. [Google Scholar]
- Colton YM. Chadee DD. Severson DW. Natural skip oviposition of the mosquito Aedes aegypti indicated by codominant genetic markers. Med Vet Entomol. 2003;17:195–204. doi: 10.1046/j.1365-2915.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- Corbet PS. Chadee DD. Incidence and diel pattern of oviposition of the mosquito, Aedes aegypti (L.) (Diptera: Culicidae) in Trinidad, W.I. in relation to solar aspect. Ann Trop Med Parasitol. 1990;84:63–78. doi: 10.1080/00034983.1990.11812434. [DOI] [PubMed] [Google Scholar]
- Corbet PS. Chadee DD. The diel pattern of entry to outdoor oviposition sites by female Aedes aegypti (L.) (Diptera: Culicidae) that then laid eggs there: a preliminary study. Ann Trop Med Parasitol. 1992;86:523–528. doi: 10.1080/00034983.1992.11812702. [DOI] [PubMed] [Google Scholar]
- Corbet PS. Chadee DD. An improved method for detecting substrate preferences shown by mosquitoes that exhibit “skip oviposition.”. Phys Entomol. 1993;18:114–118. [Google Scholar]
- Derraik JGB. Slaney D. Container aperture size and nutrient preferences of mosquitoes (Diptera: Culicidae) in the Auckland region, New Zealand. J Vec Ecol. 2005;30:73–82. [PubMed] [Google Scholar]
- Harrington LC. Scott TW. Lerdthusnee K. Coleman RC, et al. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg. 2005;72:209–220. [PubMed] [Google Scholar]
- Kaur JS. Lai YL. Giger AD. Learning and memory in the mosquito Aedes aegypti shown by conditioning against oviposition deterrence. Med Vet Entomol. 2003;17:457–460. doi: 10.1111/j.1365-2915.2003.00455.x. [DOI] [PubMed] [Google Scholar]
- Kennedy JS. On water-finding and oviposition by captive mosquitoes. Bull Entomol Res. 1942;32:279–301. [Google Scholar]
- Lester PJ. Pike AJ. Container surface area and water depth influence the population dynamics of the mosquito Culex pervigilans (Diptera: Culicidae) and its associated predators in New Zealand. J Vec Ecol. 2003;28:267–274. [PubMed] [Google Scholar]
- Lowenberger CA. Rau ME. Selective oviposition by Aedes aegypti (Diptera: Culicidae) in response to a larval parasite, Plagiorchis elegans (Trematoda: Plagiorchiidae) Envir Entomol. 1994;23:1269–1276. [Google Scholar]
- Reiter P. Amador MA. Colon N. Enhancement of the CDC ovitrap with hay infusions for daily monitoring of Aedes aegypti populations. J Am Mosq Contr Assoc. 1991;7:52–55. [PubMed] [Google Scholar]
- Reiter P. Nathan MB. Guidelines for assessing the efficacy of insecticidal space sprays for control of the dengue vector, Aedes aegypti. Bull World Health Org. 2001;2001:1–40. [Google Scholar]
- Ritchie SA. Long S. Hart A. Webb CE, et al. An adulticidal sticky ovitrap for sampling container-breeding mosquitoes. J Am Mosq Contr Assoc. 2003;19:235–242. [PubMed] [Google Scholar]
- Russell RC. Ritchie SA. Surveillance and behavioral investigations of Aedes aegypti and Aedes polynesiensis in Moorea, French Polynesia, using a sticky ovitrap. J Am Mosq Control Assoc. 2004;20:370–375. [PubMed] [Google Scholar]
- SAS Institute. SAS System for Windows. 1999.
- Scott TW. Morrison AC. Lorenz LH. Clark GG, et al. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. J Med Entomol. 2000;37:77–88. doi: 10.1603/0022-2585-37.1.77. [DOI] [PubMed] [Google Scholar]
- Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchial models, and individual growth models. J Educ Behav Stat. 1988;24:323–355. [Google Scholar]
- Soman RS. Reuben R. Studies on the preference shown by ovipositing females of Aedes aegypti for water containing immature stages of the same species. J Med Entomol. 1970;7:485–489. doi: 10.1093/jmedent/7.4.485. [DOI] [PubMed] [Google Scholar]
- Torres-Estrada JL. Rodriguez MH. Cruz-Lopez L. Arredondo-Jimenez JI. Selective oviposition by Aedes aegypti (Diptera: culicidae) in response to Mesocyclops longisetus (Copepoda: Cyclopoidea) under laboratory and field conditions. J Med Entomol. 2001;38:188–192. doi: 10.1603/0022-2585-38.2.188. [DOI] [PubMed] [Google Scholar]
- United States Naval Observatory. Astr Appl Dept. Washington, DC: 2005. Mae Sot Weather station; pp. 20392–5420. [Google Scholar]
- Watts DM. Burke DS. Harrison BA. Whitmire RW, et al. Effect of temperature on the vector efficiency of Ae. aegypti for dengue-2 virus. Am J Trop Med Hyg. 1987;23:1153–1160. doi: 10.4269/ajtmh.1987.36.143. [DOI] [PubMed] [Google Scholar]
- Wynn G. Paradise CJ. Effects of microcosm scaling and food resources on growth and survival of larval Culex pipiens. BMC Ecol. 2001. www.biomedcentral.com/1472–6785/1/3. www.biomedcentral.com/1472–6785/1/3 [DOI] [PMC free article] [PubMed]
- Zahiri N. Rau ME. Oviposition attraction and repellency of Aedes aegypti (Diptera: Culicidae) to waters from conspecific larvae subjected to crowding, confinement, starvation, or infection. J Med Entomol. 1998;35:782–787. doi: 10.1093/jmedent/35.5.782. [DOI] [PubMed] [Google Scholar]
- Zahiri N. Rau ME. Lewis DJ. Starved larvae of Aedes aegypti (Diptera: Culicidae) render waters unattractive to ovipositing conspecific females. Pop Ecol. 1997a;26:1087–1090. [Google Scholar]
- Zahiri N. Rau ME. Lewis DJ. Khanizadeh S. Intensity and site of Plagiorchis elegans (Trematoda: Plagiorchiidae) infections in Aedes aegypti (Diptera: Culicidae) larvae affect the attractiveness of their waters to ovipositing, conspecific females. Envir Entomol. 1997b;26:920–923. [Google Scholar]


