Abstract
Rickettsia felis, a flea-associated rickettsial pathogen, has been identified in many tissues, including the digestive and reproductive tissues, within the cat flea, Ctenocephalides felis. We utilized transmission electron microscopy and polymerase chain reaction to identify R. felis in the salivary glands of fed fleas and further define the distribution of R. felis within the arthropod host. We identified Rickettsia-like organisms in salivary glands using electron microscopy. Sequence analysis of portions of the Rickettsia genus-specific 17-kDa antigen gene and R. felis plasmid confirmed the morphological identification of R. felis in cat flea salivary glands. This is the first report of R. felis in tissues critical for horizontal transmission of rickettsiae. Key Words: Cat flea—Ctenocephalides felis—Rickettsia felis.
Introduction
Rickettsia felis is an intracellular gram-negative bacterium associated predominantly with the cat flea, Ctenocephalides felis. Clinical reports, coupled with serological analysis and molecular detection of rickettsial DNA in human samples, implicate R. felis as a human pathogen (Parola et al. 2005). Horizontal transmission of R. felis between fleas and vertebrate hosts (including companion animals, rodents, and opossums) has been suggested as a potential source of human exposure (Azad et al. 1997, Case et al. 2006, Psaroulaki et al. 2006). Vertical transmission of R. felis is suspected to be the primary route of maintenance in colonized cat fleas (Azad et al. 1992, Wedincamp et al. 2002); however, the potential for horizontal transmission is evident as R. felis DNA was polymerase chair reaction (PCR) amplified in the blood of laboratory cats that were exposed to R. felis-infected cat fleas and subsequently seroconverted (Wedincamp et al. 2000). The acquisition mechanism of R. felis by vertebrates and uninfected fleas in nature is unknown.
The distribution of R. felis within fleas has been examined using both transmission electron microscopy (TEM) and PCR. Specific tissues in which R. felis has been identified in cat fleas include the midgut epithelial cells (adult and larval fleas), as well as adult flea muscle cells, fat body, tracheal matrix, ovaries, and the epithelial sheath of testes (Adams et al. 1990, Bouyer et al. 2001). However, the presence of R. felis in the salivary glands of the flea host has not been confirmed.
Most of the studies characterizing the relationship between R. felis and fleas have utilized colonized fleas (Adams et al. 1990, Bouyer et al. 2001, Higgins et al. 1994, Moron et al. 2000, Pornwiroon et al. 2006, 2007, Wedincamp et al. 2000, 2002, 2003, Zavala-Castro et al. 2005). The objective of the current study was to identify R. felis in the salivary glands of cat fleas, which should allow expansion of studies relating to horizontal transmission of R. felis. In this report, we describe localization of R. felis in the salivary glands of fed fleas by TEM and PCR amplification of R. felis 17-kDa antigen gene, the conjugative plasmid pRF, but, consistent with the LSU strain of R. felis, not the smaller plasmid, pRFδ.
Methods
Source of fleas
Rickettsia felis–infected Ctenocephalides felis were maintained at the Louisiana State University, School of Veterinary Medicine (LSU-SVM) on domestic shorthair cats as previously described (Henderson et al. 1993). The prevalence of rickettsial infection in the LSU colony of C. felis has been examined (Higgins et al. 1994), and we have recently identified R. felis prevalence in this colony to be approximately 94% (Porn-wiroon et al. 2007). Unfed, R. felis-infected adult fleas were applied directly to the cat and allowed to feed for 2–4 days. A flea comb was used to brush/remove fleas off the cat host. Fleas were immobilized on ice and dissected under a standard dissecting microscope. Salivary glands were recovered in phosphate-buffered saline and stored on ice until placed into tissue fixative or frozen for subsequent DNA isolation.
Electron microscopy
Salivary glands from approximately 10 fed adult fleas were extirpated at LSU-SVM and placed in a fixative containing 1.25% formaldehyde, 2.5% glutaraldehyde, 0.03% CaCl2, and 0.03% trinitrophenol in 0.05 M cacodylate buffer, pH 7.4 (Ito et al. 1981). The fixed samples were transported to University of Texas Medical Branch (UTMB), where they were post-fixed in 1% osmium tetroxide in 0.05 M cacodylate buffer (pH 7.2), stained en bloc for 30 min with 1% uranyl acetate in 0.05 M maleate buffer, pH 5.2 at 60°C. The fixed tissues were then dehydrated in a graded series of ethanol and embedded in Poly/Bed 812 (Polysciences). Ultrathin sections were cut with diamond knives on Reichert-Leica Ultracut S ultramicrotome, mounted on copper grids, and stained at room temperature for 5 min in 2% aqueous uranyl acetate followed by 3 min in lead citrate. TEM was carried out at UTMB with a Philips 201 transmission electron microscope (Philips Electron Optics) as previously described (Bouyer et al. 2001).
DNA isolation and PCR amplification
Salivary glands from approximately 20 fed adult fleas were collected separately into a 1.5-mL microcentrifuge tube, pelleted by centrifugation, and resuspended in 200 μL of sterile phosphate-buffered saline. Genomic DNA was extracted from flea salivary glands using the DNeasy tissue kit (Qiagen) according to the manufacturer's protocol; an empty tube environmental blank treated the same as the sample was also used for genomic DNA extraction. For PCR amplification, 20 ng of genomic DNA from sample fleas, genomic DNA from R. felis (LSU; positive control for 17-kDa antigen gene and plasmid pRF) (Pornwiroon et al. 2006), environmental genomic DNA blank (genomic DNA isolation negative control), or water (PCR negative control) were used as templates for PCR in a final volume of 25 μL. PCR products were amplified using PCR Master Mix (Promega) together with gene-specific oligonucleotide primers (400 nM for each primer) for the 17-kDa antigen gene (Williams et al. 1992), pRF (primers pRFc and pRFd), pRFδ (primers pRFa and pRFd), and with the addition of the primer combination (pRFa and pRFb) to amplify the approximately 160-basepair portion of pRF (Ogata et al. 2005). The specific annealing temperatures for the primer pairs were 55°C (Rr17.61p and Rr17.492n; pRFc and pRFd) as reported by Pornwiroon et al. (2006) and 50°C (pRFa and pRFd; pRFa and pRFb).
DNA sequencing and analysis
The PCR products were gel purified and cloned into pCR4-TOPO vector (Invitrogen) according to the manufacturer's protocol. M13forward, M13reverse, and pRF36882p (Pornwiroon et al. 2006) was used in the sequencing reaction to obtain sequence data for the inserts. At least two clones of each PCR amplicon were sequenced by the dye terminator method on a 3130 Genetic Analyzer (Applied Biosystems) at LSU-SVM to generate a consensus sequence using VectorNTI software (Invitrogen). Nucleotide similarity comparisons were assessed using the GenBank database.
Results and Discussion
In this report, we demonstrate R. felis in the salivary glands of fed cat fleas by microscopic and molecular analyses. The recovery of flea salivary glands is challenging, possibly limiting examination of R. felis in these tissues. Also, the high prevalence of R. felis in the colony increased the likelihood of detecting rickettsiae in a limited number of flea salivary glands. In C. felis salivary glands, R. felis (LSU) had typical Rickettsia morphology (Fig. 1). Rickettsiae were located free in the cytosol of epithelial cells. Increased clearance of cytoplasmic contents around R. felis, usually described as “halo,” was also observed. The findings are similar to our previous report for R. felis (LSU) in the tick-derived cell line ISE6 in which there were clear areas of cytosol associated with clusters of rickettsiae. Occasionally, we observed rickettsiae with altered morphology within membrane-bound cytoplasmic vacuoles. These rickettsiae usually had irregular shape, dense cytoplasm, enlarged periplasmic spaces and represented rickettsiae being destroyed within the phagolysosomes (not shown). Also some rickettsiae contained electron-lucent vacuoles in their cytoplasm (Fig. 1B) as demonstrated for R. felis earlier (Bouyer et al. 2001, Pornwiroon et al. 2006).
FIG. 1.
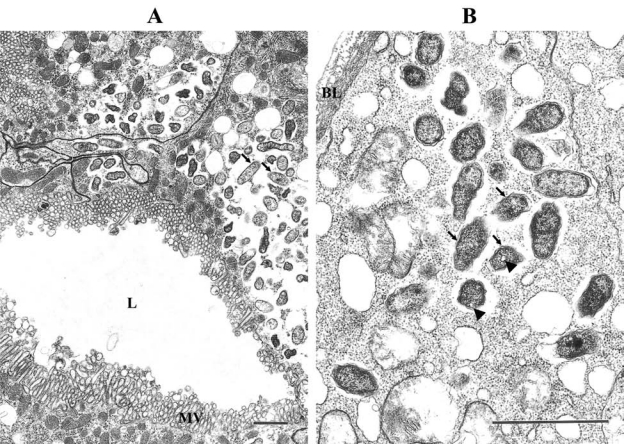
Ultrastructure of Rickettsia felis (Louisiana State University [LSU]) in cat flea salivary glands. Scale bar = 1 μm. (A) Rickettsiae free in the cytosol (arrows) of several epithelial cells. Microvilli (MV) extending into the lumen (L) of the salivary gland. (B) Rickettsiae are clustered in a basal part of a cell close to the basal lamina (BL) in an epithelial cell from a different flea. Arrowheads indicate small vacuoles in the rickettsial cytoplasm.
The presence of R. felis in the C. felis salivary glands was confirmed by PCR amplification and sequencing of the Rickettsia genus-specific 17-kDa antigen gene and R. felis plasmid (Fig. 2). Nucleotide sequences for the amplified portions of the 17-kDa antigen (434 bp) gene and pRF plasmid (1,342 bp) were identical to the sequences reported previously for R. felis (LSU) (Pornwiroon et al. 2006) and to the sequences reported for R. felis in the GenBank database (accession numbers CP000053 and AF195118 for 17-kDa antigen gene). Similar to our previous study (Pornwiroon et al. 2006), the portion of pRFδ plasmid was not amplified in all samples tested, including both the environmental blank incorporated into the gDNA isolation procedure and the negative control for the PCR (water). Additionally, we were unable to amplify rickettsial DNA from whole R. felis-negative C. felis (Heska Corp., Loveland, CO; data not shown). However, the amplification of rickettsial DNA with the pRFa and pRFb primer set indicates that the pRFa primer is valid. While pRF has been recently confirmed by pulse-field gel electrophoresis and southern blot analysis in R. felis (LSU) (Baldridge et al. 2007a), additional experimental evidence supports the lack of pRFδ in R. felis (LSU) and other R. felis strains examined (Jiang et al. 2006), and recent interpretation of the genome sequence suggests that only one plasmid may be present in the genome (Gillespie et al. 2007).
FIG. 2.
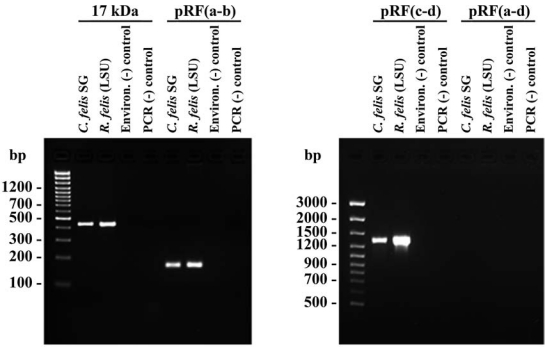
Polymerase chain reaction (PCR) amplification of genes encoding Rickettsia genus specific 17-kDa antigen and plasmid from Rickettsia felis (Louisiana State University [LSU])-infected cat flea salivary glands. In cat flea salivary gland samples and cultured R. felis (LSU), portions of genes encoding the 17-kDa antigen and pRF (primers pRFc-d), but not pRFδ (primers pRFa-d), were amplified. The amplification of a portion of pRF with the primer pair pRF(a-b) confirms the inability to detect pRFδ by PCR amplification. An environmental blank processed at the same time as samples and water served as negative controls for the genomic DNA extraction and PCR, respectively. Marker (100 bp) sizes are listed to the left of each gel.
Although there is potential for the fleas used in the current study to have acquired R. felis from the vertebrate host during the 3-day feeding period, we believe that this is not the case in this laboratory model for two reasons. First, the role of horizontal transmission for maintenance of R. felis is not clear, as horizontal transmission of viable R. felis via cat flea feeding has not been proven. Additionally, cat flea uptake of R. felis during bloodmeal acquisition, either directly from the vertebrate host or through cofeeding with infected fleas, has not been demonstrated (Wedincamp et al. 2002). Cats seroconvert after controlled exposure to previously unfed, infected fleas, indicating horizontal transmission; however, overt disease was not observed, and viable rickettsiae have not been recovered from cats (Wedincamp et al. 2000). Second, a recent study assessing R. felis infection in the LSU colony of newly-emerged unfed adult cat fleas, using whole fleas, has demonstrated approximately 94% prevalence of R. felis within this population of fleas (Pornwiroon et al. 2007). The possibility that R. felis disseminates to the salivary glands from other tissues in accompaniment with bloodmeal acquisition, versus being constitutively present in cat flea salivary glands, is interesting but unlikely. For example, vertically maintained bacteria (e.g., Wolbachia) are often identified in the salivary glands of insect hosts (Dobson et al. 1999). The potential interaction of bacteria within the flea host is intriguing; Wolbachia spp. 16S rRNA sequences have recently been amplified from the LSU colony of fleas (Pornwiroon et al. 2007). R. felis infection does impact the species richness of the microbiota (Pornwiroon et al. 2007), and the interaction between rickettsiae and other members of the bacterial community in relation to vector competence is under investigation.
The association between rickettsial infection of specific arthropod tissues and potential for horizontal transmission is not clear. Some tick-borne spotted fever group Rickettsia not associated with human disease, for example, Rickettsia peacockii, have limited tissue distribution within the invertebrate hosts (Dermacentor andersoni) in nature and are maintained via vertical transmission within the tick rather than horizontal transmission to vertebrate hosts during feeding (Burgdorfer et al. 1981, Niebylski et al. 1997). Additionally, although Rickettsia monacensis has recently been associated with human infection (Jado et al. 2007), it can be identified in the salivary glands of Ixodes scapularis in the absence of horizontal transmission (Baldridge et al. 2007b).
In summary, we report here the first ultra-structural and molecular characterization of R. felis in the salivary glands of cat fleas. The significance of R. felis, and other bacteria, in the salivary gland with respect to horizontal transmission is currently under investigation.
Acknowledgments
We thank Olga Borkhsenious and Violet Han for assistance with tissue preparation for electron microscopy. We also thank Mark Guillotte for technical assistance. This research was supported by Louisiana Board of Regents (LEQSF) and the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
References
- Adams JR. Schmidtmann ET. Azad AF. Infection of colonized cat fleas, Ctenocephalides felis (Bouche), with a rickettsia-like microorganism. Am J Trop Med Hyg. 1990;43:400–409. doi: 10.4269/ajtmh.1990.43.400. [DOI] [PubMed] [Google Scholar]
- Azad AF. Radulovic S. Higgins JA, et al. Flea-borne rickettsioses: ecologic considerations. Emerg Infect Dis. 1997;3:319–327. doi: 10.3201/eid0303.970308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad AF. Sacci JB., Jr Nelson WM, et al. Genetic characterization and transovarial transmission of a typhuslike rickettsia found in cat fleas. Proc Natl Acad Sci USA. 1992;89:43–46. doi: 10.1073/pnas.89.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge GD. Burkhardt NY. Felsheim RF, et al. Transposon insertion reveals pRM, a plasmid of Rickettsia monacensis. Appl Environ Microbiol. 2007a;73:4984–4995. doi: 10.1128/AEM.00988-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge GD. Kurtti TJ. Burkhardt N, et al. Infection of Ixodes scapularis ticks with Rickettsia monacensis expressing green fluorescent protein: a model system. J Invertebr Pathol. 2007b;94:163–174. doi: 10.1016/j.jip.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer DH. Stenos J. Crocquet-Valdes P, et al. Rickettsia felis: molecular characterization of a new member of the spotted fever group. Int J Syst Evol Microbiol. 2001;51:339–347. doi: 10.1099/00207713-51-2-339. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W. Hayes SF. Marvos AJ. Nonpathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distribution of Rickettsia rickettsii. In: Burgdorfer W, editor; Anacker RL, editor. Rickettsiae and Rickettsial Diseases. New York: Academic Press; 1981. pp. 585–594. [Google Scholar]
- Case JB. Chomel B. Nicholson W, et al. Serological survey of vector-borne zoonotic pathogens in pet cats and cats from animal shelters and feral colonies. J Feline Med Surg. 2006;8:111–117. doi: 10.1016/j.jfms.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson SL. Bourtzis K. Braig HR, et al. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem Mol Biol. 1999;29:153–160. doi: 10.1016/s0965-1748(98)00119-2. [DOI] [PubMed] [Google Scholar]
- Gillespie JJ. Beier MS. Rahman MS, et al. Plasmids and rickettsial evolution: insight from Rickettsia felis. PLoS ONE. 2007;2:e266. doi: 10.1371/journal.pone.0000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G. Foil LD. Efficacy of diflubenzuron in simulated household and yard conditions against the cat flea Ctenocephalides felis (Bouche) (Siphonoptera: Pulicidae) J Med Entomol. 1993;30:619–621. doi: 10.1093/jmedent/30.3.619. [DOI] [PubMed] [Google Scholar]
- Higgins JA. Sacci JB., Jr Schriefer ME, et al. Molecular identification of rickettsia-like microorganisms associated with colonized cat fleas (Ctenocephalides felis) Insect Mol Biol. 1994;3:27–33. doi: 10.1111/j.1365-2583.1994.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Ito S. Rikihisa Y. Techniques for electron microscopy of rickettsiae. In: Burgdorfer W, editor; Anacker RL, editor. Rickettsiae and Rickettsial Diseases. New York: Academic Press; 1981. pp. 213–240. [Google Scholar]
- Jado I. Oteo JA. Aldámiz M, et al. Rickettsia monacensis and human disease, Spain. Emerg Infect Dis. 2007;13:1405–1407. doi: 10.3201/eid1309.060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. Soeatmadji DW. Henry KM, et al. Gene sequences comparisons of Rickettsia felis from Indonesian Xenopsylla cheopis and R. felis from North Amercian Ctenocephalides felis; Presented at the 20th Meeting of the American Society for Rickettsiology; Asilomar, CA. 2006. [Google Scholar]
- Moron CG. Bouyer DH. Yu XJ, et al. Phylogenetic analysis of the rompB genes of Rickettsia felis and Rickettsia prowazekii European-human and North American flying-squirrel strains. Am J Trop Med Hyg. 2000;62:598–603. doi: 10.4269/ajtmh.2000.62.598. [DOI] [PubMed] [Google Scholar]
- Niebylski ML. Schrumpf ME. Burgdorfer W, et al. Rickettsia peacockii sp. nov., a new species infecting wood ticks, Dermacentor andersoni, in western Montana. Int J Syst Bacteriol. 1997;47:446–452. doi: 10.1099/00207713-47-2-446. [DOI] [PubMed] [Google Scholar]
- Ogata H. Renesto P. Audic S, et al. The genome sequence of Rickettsia felis identifies the first putative conjugative plasmid in an obligate intracellular parasite. PLoS Biol. 2005;3:e248. doi: 10.1371/journal.pbio.0030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P. Davoust B. Raoult D. Tick- and flea-borne rickettsial emerging zoonoses. Vet Res. 2005;36:469–492. doi: 10.1051/vetres:2005004. [DOI] [PubMed] [Google Scholar]
- Pornwiroon W. Pourciau SS. Foil LD, et al. Rickettsia felis from cat fleas: isolation and culture in a tick-derived cell line. Appl Environ Microbiol. 2006;72:5589–5595. doi: 10.1128/AEM.00532-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornwiroon W. Husseneder C. Kearney MT, et al. Comparative microbiota of Rickettsia felis-uninfected and -infected colonized cat fleas, Ctenocephalides felis. ISME J. 2007;1:394–402. doi: 10.1038/ismej.2007.38. [DOI] [PubMed] [Google Scholar]
- Psaroulaki A. Antoniou M. Papaeustathiou A, et al. First detection of Rickettsia felis in Ctenocephalides felis fleas parasitizing rats in Cyprus. Am J Trop Med Hyg. 2006;74:120–122. [PubMed] [Google Scholar]
- Wedincamp J., Jr Foil LD. Infection and seroconversion of cats exposed to cat fleas (Ctenocephalides felis Bouche) infected with Rickettsia felis. J Vector Ecol. 2000;25:123–126. [PubMed] [Google Scholar]
- Wedincamp J., Jr Foil LD. Vertical transmission of Rickettsia felis in the cat flea (Ctenocephalides felis Bouche) J Vector Ecol. 2002;27:96–101. [PubMed] [Google Scholar]
- Wedincamp J., Jr Foil LD. Rickettsia felis infection in the cat flea (Siphonaptera: Pulicidae) J Entomol Sci. 2003;38:234–239. [Google Scholar]
- Williams SG. Sacci JB., Jr Schriefer ME, et al. Typhus and typhuslike rickettsiae associated with opossums and their fleas in Los Angeles County, California. J Clin Microbiol. 1992;30:1758–1762. doi: 10.1128/jcm.30.7.1758-1762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala-Castro JE. Small M. Keng C, et al. Transcription of the Rickettsia felis ompA gene in naturally infected fleas. Am J Trop Med Hyg. 2005;73:662–666. [PMC free article] [PubMed] [Google Scholar]


