Abstract
We conducted serological studies, using epitope-blocking ELISAs directed at West Nile virus (WNV) and flavivirus antibodies, of wild birds in south-central Kansas, the first for this state, in the winters of 2003–04 through 2005–06. Overwintering migratory species (primarily the American tree sparrow and dark-eyed junco) consistently showed significantly lower seropositivity than permanent residents (primarily the northern cardinal). The cardinal showed annual variation in seropositivity between winters. Of 35 birds that were serial sampled within a single winter, one cardinal may have seroconverted between late December and mid-February, providing a preliminary suggestion of continued enzootic transmission, chronic infection, or bird-bird transfer as overwintering mechanisms. Breeding population size of the cardinal did not change after the introduction of WNV to Kansas. Of eighteen birds that were serial sampled between winters, none seroconverted. Among overwintering migrants, the Harris' Sparrow showed the highest seropositivity, possibly related to its migration route through the central Great Plains, an area of recent high WNV activity. The finding that permanent resident birds exhibit higher seropositivity than migrant birds suggests that resident birds contribute to the initiation of annual infection cycles, although this conclusion is speculative in the absence of data on viral titers and the length of viremia. Key Words: West Nile Virus—flavivirus—birds—epitope-blocking ELISA––winter.
Introduction
The New World outbreak of WNV, begun in 1999 (Lanciotti et al. 2000, Nash et al. 2001), has been remarkable in the number of bird deaths observed, with crows and jays (corvids) appearing to be especially susceptible. The pattern of reported dead crows correlates with the incidence of disease in humans in geographical (Eidson et al. 2001a,b) and statistical (Guptill et al. 2003) models. Studies based on dead birds are important, but further understanding of the spread and maintenance of WNV in the environment benefits from studies of wild bird exposure. For example, migrating birds have been suggested as important in the rapid geographic spread of WNV in both the Old World (Malkinson and Banet 2002) and New World (Rappole et al. 2000; Peterson et al. 2003), as well as in driving local WNV epidemics in humans (Kilpatrick et al. 2006). Apparently, reintroduction of WNV by migrant species to initiate an annual transmission cycle at a given location is not necessary (Tesh et al. 2004). Furthermore, the mechanism(s) by which WNV overwinters at a given location is unclear (Reeves 1974; Hubalek and Halouzka 1999); indeed, the mechanism(s) by which arboviruses overwinter poses a long-standing question (Tesh 1984).
The goal of the current study was to use wild bird surveys and re-capture studies to better understand ecological factors affecting the spread of WNV, i.e. migratory status and winter virus survivorship. We report on the results of epitope-blocking ELISAs detecting WNV antibodies in overwintering migrant and permanent resident bird species in south-central Kansas during the winters of 2003–04 through 2005–06. Serial sampling was used to detect within-winter seroconversion of overwintering passerines, that could provide preliminary support for a role of enzootic transmission and/or chronic infection as overwintering mechanisms (Reisen et al. 2006). In addition, many avian species were captured and assayed in the first general survey of seropositivity in this geographic region. In particular, we focused on the northern cardinal, recently suggested as a competent WNV reservoir (Marshall et al. 2006). Finally, to evaluate further the likelihood of the northern cardinal as a WNV reservoir, breeding population size was measured at the primary study site, before and after the introduction of WNV to Kansas.
Materials & Methods
Species collection
Avian species were captured in mist nets for blood sampling at four winter feeding stations at the Wichita State University Ninnescah Field Station during the winters of 2003–04, 2004–05, and 2005–06. The station includes 133 hectares along the Ninnescah River in southwestern Sedgwick County, Kansas (37°32′N 97°41′W). Captured species (Table 1) included common members of the avian community wintering in riparian, scrubland, and grassland habitats. Individual birds were banded upon initial capture with a uniquely numbered aluminum U.S. Geological Survey numerical band on the left leg. Serial sampling of banded individual birds was conducted within the winters 2004–05 and 2005–06, with at least 10 d between sampling. Only northern cardinals and birds banded in earlier winters were sampled during winter 2005–06.
Table 1.
Rate of Positive Reactions in Epitope-Blocking ELISAs Using WNV-Specific (WNV) and Flavivirus Group Reactive (FV) Primary Antibodies in Permanent Resident and Overwintering Migratory Species in Two Winters in South-Central Kansas
| |
|
Percent seropositive (n sampled, n positive) |
|||||
|---|---|---|---|---|---|---|---|
| |
|
2003–04 winter |
2004–05 winter |
||||
| Species | Scientific name (migratory status) | WNV | FV | WNV+FV | WNV | FV | WNV+FV |
| Red-bellied woodpecker | Melanerpes carolinus (resident) | na | na | na | 0 (1, 0) | 0 (1, 0) | 0 (1, 0) |
| Downy woodpecker | Picoides pubescens (resident) | na | na | na | 0 (5, 0) | 20.0 (5, 1) | 0 (5, 0) |
| Blue jay | Cyanocitta cristata (migratory) | 0 (3, 0) | 0 (3, 0) | 0 (3, 0) | 50.0 (2, 1) | 50.0 (2, 1) | 50.0 (2, 1) |
| Black-capped chickadee | Poecile atricapillus (resident) | na | na | na | 0 (1, 0) | 0 (1, 0) | 0 (1, 0) |
| Eastern bluebird | Sialia sialis (migratory) | na | na | na | 100.0 (1, 1) | 100.0 (1, 1) | 100.0 (1, 1) |
| American tree sparrow | Spizella arborea (migratory) | 2.6 (192, 5) | 2.1 (192, 4) | 1.0 (192, 2) | 1.3 (151, 2) | 4.6 (151, 7) | 0.7 (151, 1) |
| Song sparrow | Melospiza melodia (migratory) | 12.5 (16, 2) | 0 (16, 0) | 0 (16, 0) | 5.0 (21, 1) | 0 (21, 0) | 0 (21, 0) |
| White-throated sparrow | Zonotrichia albicollis (migratory) | na | na | na | 0 (7, 0) | 0 (7, 0) | 0 (7, 0) |
| Harris' sparrow | Zonotrichia querula (migratory) | 13.0 (23, 3) | 39.1 (23, 9) | 13.0 (23, 3) | 2.0 (54, 1) | 0 (54, 0) | 0 (54, 0) |
| Dark-eyed junco | Junco hyemalis (migratory) | 0 (14, 0) | 7.1 (14, 1) | 0 (14, 0) | 0 (92, 0) | 1.1 (92, 1) | 0 (92, 0) |
| Northern cardinal | Cardinalis cardinalis (resident) | 42.8 (28, 12) | 46.4 (28, 13) | 35.7 (28, 10) | 19.1 (68, 13) | 10.3 (68, 7) | 8.8 (68, 6) |
| Red-winged blackbird | Aegelaius phoenicius (migratory) | na | na | na | 50.0 (2, 1) | 50.0 (2, 1) | 50.0 (2, 1) |
| American goldfinch | Carduelis tristis (migratory) | na | na | na | 0 (2, 0) | 0 (2, 0) | 0 (2, 0) |
| Migrants | 4.0 (248, 10) | 5.6 (248, 14) | 2.0 (248, 5) | 2.1 (332, 7) | 3.3 (332, 11) | 1.2 (332.4) | |
| Residents | 42.8 (28, 12) | 46.4 (28, 13) | 35.7 (28, 10) | 17.3 (75, 13) | 10.7 (75, 8) | 8.0 (75, 6) | |
| Total | 8.0 (276, 22) | 9.8 (276, 27) | 5.4 (276, 15) | 4.9 (407, 20) | 4.7 (407, 19) | 2.5 (407, 10) | |
Breeding bird populations at the field station are routinely estimated each May with the spot-mapping method (Koskimies and Vaisanen 1991). To estimate the effect of WNV mortality on abundance, annual report data are presented for the northern cardinal. From one to four plots were censused each year, with the average number of cardinals per plot taken as a measure of breeding population density.
Serum collection
Immediately after capture, blood samples were acquired using a sterile 1-ml syringe fitted with a needle (27 g, 5/8 in) to puncture the left or right brachial vein of the bird. Whole blood was collected in 70-μl microhematocrit capillary tubes and allowed to coagulate for at least 15 min. After collection, a piece of paper towel was applied to the puncture wound to promote clotting. When the wound stopped bleeding, the bird was released and observed for obvious flight impairment. Individuals were in captivity no longer than 30 min and were kept warm in holding bags before sampling to minimize stress.
After coagulation of the blood samples, the microhematocrit capillary tubes were clarified using a specialized centrifuge (Damon/IEC Division) for 3 min at 8 × g at room temperature. The serum was then collected using a syringe, and placed in externally threaded cryotubes as mandated by the Centers for Disease Control and Prevention (CDC 2003). Samples were stored briefly at 4°C, transported on ice from the field, and then stored at −80°C until laboratory evaluation.
Epitope-blocking ELISA
Serum samples were assayed for flavivirus antibodies using an epitope-blocking ELISA, as previously described (Hall et al. 1995; Blitvich et al. 2003a,b). Briefly, two replica 96-well microtiter plates were prepared, one for each of two primary mouse IgG antisera, MAb 3.1112G and MAb 6B6C-1 (Chemicon). MAb 3.1112G is specific for a WNV NS1 epitope and MAb 6B6C-1 is flavivirus group reactive, detecting an E protein epitope (Hall et al. 1995; Blitvich et al. 2003a). Wells were coated with WNV antigen (supplied by the Arbovirus Infectious Disease Laboratory at Colorado State University) at empirically determined working dilutions in carbonate-bicarbonate buffer (50 mM sodium carbonate, 50 mM sodium bicarbonate, pH 9.6) with incubation overnight at 4°C (Blitvich et al. 2003b). Coated plates were washed 4 times with PBS (pH 7.4) supplemented with 0.1% (v/v) Tween 20, using an automated plate washer (BioTek Instruments ELx 50 Automated Stripwasher). Blocking buffer (PBS supplemented with 0.1% (v/v) Tween 20 and 5% (w/v) non-fat dry milk) was added after washing and the plates were incubated for 40 min at 37°C. After washing as above, 1:10 dilutions of serum samples in blocking buffer were added in triplicate to the coated wells and incubated at 37°C for 2 h. Each plate included four negative-control wells of uninfected chicken serum (Fisher), and two positive-control wells of seropositive horse serum (Arbovirus Infectious Disease Laboratory). After washing, appropriate monoclonal antibody dilutions were added in blocking buffer, and incubated at 37°C for 1 h. Quantification of primary monoclonal antibody binding was performed with horseradish peroxidase-conjugated rabbit anti-mouse IgG secondary antibody (Zymed Laboratories) diluted to 1:2000 in blocking buffer and then incubated at 37°C for 1 h. After washing as above, ABTS (2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) solution was added to induce a color reaction with horseradish peroxidase. The optical density of each well was determined using an automated plate reader (BioTek Instruments ELx 800 Universal Microplate Reader) at 405 nm (Blitvich et al. 2003b).
The percent inhibition of primary antibody binding was calculated (Hall et al. 1995) as follows:
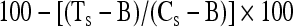 |
where TS is the mean optical density from the test serum, CS is the mean optical density from the negative-control serum (from uninfected chickens), and B is the background optical density. The assays were acceptable when the mean optical density of CS exceeded 0.3. A percent inhibition value of ≥30% was the threshold for scoring a positive response (Blitvich et al. 2003b). Mean values of triplicate samples are reported, although variances were very small among replicates.
Results
General survey
In the following analyses, dark-eyed juncos and American tree, song, Harris', and white-throated sparrows are considered overwintering migrants, as south-central Kansas is south of the breeding range of all five species. The red-winged blackbird, eastern bluebird and American goldfinch are considered possible wintering migrants, as at least some of their species populations are well known to be migratory (Yasukawa and Searcy 1995; Gowaty and Plissner 1998; Middleton 1998, respectively). The migratory status of the blue jay is less clear, but some individuals depart for the winter throughout the breeding range (Tarvin and Woolfenden 1999). The red-bellied and downy woodpeckers and the black-capped chickadee are assumed to be permanent residents, showing no evidence of regular migration throughout their breeding range, and occasional irruptive or other irregular long-distance movements are largely confined to northern regions, distant from our study site in south-central Kansas (Shackelford et al. 2000; Jackson and Ouellet 2000; Smith 1993, respectively,). The northern cardinal is nonmigratory throughout its breeding range, and 90% of banded birds recovered dead were found within the same 10-minute block of latitude/longitude of where they were banded (Halkin and Linville 1999). These are general categories, and unusual dispersal or other movements of individual birds, or birds remaining on the breeding grounds in atypical fashion, cannot be ruled out.
A total of 683 wild birds was sampled during the two main winters of the study: 407 overwintering migrants, and 276 permanent residents. Sampling was conducted on days dispersed throughout each winter, to determine whether the abundance of seropositive birds increased at any time during the winter months; no clear relationship was observed. During each winter, all common species in the winter bird community were sampled. Of 13 wintering bird species sampled in the winters of 2003–04 and 2004–05, nine were seropositive in one or both ELISAs (Table 1). Among permanent resident species, the northern cardinal consistently showed the highest seropositivity (Table 1). The most frequently sampled wintering migrant, the American tree sparrow, showed low seropositivity, as did most other migrants wintering at the field station (Table 1). A clear exception was the Harris' sparrow in the 2003–04 winter. The frequencies of positive ELISAs with WNV-specific or flavivirus group reactive MAbs, or to both primary antibodies, differed significantly among permanent residents and overwintering migrants in the winters of 2003–04 (x2 = 52.648, 63.414, and 56.938, respectively; all P < 0.001, Table 1) and 2004–05 (x2 = 30.169, 7.439, and 12.247, respectively; all P < 0.01, Table 1). Two bird species, a permanent resident and a migrant, showed significant annual variation in seropositivity. For simplicity, this analysis is restricted to birds giving a positive response in both ELISAs. For the northern cardinal (permanent resident), 9 of 28 birds were seropositive in both ELISAs in the winter of 2005–06. Northern cardinal seropositivity did not differ significantly between the 2003–04 and 2005–06 winters (x2 = 0.010, P < 0.95), hence these data were pooled. In addition, there was no significant sex difference in seropositivity within winters for the cardinal (all x2 ≥ 1.334, all P < 0.10), justifying pooling data from males and females. The frequency of positive responses in both ELISAs differed significantly between 2003–04+ 2005–06 (pooled) and 2004–05, being higher in the former (33.9%) than the latter (8.8%) (x2 = 13.911, P < 0.001). In the Harris' sparrow (overwintering migrant), seropositivity was significantly higher in 2003–04 (13.0%) than in 2004–05 (0%, Table 1; x2 = 7.281, P < 0.01).
Serial sampling
A total of 35 birds of seven species was serial sampled within a single winter (Table 2). A striking feature of this data set is the potential seroconversion of one northern cardinal (35537), that may have converted from seronegative on 28 December 2004 to seropositive in an ELISA on 19 February 2005. Both 6B6C tests were at or below 10.3% inhibition, and the 3.1112G December and February tests were at 17.1 and 29.6% inhibition, respectively, the last result only marginally below the 30% cutoff.
Table 2.
Seroconversion from Negative (neg) to Positive (pos) Status in Epitope-Blocking ELISAs for Both MAb 3112 and MAb 6B6C-1, Within a Single Winter, in One Piciform and Six Passerine Bird Species, Sampled in South-Central Kansas, During Winters of 2003–04, 2004–05, and 2005–06
| |
Category |
||||
|---|---|---|---|---|---|
| Common name | Neg-neg | Neg-pos | Pos-neg | Pos-pos | Neg-neg-neg |
| Red-bellied woodpecker | 1 | 0 | 0 | 0 | na |
| American tree sparrow | 4 | 0 | 0 | 0 | na |
| Song sparrow | 2 | 0 | 0 | 0 | na |
| White-throated sparrow | 1 | 0 | 0 | 0 | na |
| Harris' sparrow | 3 | 0 | 0 | 0 | 2 |
| Dark-eyed junco | 13 | 0 | 0 | 0 | 1 |
| Northern cardinal | 6 | 1 | 0 | 1 | na |
| Total | 30 | 1 | 0 | 1 | 3 |
Species are presented in taxonomic order; scientific names are given in Table 1.
A total of 18 birds of four species was serial sampled between winters, distributed as follows: 11 American tree sparrows; 4 dark-eyed juncos; 1 Harris' sparrow; 2 northern cardinals. The 16 non-cardinals were seronegative initially and remained seronegative in a subsequent year; for the cardinal, both birds were seropositive initially and remained seropositive in a subsequent year.
Northern cardinal population census
There was little variation in the estimated size of the breeding population of northern cardinals at the field station, when comparing data from before the WNV invasion (2000–01), the year of WNV invasion (2002), and after WNV invasion in Kansas (2003–05). On average, between five and six birds were recorded per plot.
Discussion
In the current study, permanent resident bird species exhibited high seropositivity compared with most wintering migratory species based on epitope-blocking ELISAs that detect WNV antibodies. It is possible that permanent resident bird species may contribute to the initiation of annual WNV infection cycles, with the potential for long contact periods with vectors in the area, although the current study does not directly address this issue. The northern cardinal was the most abundant permanent resident species sampled and it also had the highest seropositivity among all species, permanent resident or overwintering migrant. The northern cardinal is abundant in both rural and urban locales, and may nest in moist areas where mosquitoes can be abundant. It should be pointed out that high seropositivity could be manifest as a dampening of the force of transmission that could explain the between-winter variations in seropositivity. Seroprevalence in the cardinal was high in three other studies, in Ohio (Marshall et al. 2006; 25%), Louisiana (Komar et al. 2005; 48%), and Illinois (Ringia et al. 2004; 12%). High rates of seropositive northern cardinals suggest that high rates of survival are likely, in contrast to the American crow that typically succumbs to WNV within a week after experimental infection (Komar et al. 2003)
This conclusion is further evidenced by bird census data from the field site, showing no change in population density following the introduction of WNV. Other birds from unaffected sites may have moved into the study site to fill breeding vacancies at the field station, although there is no a priori reason to expect that surrounding cardinal populations were not also exposed to WNV. In addition, northern cardinals are rarely reported to health officials in dead bird surveys (Bernard et al. 2001; Eidson et al. 2001a,b; Marfin et al. 2001; Blackmore et al. 2003), even though it is a relatively common bird species and the same general size as the blue jay, which is frequently reported. Those cardinals exposed to WNV may develop viral titers high enough to infect vectors, and then recover. A caveat is that to determine the actual role of a given bird species in contributing to local viral transmission, both the period of viremia and viral titer need to be known, and the current study did not measure these parameters. However, this species is apparently capable of infecting appreciable numbers of mosquito vectors, and was a competent WNV reservoir in Louisiana (Komar et al. 2005).
The present study found evidence that a northern cardinal may have seroconverted between two samplings in the same winter (28 December 2004 and 19 February 2005) suggesting overwinter survival of WNV at the study site. Three mechanisms have been proposed for the overwintering of WNV: (1) continued enzootic transmission, (2) chronic infections in living birds, and (3) vertical transmission of virus (Reisen et al 2006). Of these, within-winter seroconversion of an individual bird is consistent with the first two mechanisms. At winter feeding stations of the type used in the current study, infected individuals may consume food contaminated with fecal materials harboring WNV or experience more direct contact with infected birds or scat. Outbreaks due to in-contact transmission have been suggested for chickens, geese, and shrikes (Langevin et al. 2001; Austin et al. 2004; Bertelson et al. 2004), and infection by an oral route, whether by imbibing inoculating solution or ingesting dead mosquito, mice, or bird flesh, led to viremia in American crows, common grackles, house finches, and house sparrows (Komar et al. 2003). If transmission occurs among a group of birds over the course of a winter, the transfer of live virus without vectors represents another possible overwintering mechanism. At present, it may only be concluded that further data are needed to evaluate these possibilities. Some indication of the potential importance of non-mosquito transmission in the spread and initiation of viral activity is given by a mathematical modeling study (Naowarat and Tang 2004). Here earlier models of annual transmission cycles were modified to include bird-to-bird transmission through aerosols.
The Harris' sparrow was the migratory species that showed the highest rate of seropositive individuals. Unlike the other migrants sampled, it has a very limited north-south migration corridor, restricted to the central plains in North America. In this region, particularly North Dakota and Saskatchewan, WNV infection rates among breeding and fall migratory birds are relatively high (Bell et al. 2006). Migratory Harris' sparrows may therefore become exposed during migration between their arctic/subarctic breeding grounds and lower central Great Plains wintering grounds. Exposure may vary annually, due to yearly variation in temperature-dependent transmission cycles in the region (Bell et al. 2006). All other migratory species (including presumed migrants) sampled showed lower seropositivity, with the American tree sparrow, dark-eyed junco and song sparrow the most frequently sampled. Of the 16 birds of definite migratory status and that were serial sampled within a given winter, none sero-converted. Without survivorship curves of WNV-inoculated birds, conclusions concerning their role in geographic viral transfer are problematic, although recent experiments indicate that migratory birds may show normal behavior when viremic (Owen et al. 2006).
Evidence suggests that WNV is carried over long distances by migrating birds (Rappole et al. 2000; Peterson and Roehrig 2001; Peterson et al. 2003; Owen et al. 2006). What has been called the “migrant bird as introductory host” theory (Rappole and Hubalek 2003) is widely accepted, although there are some indications that this may not be suitable for explaining the New World outbreak. If migrant birds were central to the spread of WNV in the New World, then it might be expected to have occurred more quickly. Migrating birds can cover hundreds of km each day, while the spread of WNV was closer to 70 km per month (Rappole and Hubalek 2003). In addition, typical migrations are on the north-south axis, while the virus spread virtually east to west. The most susceptible species (i.e., crows) die from the disease and are not likely to move large distances while viremic, especially since viremia is usually for a short period. The spread of WNV from the initial New York outbreak occurred in concentric circles from the epicenter and does not appear to follow the pattern expected for dissemination by migrant birds. The dissemination of WNV is probably through a combination of mosquitoes, resident birds, and migratory birds, that results in the introduction and maintenance of the virus in new and old areas.
In the present study, cardinals showed significant annual variation in seropositivity, suggesting annual variation in local ecological factors underlying WNV outbreaks in south-central Kansas. WNV was first detected in Kansas in 2002, with 22 human cases confirmed by the Kansas Department of Heath and Environment. From 2003–2006, confirmed human cases in Kansas numbered 90, 25, 46, and 90, respectively. No apparent relationship exists between seropositivity in cardinals and reported cases in humans, indicating that seropositivity in the most commonly seropositive bird species is not a good predictor of WNV infection rates in humans.
Acknowledgements
The authors are grateful for the kind assistance of Maria Alba, Barry Beaty, Bradley Blitvich, and Nicole Marlenee of Colorado State University, for training students, supplying antigen, and performing some of the ELISAs reported here. We also thank David Fields, Micaela Fisher, Patrick Herd, Chris Stanton, and Trista Newville for their assistance in the field, and Donald Distler, Director of the WSu Field Station, for assistance with logistical support. This research was supported by grants from NIH NCRR through the Kansas Biomedical Research Infrastructure Network (P20 RR16475). This is contribution number 14 of the Nin-nescah Field Station.
References
- Austin RJ. Whiting TL. Anderson RA. Drebot MA. An outbreak of West Nile virus-associated disease in domestic geese (Anser anser domesticus) upon initial introduction to a geographic region, with evidence of bird to bird transmission. Can Vet J. 2004;45:117–123. [PMC free article] [PubMed] [Google Scholar]
- Bell JA. Brewer C. Mickelson NJ. Garman GW. Vaughan JA. West Nile virus epizootiology, central Red River Valley, North Dakota and Minnesota, 2002–2005. Emerg Infect Dis. 2006;12:1245–1247. doi: 10.3201/eid1208.060129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K. Maffei JG. Jones S. Kauffman E, et al. West Nile virus infection in birds and mosquitoes, New York State, 2000. Emerg Infect Dis. 2001;7:679–685. doi: 10.3201/eid0704.010415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen MF. Ølberg R-A. Crawshaw GJ. Dibernardo A, et al. West Nile virus infection in the eastern loggerhead shrike (Lanius ludovicianus migrans): pathology, epidemiology, and immunization. J Wildlife Dis. 2004;40:538–542. doi: 10.7589/0090-3558-40.3.538. [DOI] [PubMed] [Google Scholar]
- Blackmore CGM. Stark LM. Jeter WC. Oliveri RL, et al. Surveillance results from the first West Nile virus transmission season in Florida, 2001. Am J Trop Med. 2003;69:141–150. [PubMed] [Google Scholar]
- Blitvich BJ. Bowen RA. Marlenee NL. Hall RA, et al. Epitope-blocking enzyme-linked immunosorbent assays for the detection of serum antibodies to West Nile virus in domestic mammals. J Clin Microbiol. 2003a;41:2676–2679. doi: 10.1128/JCM.41.6.2676-2679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitvich BJ. Marlenee NL. Hall RA. Calisher CH, et al. Epitope-blocking enzyme-linked immunosorbent assays for the detection of serum antibodies to West Nile virus in multiple avian species. J Clin Microbiol. 2003b;41:1041–1047. doi: 10.1128/JCM.41.3.1041-1047.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Infectious Diseases. Fort Collins, Co. Epidemic/Epizootic West Nile Virus in the United States: Guidelines for Surveillance, Prevention, and Control. 2003. 3rd revision.
- Eidson M. Komar N. Sorhage F. Nelson R, et al. Crow deaths as a sentinel surveillance system for West Nile virus in the northeastern United States, 1999. Emerg Infect Dis. 2001a;7:615–620. doi: 10.3201/eid0704.010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson M. Kramer L. Stone W. Hagiwara Y, et al. Dead bird surveillance as an early warning system for West Nile virus. Emerg Infect Dis. 2001b;7:631–635. doi: 10.3201/eid0704.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowaty P. Plissner JH. Eastern Bluebird (Sialia sialis) In: Poole A, editor; Gill F, editor. The Birds of North America. The Birds of North America, Inc.; Philadelphia, PA: 1998. No. 381. [Google Scholar]
- Guptill SC. Julian KG. Campbell GL. Price SD. Marfin AA. Early-season avian deaths from West Nile virus as warning of human infection. Emerg Infect Dis. 2003;9:483–484. doi: 10.3201/eid0904.020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkin SL. Linville SU. Northern Cardinal (Cardinalis cardinalis) In: Poole A, editor; Gill F, editor. The Birds of North America. The Birds of North America, Inc; Philadelphia, PA: 1999. No. 440. [Google Scholar]
- Hall RA. Broom AK. Hartnett AC. Howard MJ. Mackenzie JS. Immunodominant epitopes on the NS1 protein of MVE and KUN viruses serve as targets for a blocking ELISA to detect virus-specific antibodies in sentinel animal serum. J Virol Meth. 1995;51:201–210. doi: 10.1016/0166-0934(94)00105-p. [DOI] [PubMed] [Google Scholar]
- Hubalek Z. Halouzka J. West Nile fever—A reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 1999;5:643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JA. Ouellet HR. Downy Woodpecker (Picoides pubescens) In: Poole A, editor; Gill F, editor. The Birds of North America. The Birds of North America, Inc; Philadelphia, PA: 2002. No. 613. [Google Scholar]
- Kilpatrick AM. Kramer LD. Jones MJ. Marra PP. Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biology. 2006;4:e82. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N. Langevin S. Hinten S. Memeth N, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N. Panella NA. Langevin SA. Brault AC. Amador M. Edwards E. Owen JC. Avian hosts for West Nile virus in St. Tammany Parish, Louisiana, 2002. Am J Trop Med Hug. 2005;73:1031–1037. [PubMed] [Google Scholar]
- Koskimies P. Vaisanen RA. Zoological Museum, Finnish Museum of Natural History. University of Helinski; 1991. Monitoring bird populations Helinski; 145 pp. [Google Scholar]
- Lanciotti RS. Kerst AJ. Nasci RS. Godsey MS, et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin SA. Bunning M. Davis B. Komar N. Experimental infection of chickens as candidate sentinels for West Nile virus. Emerg Infect Dis. 2001;7:726–729. doi: 10.3201/eid0704.010422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkinson M. Banet C. The role of birds in the ecology of West Nile virus in Europe and Africa. Curr Top Microbiol Immunol. 2002;267:309–322. doi: 10.1007/978-3-642-59403-8_15. [DOI] [PubMed] [Google Scholar]
- Marfin AA. Petersen LR. Eidson M. Miller J, et al. Widespread West Nile virus activity, eastern United States, 2000. Emerg Infect Dis. 2001;7:730–735. doi: 10.3201/eid0704.010423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JS. Zuwerink AZ. Restifo RA. Grubb TC. West Nile virus in the permanent-resident bird community of a fragmented Ohio landscape. Ornithol Monogr. 2006;60:79–85. [Google Scholar]
- Middleton ALA. American Goldfinch (Carduelis tristis) In: Poole A, editor; Gill F, editor. The Birds of North America. The Birds of North America, Inc; Philadelphia, PA: 1993. No. 80. [Google Scholar]
- Naowarat S. Tang I. Effect of bird-to-bird transmission of the West Nile virus on the dynamics of the transmission of this disease. SE Asian J Trop Med Public Health. 2004;35:162–166. [PubMed] [Google Scholar]
- Nash DF. Mostashari A. Fine J. Miller D, et al. The outbreak of West Nile virus infection in the New York City area in 1999. New Eng J Med. 2001;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- Owen J. Moore F. Panella N. Edwards E, et al. Migrating birds as dispersal vehicles for West Nile virus. Eco-Health. 2006;3:79–85. [Google Scholar]
- Peterson LR. Roehrig JT. West Nile virus: A reemerging global pathogen. Emerg Infect Dis. 2001;7:611–622. doi: 10.3201/eid0704.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AT. Vieglais DA. Andreasen JK. Migratory birds modeled as critical transport agents for West Nile virus in North America. Vector-Borne Zoonot Dis. 2003;3:27–37. doi: 10.1089/153036603765627433. [DOI] [PubMed] [Google Scholar]
- Rappole JH. Derrickson SR. Hubalek Z. Migratory birds and spread of West Nile virus in the Western Hemisphere. Emerg Infect Dis. 2000;6:319–328. doi: 10.3201/eid0604.000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappole JH. Hubalek Z. Migratory birds and West Nile virus. J Appl Microbiol. 2003;94:47s–58s. doi: 10.1046/j.1365-2672.94.s1.6.x. [DOI] [PubMed] [Google Scholar]
- Reeves WC. Overwintering of arboviruses. Prog Med Virol. 1974;17:193–220. [PubMed] [Google Scholar]
- Reisen WK. Fang Y. Lothrop HD. Martinez VM, et al. Overwintering West Nile virus in southern California. J Med Entomol. 2006;43:344–355. doi: 10.1603/0022-2585(2006)043[0344:oownvi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ringia AM. Blitvich BJ. Hyun-Young K. Van de Wyn-gaerde M, et al. Antibody prevalence of West Nile virus in birds, Illinois, 2002. Emerg Infect Dis. 2004;10:120–124. doi: 10.3201/eid1006.030644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford CE. Brown RE. Conner RN. Red-bellied Woodpecker (Melanerpes carolinus) In: Poole A, editor; Gill F, editor. The Birds of North America. The Birds of North America, Inc; Philadelphia, PA: 2000. No. 500. [Google Scholar]
- Smith SM. Black-capped Chickadee (Parus atricapillus) In: Poole A, editor; Gill F, editor. The Birds of North America. The Birds of North America, Inc; Philadelphia, PA: 1993. No. 39. [Google Scholar]
- Tarvin KA. Woofenden GE. Blue Jay (Cyanocitta cristata) In: Poole A, editor; Gill F, editor. The Birds of North America. The Birds of North America, Inc; Philadelphia, PA: 1999. No. 469. [Google Scholar]
- Tesh RB. Transovarial transmission of arboviruses their invertebrate vectors. In: Harris KG, editor. Current Topics in Vector Research. Vol. 2. New York: Praeger Scientific; 1984. pp. 57–76. [Google Scholar]
- Tesh RB. Parsons R. Siirin M. Randle Y, et al. Year-round West Nile virus activity, Gulf Coast region, Texas and Louisiana. Emerg Infect Dis. 2004;10:1649–1652. doi: 10.3201/eid1009.040203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa K. Searcy WA. Red-winged Blackbird (Aegelaius phoenicius) In: Poole A, editor; Gill F, editor. Halkin, SL, The Birds of North America. The Birds of North America, Inc; Philadelphia, PA: 1995. No. 184. [Google Scholar]


