Abstract
Alpha-particle-emitting elements are of increasing importance as environmental and occupational carcinogens, toxic components of radiation dispersal devices and accidents, and potent therapeutics in oncology. Alpha particle radiation differs from radiations of lower linear energy transfer in that it predominantly damages DNA via direct action. Because of this, radical scavengers effective for other radiations have had only limited effect in mitigating alpha particle toxicity. We describe here a simple assay and a pilot screen of 3,119 compounds in a high-throughput screen (HTS), using the alpha-particle-emitting isotope, 225Ac, for the discovery of compounds that might protect mammalian cells from alpha particles through novel mechanisms. The assay, which monitored the viability of a myeloid leukemic cell line upon alpha particle exposure, was robust and reproducible, yielding a Z' factor of 0.66 and a signal-to-noise ratio of nearly 10 to 1. Surprisingly, 1 compound emerged from this screen, epoxy-4,5-α-dihydroxysantonin (EDHS), that showed considerable protective activity. While the value of EDHS remains to be determined, its discovery is a proof of concept and validation of the utility of this HTS methodology. Further application of the described assay could yield compounds useful in minimizing the toxicity and carcinogenesis associated with alpha particle exposure.
Introduction
Alpha particles are a form of extremely potent, short-ranged (50–80 μm in aqueous), and highly energetic (5–8 MeV) radiation capable of inducing gross chromosomal changes and killing individual cells.1 Depositing a large amount of its energy over such a short range, or high linear energy transfer (LET), distinguishes alpha emissions from the more common, low LET, gamma and beta radiations. It is precisely these properties that make alpha particle emitters highly lethal toxins, carcinogens, and, when targeted properly, potent therapeutic agents.
Targeted alpha-particle-emitting nuclides have shown great promise in a number of neoplastic disease models2,3 and are currently in human clinical trials for leukemia,4 ovarian cancer,5 gliomas,6 and bone metastases from refractory disease.7 Alpha emitters are >100 times as potent as traditional radiopharmaceuticals in current clinical use.1 The therapeutic window for such agents is limited in part by the extent of alpha-particle-mediated damage to normal tissues through nonspecific targeting, tissue diffusion, or through the course of metabolism and clearance.
In contrast to the therapeutic potential of alpha-emitting nuclides, 210Po was recently used in a highly publicized poisoning case in only sub-microgram quantities, which led to acute radiation sickness and death.8 Additionally, alpha-emitting isotopes have gained notoriety for their potential use by terrorists in radiation dispersal devices, commonly known as “dirty bombs,” where alpha particle emitters could enter the body following a conventional explosion.9
A far more common source of alpha particle exposure is 222Rn, which is found in appreciable concentrations in ground water, soil, and air, and can pose significant carcinogenic hazards when solid, charged alpha-emitting daughters adhere to sensitive mucosal epithelium. 222Radon and its progeny are responsible for 50% of environmental radiation exposure.10 The increased risk of lung cancer from industrial exposure to alpha particles such as in uranium, iron, and tin mines has been known for a number of years,11–13 and definitive evidence linking lung cancer with even moderate household exposure to 222Ra has been reported.14 222Radon is estimated to be the second leading cause of lung cancer in the United States behind cigarette smoke, and the number one cause in nonsmokers,15 underscoring the carcinogenicity of internalized alpha particle emitters.
Compounds that protect tissues from gamma radiation have been identified and characterized, dating back to the 1950s16,17 and share common chemical characteristics of radical scavenging and hydrogen donation. While these compounds can be highly effective for low-LET radiations, they offer only limited protection from alpha particle radiation.18,19 This evidence has led to the widely held assumption that alpha particle direct action is not easily mitigated pharmacologically,19 perhaps casting doubt on the potential for successful alternative strategies of protection. The lack of available cytoprotective compounds for alpha particles may be compounded by the scarcity and expense of appropriate alpha-emitting reagents and devices, which has ostensibly limited their study. In spite of these shortcomings, recent evidence has identified other pathways that might be important to radiation protection that do not necessarily involve free radical metabolism, and suggests a more biologically complex mechanism for cellular protection20,21 that might be exploited pharmacologically.
Further evidence that there are mitigable factors in alpha particle toxicity are observations suggesting that DNA double-strand breaks (DSBs) incurred by cells exposed to alpha particles can be partially repaired at high doses,22 and at lower, therapeutically relevant doses, these DSBs are nearly eliminated.23 Moreover, radiosensitivities between mammalian cell types can range widely (Do = 0.34–1.16 Gy), and can have probabilities of surviving a single alpha particle traversal of >90%,24 phenomena not sufficiently explained by differences in radical scavenging potential. Consistent with these data, we have recently found that cells selected for resistance to the vinca alkaloid chemotherapeutic, vincristine, are relatively cross-resistant to alpha radiation, suggesting a biological mechanism of resistance. It follows that with the high-throughput screen (HTS) assay described in this report, we could potentially identify pharmacologic agents that can recapitulate this resistance in sensitive cells.
The goal of this report is to introduce an assay to identify small molecules that protect cells from alpha radiation. Identification of one or more such compounds could have a significant impact on the lethal effects of radiation dispersal devices and environmental exposure, enhancing current alpha particle radiotherapies, and, importantly, elucidating biologic mechanisms of alpha-particle-induced mutagenesis and carcinogenesis. As a proof of concept, we developed a novel assay to screen a library of 3,119 compounds against alpha-irradiated cells, monitoring for protection of cell viability. We validated potential protective hits through full titrations across 2 viability assays and demonstrated reproducibility of screen results. One compound, epoxy-4,5-α-dihydroxysantonin (EDHS), showed significant protective activity up to 48 h within the context of these assays, validating this HTS methodology as a useful tool in the exploration of molecules that might protect cells from alpha particle cytotoxicity.
Materials and Methods
Reagents
Sodium dodecyl sulfate (SDS), dimethylsulfoxide (DMSO), phosphate-buffered saline (PBS), staurosporine, and cell culture additives were purchased from Sigma-Aldrich.
Cell Culture
HL60 was obtained from the American Type Culture Collection, and further confirmed by phenotype, CD33 surface expression, and karyotype with G-band analysis. The resistant cell line, RV+ was a generous gift from Dr. Melvin S. Center, who developed the cell line,25 and were provided by Dr. Ellin Berman (Memorial Sloan-Kettering Cancer Center, New York, NY); these were confirmed as described with HL60 with detection of p-glycoprotein surface expression by flow cytometry. Cells were maintained at densities between 105 mL−1 and 106 mL−1, in complete Roswell Park Memorial Institute (RPMI) medium (containing 10% fetal bovine serum, 10 mM hydroxyethyl-piperazine ethane sulfonic acid (HEPES), nonessential amino acids, penicillin, and streptomycin) at 37°C, with 5% CO2. Although phenotypic stability has been demonstrated out to 6 months, RV+ were selected in the presence of 250 ng/mL vincristine (Oncovin®; Eli Lilly) over 4 passages and then stored in liquid N2. Cultures were discarded every 3 months and replaced with freshly thawed RV+. Thawed RV+ were reselected with 250 ng/mL vincristine to ensure that there was no phenotypic reversion to HL60. All assays were performed at least 3 passages following vincristine treatment.
225Ac Preparation
As described previously,26 5 mCi of dried 225Ac(NO3)3 (Oak Ridge National Laboratories) was reconstituted in 100 μL of 0.2 N HCl. After 6 h of nuclide equilibration, isotope quantity was measured using a CRC®-25 (Capintec, Inc.) dose calibrator. A bolus was transferred to complete RPMI to desired activity/mL (2 × final concentration) and confirmed after 6-h equilibration in dose calibrator and Packard Cobra II gamma counter (PerkinElmer) corrected for 213Bi gamma emissions.
Alpha Particle HTS Assay
HL60 in exponential growth phase were washed and resuspended in complete RPMI to a density of 7 × 104/mL (2 × final density). An equivalent volume of complete RPMI containing 300 nCi/mL 225Ac(NO3)3 was combined with cell suspension for a final density of 3.5 × 104/mL and final activity concentration of 150 nCi/mL, equivalent to 11.4 pM 225Ac+3. Next, HL60 225Ac cell suspensions were dispensed into 384-well assay plates (Corning, Inc.) containing compounds at a cell seeding density of 1,200 cells per well in 35 μL using the Flexdrop™ IV (PerkinElmer). For prevalidation, the assay was run with three 384-well high control plates containing 1% DMSO (v/v) and three 384-well low control plates containing 25 μM staurosporine with 0.1% SDS (v/v) in 1% DMSO (v/v). Staurosporine, a broad and potent kinase inhibitor, is added to cells in combination with 0.1% SDS, to ensure maximum cell penetration of stauosporine, disruption of membrane function, and dispersion of protein-protein interactions, essentially killing all cells, as a model of maximum cytotoxicity. For the validation screen, compounds were prearrayed at 5 μL into 384-well screen plates using the PP-384-M Personal Pipettor (Apricot Designs) for a final testing concentration of 10 μM in 1% DMSO (v/v). In addition, each 384-well screen plate carries high control wells containing 1% DMSO (v/v) and low control wells containing 25 μM staurosporine with 0.1% SDS (v/v) in 1% DMSO (v/v). Plates were incubated at 37°C, 5% CO2, for 24 h. At 24-h posttreatment, 5 μL of Alamar Blue (AB) reagent, a redox indicator that fluoresces in the presence of metabolically active cells,27 was added to the cells with the Flexdrop and further incubated for 24h. The resulting AB fluorescence intensity was read on the LEADseeker™ Multimodality Imaging System (GE Healthcare), excitation, 570 nm, and emission, 585 nm.
Confirmatory and Optimization Assays
AB assays were performed exactly as described above with select compounds, with the exception that compounds were titrated before the assay by 2-fold serial dilutions in DMSO and distributed by PP-384-M Personal Pipettor (Apricot Designs), including a DMSO vehicle control, and read on the LEADseeker as above. When analogous assays were performed in a 384-well format for assay optimization with increasing amounts of 225Ac, 5-fold serial dilutions at of isotope (2 × final concentration) were performed manually within sterile conical tubes containing a complete culture medium. Manual multichannel pipettor was used to dispense 20 μL of isotope dilutions to all wells. Cells suspended in a fresh complete culture medium at 7 × 104/mL (2 × final density) containing either 2% DMSO (v/v) (2 × final concentration) or the complete medium only were added to isotope dilutions at an equal volume. Similar to above assays, 5 μL of AB reagent was added by manual multichannel pipettor to each well after a 24-h incubation at 37°C. After an additional 24 h, plates were read for fluorescence on a Spectramax M2 (Molecular Devices), excitation, 570 nm, and emission, 585 nm.
Similarly, for the ATP-Lite viability assay, HL60 or RV+ cells were adjusted to a density of 7 × 104/mL (2 × final density) and combined with an equivalent volume of 300 nCi/mL 225Ac. Ninety microliters of cell-radionuclide mixture was added to opaque 96-well plates preseeded with 10 μL of titrated compound in DMSO, or DMSO vehicle alone. After 24-h incubation at 37°C, 5% CO2, according to manufacturer's instruction, 50 μL of ATP-Lite™ (PerkinElmer) lysis buffer was added to each well. Plates were then placed on an orbital shaker at 700 rpm for 5 min at room temperature. Subsequently, 50 μL of ATP-Lite luciferase buffer reagent was added, and promptly read on a Topcount™ chemiluminescence plate reader (PerkinElmer), 1 s/well.
Chemical Compound Libraries for the Validation Screen
The library used for the pilot screen combines 3,119 chemicals obtained commercially from Prestwick and MicroSource. The Prestwick Chemical Library is a unique collection of 1,119 high-purity chemical compounds, all off patent and carefully selected for structural diversity and broad spectrum, covering several therapeutic areas from neuropsychiatry to cardiology, immunology, rheumatology, and anesthesia, with known safety and bioavailability in humans. The library contains 90% of marketed drugs and 10% bioactive alkaloids or related substances. The MicroSource Library contains 2,000 biologically active and structurally diverse compounds from known drugs, experimental bioactives, and pure natural products. The library includes a reference collection of 160 synthetic and natural toxic substances (inhibitors of DNA/RNA synthesis, protein synthesis, cellular respiration, and membrane integrity), a collection of 80 compounds representing classical and experimental pesticides, herbicides, and endocrine disruptors, and a unique collection of 720 natural products and their derivatives. The collection includes simple and complex oxygen heterocycles, alkaloids, sesquiterpenes, diterpenes, pentacyclic triterpenes, sterols, and many other diverse representatives. The screening compounds were stored in 384-well microtiter plates as 10 mM stock solutions in 100% DMSO (v/v) in dedicated Biophile freezers at −20°C and constantly purged with nitrogen to avoid any condensation leading to ice buildup. From these master plates daughter plates were made as 1 mM in 100% DMSO (v/v), from which intermediate plates were made as 0.1 mM in 10% DMSO (v/v). Immediately before the assay, assay plates were made from the intermediate plates as described in the above section. Once the screen was completed, fresh material was purchased from the appropriate vendors and used for confirmatory assays at which point we systematically perform liquid chromatography/mass spectrometry (LC/MS) on each compound purchased, and confirmed its identity and chemical integrity.
Liquid Dispensing Systems, Automation System, and Screening Data Management
The assay was performed on a fully automated linear track robotic platform (CRS F3 Robot System; Thermo CRS) using several integrated peripherals for plate handling, cell incubators, liquid dispensing, and detection systems. Compounds were plated using a custom-designed 384 head on a PP-384-M Personal Pipettor (Apricot Designs). The addition of cell suspensions and the AB (Biosource) reagent addition was performed using the FlexDrop as described before.28 Screening data files were obtained from the automated LEADseeker Multimodality Imaging System (GE Healthcare) for the AB viability assay. The obtained screening data files were subsequently loaded into the HTS Core Screening Data Management System, a custom-built suite of modules for compound registration, plating, and data management, powered by ChemAxon Cheminformatic tools (ChemAxon). Chemical screening data were loaded into ORIS ChemScan and further analyzed. The summary of the identified positives was exported as chemical structure data files for further analysis and reporting.
Nuclide Accumulation
HL60 or RV+ cell cultures at a density of 5 × 105/mL were incubated with 150 nCi/mL 225Ac in the culture medium at 37°C up to 24 h. At prescribed time points, aliquots were removed and quickly centrifuged at 400 g, 4°C, and washed 4 times with cold PBS. After final spin, supernatant was aspirated as close to the pellet as possible without disturbance before counting daughter 213Bi gamma emissions between 185 and 480 keV.
Statistical Analysis
The Z′ factor was used to assess assay performance for the small molecule drug screen, calculated as described previously.29 Dose–response curve fitting for assay optimization and EC50 calculation was accomplished by using nonlinear regression, sigmoidal dose–response model, using Prism (GraphPad Software, Inc.). One-way ANOVA and Bonferroni's multiple comparison posttests were employed using Prism software to determine statistical significance of radioprotection where indicated.
Results
Assay Optimization
Preliminary results indicated that for maximum AB signal output, HL60 must be incubated with AB reagent for 24 h (data not shown). No additional signal was gained by increased AB incubation time. To eliminate potential confounding results with AB redox and fluorescence output, 24 h was allowed for experimental agents to act before cell viability was assayed for an additional 24 h. Thus, optimal cell density and alpha particle toxicity EC50 concentrations were determined by titrating cell number against increasing concentrations of the alpha-emitting isotope, 225Ac, in 384-well plates, by incubating for 24 h with isotope, and then adding AB reagent before reading fluorescence at 48 h at 5:5 nm. At >9,600 cells per well, there is clear saturation of fluorescence, likely due to the limits of AB fluorescence linearity (Fig. 1A). Below 1,200 cells per well was expectedly suboptimal, as we observed a precipitous baseline drop in cell viability in the absence of isotope. To minimize the potential for assay saturation, to limit the amount of isotope used in the assay on a larger scale, and to maximize signal-to-noise ratio, we fixed the assay conditions at 1,200 cells per well for all subsequent experiments. By using a one-site inhibition model of nonlinear regression, we calculated an EC50 concentration of 225Ac under these conditions to be approximately 150 nCi/mL, and used this concentration thereafter for assays (Fig. 1B). Upon equilibration of 225Ac decay products, stable 209Bi will be equal to the amount of 225Ac decayed. Over the length of the assay, this is maximally 1.47 pM (0.331 fg/mL) 209Bi, given the 10-day half-life of 225Ac. Reports have shown that other heavy metals are cytotoxic in the μg/mL range for this time frame,30 6 to 9 orders of magnitude more than what is present in our assay. Therefore, the nonradioactive effects of 209Bi are likely irrelevant.
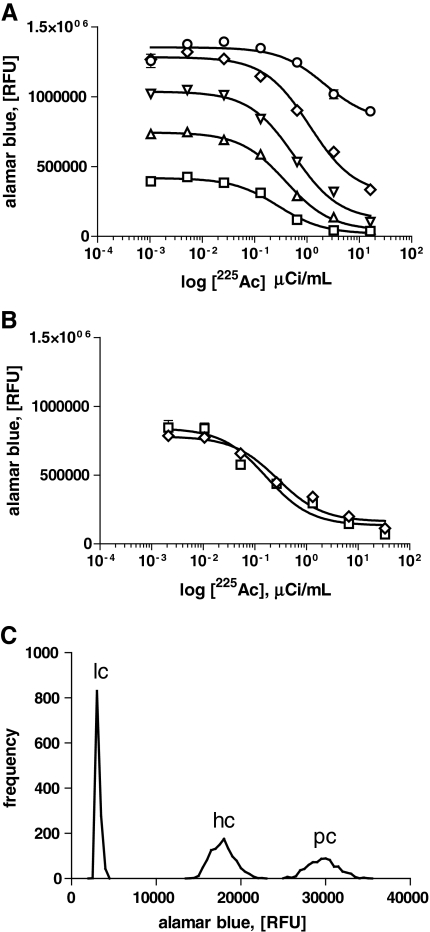
Fig. 1.
Assay optimization and prevalidation. (A) HL60 cell density was titrated from 19,200 cells/well (circle) (EC50 = 2201 nCi/mL, 95% CI 1,114–4,347 nCi/mL); 9600 cells/well (diamond) (EC50 = 1,130 nCi/mL, 95% CI 903.5–1,413 nCi/mL); 4,800 cells/well (inverted triangle) (EC50 = 573.5 nCi/mL, 95% CI 469.1–701.2 nCi/mL); 2400 cells/well (triangle) (EC50 = 392.7 nCi/mL, 95% CI 331.6–465.1 nCi/mL); and 1,200 cells/well (square) (EC50 = 252.2 nCi/mL, 95% CI 214.3–371.5 nCi/mL) in the presence of increasing amounts of 225Ac to determine optimal Alamar Blue conditions at 48 h. (B) Dimethylsulfoxide (DMSO) at 1% final concentration (v/v) (diamond) (EC50 = 166.8 nCi/mL, 95% CI 82.2–338.5 nCi/mL) had no discernable effect on HL60 225Ac toxicity compared to the normal culture medium (square) (EC50 = 275.8 nCi/mL, 95% CI 165.9–458.4 nCi/mL) at 48 h. (C) Prevalidation results of HL60 representing triplicate 384-well plates. Untreated cells containing only 1% DMSO (v/v) served as positive controls (pc), and compared to high controls (hc) containing EC50 225Ac (150 nCi/mL), and low controls (lc) containing 25 μM staurosporine in 0.1% sodium dodecyl sulfate. In all panels, data presented are the mean value (n ≥ 3), and error bars indicate ± standard error of the mean (SEM). EC50 values were determined by nonlinear regression curve fitting, sigmoidal dose–response model, using GraphPad Prism software.
Compound library stocks were formulated in 10% DMSO (v/v) at 100 μM, to be added at 10% of final assay volume for a 10 μM compound concentration in 1% DMSO (v/v). Because HL60 are known to be sensitive to terminal differentiation even at low concentrations of DMSO,31 we assayed for viability at 1% DMSO (v/v) in the presence of increasing amounts of 225Ac under HTS conditions. Compared to wells that contained no DMSO, 1% DMSO (v/v) had no significant effect on alpha particle toxicity (Fig. 1B).
Prevalidation
Prevalidation was used to determine the functionality, range, and robustness of the assay, indicated by the Z′ factor value from the collated data. In triplicate, 384-well plates containing 1% DMSO (v/v) alone (positive control, Fig. 1C); 150 nCi/mL 225Ac with 1% DMSO (v/v) (high control, Fig. 1C); or 150 nCi/mL 225Ac and 25 μM staurosporine in 0.1% SDS (w/v) (low control, Fig. 1C) were incubated for a total of 48 h as in the optimization assay. The raw AB fluorescence signal means and standard deviations (positive control mean 29,428.08 ± 1,810.28 relative fluorescent units (RFUs); high control mean 17,585.02 ± 1,452.35 RFUs; and low control mean 2,925.41 ± 230.82 RFUs) were used to calculate Z′ and signal-to-noise ratio for the assay. This assay yielded a Z′ factor of 0.66, a signal-to-noise ratio of nearly 10 to 1, along with an EC50 for alpha particle toxicity that was within the predictable limits established during optimization (Fig. 1C). The resulting optimized assay conditions are depicted step by step in Table 1.
Table 1.
Actinium-Based High-Throughput Screen Assay Protocol
| Step | Parameter | Value | Description |
|---|---|---|---|
|
1 |
Library compounds |
5 μL |
100 μM in 10% DMSO |
|
2 |
Low control compounds |
5 μL |
250 μM in 10% DMSO, 1% sodium dodecyl sulfate |
|
3 |
High control |
5 μL |
10% DMSO alone |
|
4 |
Positive control |
40 μL |
1,200 HL60 cells in complete RPMI containing 1% DMSO |
|
5 |
Plate cell–radioactivity mixture |
35 μL |
1,200 HL60 cells in complete RPMI containing 150 nCi/mL225Ac |
|
6 |
Incubation time |
24 h |
37°C, 5% CO2 |
|
7 |
Viability reagent |
5 μL |
Alamar Blue (resazurin) |
|
8 |
Incubation time |
24 h |
37°C, 5% CO2 |
| 9 | Assay readout | 570 nm exc and 585 nm em | LEADseeker Multimodality Imaging System |
Dispensing on the PP-384-M Personal Pipettor using a custom 384 head.
Dispensing on the PP-384-M Personal Pipettor using a custom pin tool head.
Dispensing on the PP-384-M Personal Pipettor using a custom pin tool head.
Cells prepared in media and dispensing performed with Flexdrop IV.
Radioactivity and cells are prepared at 2× final and combined 1:1 immediately before dispensing with Flexdrop IV.
384-well assay plates stored in the Stericult, an automated temperature-controlled incubator.
Dispensing with Flexdrop IV.
384-well assay plates stored in the Stericult, an automated temperature-controlled incubator.
Readout performed on a fully automated linear track platform with CRS F3 robot.
Validation Assay and Compound Selection
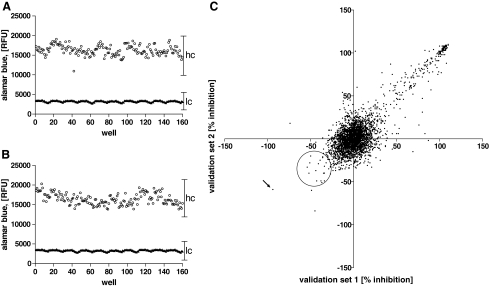
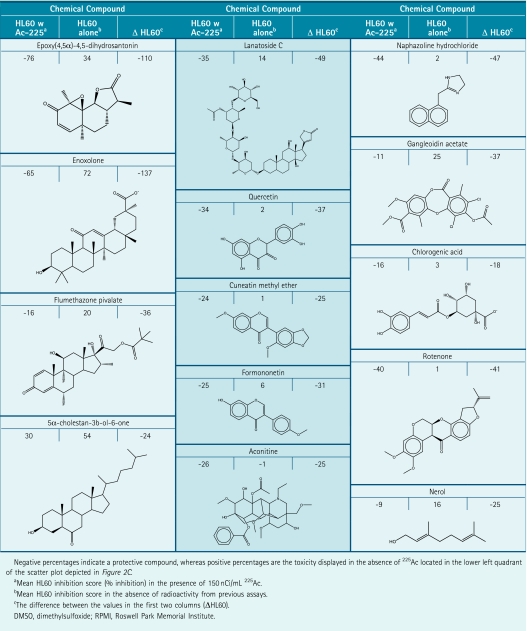
Internal high and low controls of the validation assay, analogous to conditions for prevalidation, were acceptable, yielding consecutive Z′ factors of 0.68 and 0.63, respectively, with a signal-to-noise ratio of approximately 5 to 1 compared to wells containing EC50 concentrations of 225Ac (Fig. 2A, B, respectively). The validation run involved screening 3,119 compounds in duplicate assay plates, run successively, where compounds were assayed at a single concentration of 10 μM, in the presence or absence of an EC50 225Ac (150 nCi/mL). Percent compound inhibition was calculated using RFUs for each well and plotted on opposing axes to determine reproducibility (Fig. 2C). The selection process was based on comparing cellular responses to alpha particle radiation with regard to the response in the absence of radiation (Table 2). Selection of protective compounds in the presence of the alpha-emitter 225Ac was implemented via 3 basic criteria: (1) reasonable correlation between consecutive replicates, defined by a coefficient of variance of >50%, (2) a difference in HL60 viability between irradiated and nonirradiated samples ≥25%, where the compound must display at least an absolute 8% increase in viability in the presence of 225Ac, and (3) display no mitogenic effect in the absence of 225Ac that exceeds the radioprotective effect.
Fig. 2.
High-throughput assay results. Collated internal high (hc, open circle) and low (lc, triangle) control data from successive (A and B) validation runs with LEADseeker™ Multimodality Imaging System. (C) Duplicate results from same assays for 3,119 validation compounds were plotted on opposite axes as indicated, expressed as percent inhibition of growth. Protective compounds are in the lower left quadrant, toxic compounds are in the upper right quadrant, and compounds with no effect are at the center. Protective compounds were selected from the indicated circular region, and epoxy-4,5-α-dihydroxysantonin was found to be the most protective compound (arrow).
Table 2.
Summary of 13 Protective Compounds That Were Selected in the Validation Run
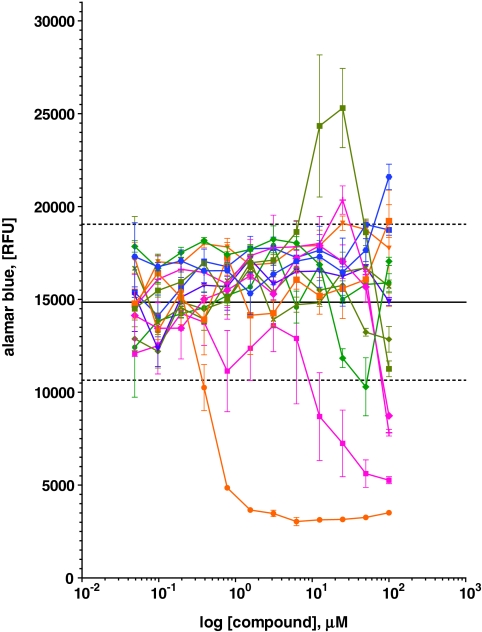
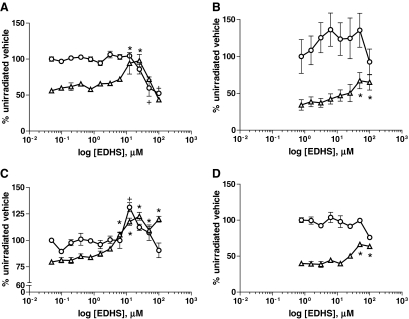
Thirteen compounds were selected based on these criteria (summarized in Table 2) that were titrated under similar conditions to confirm screen results and to determine a dose response (Fig. 3). Out of these 13 compounds, only EDHS could be confirmed as having a protective effect over a range of doses (Fig. 4A), which was subsequently confirmed in a 24-h ATP-based viability assay (Fig. 4B). Although toxicity is minimal up to 12.5 μM, viability is slightly diminished at this concentration in the absence of alpha particle exposure, suggesting that the protective effects might be partially masked by an unrelated toxic cellular response. Despite this, EDHS provides up to a 75% increase in cell survival to HL60 within a 48-h window.
Fig. 3.
Titration of select compounds with Alamar Blue (AB) reagent. Thirteen selected compounds were titrated in the presence of 225Ac and assayed by AB reagent to confirm screen results. Epoxy-4,5-α-dihydroxysantonin was found to have a clear dose response (olive square) that was over 2 standard deviations (dashed lines) above the mean (solid line), whereas others, lanatoside C (orange circle) and rotenone (magenta square), were found to be toxic. Remaining compounds are 5α-cholestan-3β-ol-6-on (green diamond), quercetin (light green diamond), nerol (magenta diamond), enoxolone (magenta cross), gandleoidin acetate (green circle), flumethazone pivalate (orange square), formononetin (violet inverted triangle), naphazoline hydrochloride (blue circle), aconitine (blue square), and cuneatin methyl ether (olive diagonal cross). All values are means (n = 5), and error bars indicate ± SEM.
Fig. 4.
Epoxy-4,5-α-dihydroxysantonin (EDHS) protective and toxic effects. (A) Forty-eight-hour Alamar Blue (AB) assay with HL60 compared to (B) a 24-h ATP-Lite assay, (C) RV+ in AB at 48 h, and (D) RV+ in a 24-h ATP-Lite assay, with 150 nCi/mL 225Ac (triangle) or no radioactivity (circle), and normalized to vehicle control with no 225Ac. *P value < 0.05 for 225Ac-treated samples. +P value of <0.05 for unirradiated samples, as compared to vehicle control, by one-way ANOVA with Bonferroni's multiple comparison posttest. For all panels, values are means (n ≥ 3), and error bars indicate ± SEM.
Two other compounds, according to our criteria, were not only false-positive for protection, but actually sensitizing. Lanatoside and rotenone, although they scored at least 25% protection (Table 2), when titrated in the confirmatory AB assay, were clearly toxic (Fig. 3). These compounds were not carried forward into the ATP-based viability assays. Out of the 13 compounds selected, this indicates a rate of erroneous identification of sensitizing compounds of 0.15.
Comparison to RV+ Radioresistant Cell Line
We previously established that RV+, a phenotypically stable multidrug-resistantance-expressing cell line, derived from HL60 was 6-fold more resistant to 225Ac alpha emissions in solution and is significantly resistant to external alpha radiation (data not shown). To demonstrate whether the EDHS-induced alpha particle radiation protection observed in HL60 was related to RV+ resistance, we treated RV+ with EDHS in the presence of 225Ac, analogous to HL60 treatment. EDHS provided similar protection with less overall toxicity to RV+ cells in AB (Fig. 4C) and ATP-Lite (Fig. 4D) assays, suggesting that EDHS is not mimicking the RV+ phenotype in HL60. EDHS appears to have some slight mitogenic effect in the AB assay for RV+ only, for there is a significant increase in cell growth in the absence of radiation at 12.5 μM, and also recovery beyond the unirradiated baseline beginning at the same concentration (Fig. 4C).
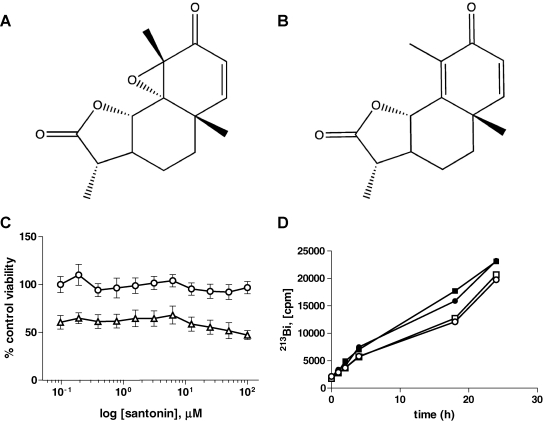
Epoxy(4,5α)-4,5-Dihydrosantonin Compared to Santonin
The parent compound of EDHS, santonin (Fig. 5), an anthelmintic sesquiterpene lactone derived from the Levant wormseed plant,32 was used to determine if the highly reactive epoxide moiety of EDHS (Fig. 5) was related to its biological activity. Up to 100 μM, santonin had no discernible effect on RV+ alpha particle cytotoxicity in an ATP viability assay, nor did it display mitogenic effects in the absence of radionuclide (Fig. 5C). Importantly, we demonstrated by the AB and ATP-Lite assays (Fig. 4A, B, D), with the exception of the mild effect on RV+ detected in the AB assay (Fig. 4C), that EDHS has no obvious mitogenic effects in the absence of 225Ac. However, because of the isolated observation in RV+ that EDHS has some mitogenic potential, we cannot rule out the possibility that ingrowth of surviving cells during the incubation period plays some role in the resultant increased viability.
Fig. 5.
Comparison of epoxy-4,5-α-dihydroxysantonin (EDHS) (A) to its parent compound, santonin (B). The structure of the sesquiterpene lactone parent compound santonin compared to the epoxidated derivative, EDHS. (C) In an ATP-Lite assay with the HL60 variant, RV+ cells, no protective or mitogenic activity was detected in the absence of 225Ac (circle) and presence (triangle) of isotope. Means were not significantly different among all groups. (D) Treatment of HL60 (open) or RV+ cells (closed) with EDHS has no effect on nuclide accumulation. EDHS (square) or 1% dimethylsulfoxide (v/v) (circle) was incubated along with 225Ac for indicated time points before pellets were washed and counted for gamma activity. For each panel, values are means (n ≥ 3), and error bars indicate ± SEM.
Nuclide Accumulation
One possible explanation for the increased viability at 48 h is that EDHS is attenuating isotope accumulation within the cells, therefore altering microdosimetry to the cells by reducing the number of alpha tracks through the DNA. To rule out the possibility that EDHS was affecting alpha particle microdosimetry, HL60 was incubated with 225Ac over a 24-h period in the presence and absence of 20 μM EDHS. At various points, cell suspensions were sampled, washed of nuclide-containing media, and pellets were counted for gamma emission as a measure of radionuclide accumulation. Over the course of 24 h, EDHS had no effect on radionuclide accumulation in either HL60 or RV+ cell lines (Fig. 5D).
Discussion
The success of HTS depends on a robust, reproducible assay, scalable to 384- or 1,536-well culture plates, which is indicated by a Z′ factor score of 0.66.29 We confirmed the utility of the assay with the identification of 1 hit, EDHS. Extensive experience with 225Ac physiochemical properties, along with its potential for direct clinical application, made it an ideal choice for HTS. 225Actinium has a half-life of 10 days and decays to 209Bi via 6 atoms, yielding 4 alpha particles per 225Ac disintegration, an attribute that likely adds to its potency. It is capable of delivering a large cellular dose within a reasonable timeframe, with only a small amount of material needed to achieve toxicity. We determined that for an EC50 concentration at 48 h, 150 nCi/mL is required in an assay volume of 40 μL. For the current screen of only 3,119 compounds run in duplicate in a 384-well format, the entire experiment required only 38 μCi of 225Ac (total cost < 50 USD). Extrapolated to a full size screen of 106 compounds or more, the total radioactivity is still obtained and discarded economically (total cost <15,000 USD; total waste <13 mCi). Although a 384-well format is entirely feasible for a screen of this magnitude, scaling to a 1,536-well format, which has been done successfully with assays using the AB reagent,28,33 would reduce the total radioactivity by a factor of at least 4. This is a considerable cost savings for isotope use and for waste disposal, as well as a time saving measure for plate loading and data acquisition. However, the smaller well format necessitates using charge-coupled-device-type plate readers that have been known to limit the dynamic range and signal-to-noise ratio of assays, reducing the Z′ factor. Despite this drawback, it is an option worth exploring should the assay be utilized in a full screen.
We demonstrated an EDHS dose response that peaks at 20 μM, offering a nearly 75% gain in viability at the 48-h mark. The assay did erroneously yield 2 sensitizing compounds, lanatoside and rotenone, which were only marginally cytotoxic in the absence of radiation according to our initial screen (Table 2), but shown to be inhibitory in the confirmatory assay (Fig. 3) and in independent studies.34,35 Our selection criteria were somewhat arbitrary in stringency, for we adjusted thresholds to yield a reasonable amount of confirmatory assays for the purposes of demonstration (in this case, 13). Although a false-positive rate of 0.92 (12/13), where those compounds that were deemed protective from the original screen showed no effect or a clear inhibitory dose response in a confirmatory assay, might be cumbersome in a larger scale screen, it could easily be avoided by applying more stringent criteria for selection (e.g., setting a higher threshold for protection). Regardless of selection criteria, the overall confirmed hit rate for the assay is 0.0003 (1/3,119), which should yield a reasonable level of confirmed hits given a larger scale screen.
The myeloid leukemia cell line, HL60, is a robust and well-characterized cell line that has the potential for developing chemoresistance. Stable variants of HL60 have been developed, including the RV+ line, which is a classic multidrug-resistant phenotype, characterized by the high expression of p-glycoprotein, which we have identified as being cross-resistant to alpha particle radiation (data not shown). This observation was the initial impetus to conducting an HTS. We reasoned, first, that resistance must be mediated by a pharmacologically modulatable biochemical process within the cell and, second, that we might be able to mirror this resistance phenotype with a small molecule. Ascertaining the mechanism of the small molecule also might reveal a new biological pathway involved in radioresistance to alpha particles. Surprisingly, EDHS seems to protect RV+ to the same extent at which it protects HL60. This not only suggests that the EDHS pathway is unrelated to the RV+ resistance mechanism, it points to the ability of EDHS to protect cells that overexpress the ATP-binding cassette (ABC) transporter p-glycoprotein (p-gp). This is important because clonogenic stem cells, including hematopoietic progenitors, are critical to tissue regeneration after radiation injury and have a propensity to express ABC transporter molecules at higher levels than more differentiated cells.36 Paradoxically, the essential ability of these long-lived cells to banish metabolic and xenobiotic toxins might exclude a protective compound administered following an irradiative insult, rendering it ineffective. The data here supports the notion that EDHS is not a p-gp substrate, and thus is unlikely to be transported out of cells.
By the AB assay, EDHS has significant toxicity at 48 h in concentrations exceeding 25 μM (Fig. 4A). Incidentally, it is at this threshold that the radioprotection appears to be reaching its maximum. At concentrations above 25 μM, EDHS still shows significant protective effects, whereas at the same concentration in the absence of 225Ac, HL60 proliferation begins to wane. This is an indication that protective effects of EDHS are partly masked by cytotoxicity, something that might be ameliorated by medicinal chemistry modifications. In contrast, RV+ showed reduced cytotoxicity at the highest concentrations (Fig. 4C), and shows a classic saturation of effect beyond 25 μM, supporting the idea that the more sensitive HL60 could benefit from additional protection with decreased cytotoxicity. The AB assay for RV+ (Fig. 4C) also reveals the possibility of EDHS mitogenicity, as there is a significant elevation of viability at a single concentration of EDHS (25 μM) in the absence of radionuclide. However, this is a single point, and was not duplicated in the complementary ATP-based assay, so it is likely an experimental anomaly. There is also the appearance that EDHS might induce the irradiated samples to exceed 100% viability of unirradiated cells. These changes above 100% are not statistically significant, nor were they duplicated in the ATP-Lite assay, yet they could represent a trend toward mitogenicity. However, the increased viability (∼80%) compared to HL60 (∼50%), expected given the relative radioresistance of the RV+ line, might explain the sustained protective effects lingering near the 100% viability mark.
In comparing EDHS to its parent compound, santonin, we learned that its epoxide moiety is likely responsible for its activity, if not some of its cytotoxic effects. Santonin had no discernible toxic or protective effects across a wide range of doses in RV+ cells (Fig. 5C). Although the full mechanism of EDHS is unknown, we were able to rule out the possibility that EDHS activity is based on alterations in microdosimetry through reduced radionuclide accumulation. Because alpha particle range is only that of a few cell diameters, the absorbed dose to the nucleus (Gy) is highly dependent upon the amount of isotope accumulated in or on the surface of the cell. A compound that merely inhibited, for example, pinocytosis, would not be radioprotective per se, for it would only reduce the effective dose to the cell. After repeated washing, cell pellets of both HL60 and RV+ retained nearly identical amounts of 225Ac, whether they were treated with EDHS or the vehicle control of DMSO (Fig. 5D). These findings notwithstanding, it would be premature to call EDHS a bona fide alpha particle protectant. It has yet to undergo the rigors of testing on multiple cell lines, normal tissues, and, importantly, clonogenic assays. Failure to demonstrate increased clonogenic survival does not preclude its potential research or clinical utility.
One drawback to this viability-based HTS assay is its inability to reveal a specific biochemical target, thus making structure–activity relationships difficult to draw. However, the myriad pathways that might contribute to alpha particle resistance—some yet unknown—make using a more narrowly defined HTS assay in this case an inefficient approach to discovery. While the assay described here will certainly require a significant amount of postassay research to determine mechanism beyond radioprotection (e.g., affinity chromatography or functional genomic screens to pull down specific cellular targets), with a cell viability approach we are avoiding the costly choice of an irrelevant pathway on which to focus. Because of this pragmatic approach, the question remains whether a compound protects against carcinogenicity. While both carcinogenesis and cell viability after alpha particle exposure are linked causally to DNA damage, a compound that protects cells from alpha particle radiation does not necessarily indicate a compound that can reduce carcinogenic potential. It is conceivable that a compound that protects cells from alpha-particle-mediated death might even enhance alpha particle carcinogenicity by sustaining a cell that has incurred an otherwise lethal oncogenic mutation. As a result, any compound detected in the HTS, including EDHS, will necessarily require a detailed evaluation of radioprotection weighed against potential carcinogenicity.
To reduce side effects anticipated in alpha particle radioimmunotherapy, we recently explored pharmacologic strategies to protect the renal tissue from internally delivered alpha particle radiation in the context of radioimmunotherapy.37 However, these approaches did not address radiation toxicity on a cellular level. We sought to build on this foundation by devising an HTS to look for novel agents to protect against the alpha-particle-mediated toxicity and carcinogenesis. Here, we demonstrated such a screen, and, with the identification of a potentially protective compound, EDHS, we have provided justification to expand to a full scale screen. If the current validation screen is predictive, there is a potential for multiple hits that might have protective effects. Candidate compounds uncovered through a broader screen, including EDHS, might be used to mitigate systemic radiation toxicity or carcinogenesis resulting from environmental or occupational exposure to alpha emitters, alpha particle radioimmunotherapy, or radiation dispersal devices.
Acknowledgments
The authors wish to thank members of the HTS Core Facility for their help during the course of this study. The HTS Core Facility is partially supported by Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, the Experimental Therapeutics Center of the Memorial Sloan-Kettering Cancer Center, the William Randolph Hearst Fund in Experimental Therapeutics, the Lillian S. Wells Foundation, and by an NIH/NCI Cancer Center Support Grant 5 P30 CA008748-44. Supported by NIH CA 33049 and CA 55349.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.McDevitt MR. Sgouros G. Finn RD, et al. Radioimmunotherapy with alpha-emitting nuclides. Eur J Nucl Med. 1998;25(9):1341–1351. doi: 10.1007/s002590050306. [DOI] [PubMed] [Google Scholar]
- 2.McDevitt MR. Ma D. Lai LT, et al. Tumor therapy with targeted atomic nanogenerators. Science. 2001;294(5546):1537–1540. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 3.McDevitt MR. Barendswaard E. Ma D, et al. An alpha-particle emitting antibody ([213Bi]J591) for radioimmunotherapy of prostate cancer. Cancer Res. 2000;60(21):6095–6100. [PubMed] [Google Scholar]
- 4.Jurcic JG. McDevitt MR. Sgouros G. Larson SM. Scheinberg DA. Antibody targeted alpha-particle therapy of leukemia. Blood. 2002;100:1233–1239. [PubMed] [Google Scholar]
- 5.Andersson H. Cederkrantz E. Back T, et al. Intraperitoneal {alpha}-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of 211At-MX35 F(ab')2—a phase I study. J Nucl Med. 2009;50:1153–1160. doi: 10.2967/jnumed.109.062604. [DOI] [PubMed] [Google Scholar]
- 6.Zalutsky MR. Reardon DA. Akabani G, et al. Clinical experience with alpha-particle emitting 211At: treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J Nucl Med. 2008;49(1):30–38. doi: 10.2967/jnumed.107.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nilsson S. Franzen L. Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8(7):587–594. doi: 10.1016/S1470-2045(07)70147-X. [DOI] [PubMed] [Google Scholar]
- 8.McFee RB. Leikin JB. Death by polonium-210: lessons learned from the murder of former Soviet spy Alexander Litvinenko. Semin Diagn Pathol. 2009;26(1):61–67. doi: 10.1053/j.semdp.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Shin H. Kim J. Development of realistic RDD scenarios and their radiological consequence analyses. Appl Radiat Isot. 2009;67(7–8):1516–1520. doi: 10.1016/j.apradiso.2009.02.054. [DOI] [PubMed] [Google Scholar]
- 10.United Nations Scientific Committee on the Effects of Atomic Radiation. Annex B: Exposures from Natural Radiation Sources. Vol. 1. UNSCEAR; Vienna, Austria: 2000. Sources. [Google Scholar]
- 11.Fabrikant JI. Radon and lung cancer: the BEIR IV Report. Health Phys. 1990;59(1):89–97. doi: 10.1097/00004032-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Radford EP. Renard KG. Lung cancer in Swedish iron miners exposed to low doses of radon daughters. N Engl J Med. 1984;310(23):1485–1494. doi: 10.1056/NEJM198406073102302. [DOI] [PubMed] [Google Scholar]
- 13.Xuan XZ. Lubin JH. Li JY, et al. A cohort study in southern China of tin miners exposed to radon and radon decay products. Health Phys. 1993;64(2):120–131. doi: 10.1097/00004032-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Darby S. Hill D. Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ. 2005;330(7485):223. doi: 10.1136/bmj.38308.477650.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radon Health Risks. www.epa.gov/radon/healthrisks.html www.epa.gov/radon/healthrisks.html
- 16.Patt HM. Smith DE. Jackson E. The effect of cysteine on the peripheral blood of the irradiated rat. Blood. 1950;5(8):758–763. [PubMed] [Google Scholar]
- 17.Howell RW. Goddu SM. Bishayee A. Rao DV. Radioprotection against lethal damage caused by chronic irradiation with radionuclides in vitro. Radiat Res. 1998;150(4):391–399. [PMC free article] [PubMed] [Google Scholar]
- 18.Narra VR. Harapanhalli RS. Goddu SM. Howell RW. Rao DV. Radioprotection against biological effects of internal radionuclides in vivo by S-(2-aminoethyl)isothiouronium bromide hydrobromide (AET) J Nucl Med. 1995;36(2):259–266. [PubMed] [Google Scholar]
- 19.Hall EJ. Giaccia AJ. Radiobiology for the Radiologist. Lippincott Williams & Wilkins; Philadelphia: 2006. [Google Scholar]
- 20.Strom E. Sathe S. Komarov PG, et al. Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nat Chem Biol. 2006;2(9):474–479. doi: 10.1038/nchembio809. [DOI] [PubMed] [Google Scholar]
- 21.Burdelya LG. Krivokrysenko VI. Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320(5873):226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenner TJ. deLara CM. O'Neill P. Stevens DL. Induction and rejoining of DNA double-strand breaks in V79-4 mammalian cells following gamma- and alpha-irradiation. Int J Radiat Biol. 1993;64(3):265–273. doi: 10.1080/09553009314551421. [DOI] [PubMed] [Google Scholar]
- 23.Leatherbarrow EL. Harper JV. Cucinotta FA. O'Neill P. Induction and quantification of gamma-H2AX foci following low and high LET-irradiation. Int J Radiat Biol. 2006;82(2):111–118. doi: 10.1080/09553000600599783. [DOI] [PubMed] [Google Scholar]
- 24.Goodhead DT. Mechanisms for the biological effectiveness of high-LET radiations. J Radiat Res (Tokyo) 1999;40(Suppl):1–13. doi: 10.1269/jrr.40.s1. [DOI] [PubMed] [Google Scholar]
- 25.McGrath T. Center MS. Adriamycin resistance in HL60 cells in the absence of detectable P-glycoprotein. Biochem Biophys Res Commun. 1987;145(3):1171–1176. doi: 10.1016/0006-291x(87)91560-9. [DOI] [PubMed] [Google Scholar]
- 26.McDevitt MR. Ma D. Simon J. Frank RK. Scheinberg DA. Design and synthesis of 225Ac radioimmunopharmaceuticals. Appl Radiat Isot. 2002;57(6):841–847. doi: 10.1016/s0969-8043(02)00167-7. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama T. Watanabe M. Teramoto T. Kitajima M. Slope analysis of CA19-9 and CEA for predicting recurrence in colorectal cancer patients. Anticancer Res. 1997;17(2B):1379–1382. [PubMed] [Google Scholar]
- 28.Shum D. Radu C. Kim E, et al. A high density assay format for the detection of novel cytotoxic agents in large chemical libraries. J Enzyme Inhib Med Chem. 2008;23(6):931–945. doi: 10.1080/14756360701810082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang JH. Chung TD. Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4(2):67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 30.Tchounwou PB. Yedjou CG. Foxx DN. Ishaque AB. Shen E. Lead-induced cytotoxicity and transcriptional activation of stress genes in human liver carcinoma (HepG2) cells. Mol Cell Biochem. 2004;255(1–2):161–170. doi: 10.1023/b:mcbi.0000007272.46923.12. [DOI] [PubMed] [Google Scholar]
- 31.Collins SJ. Ruscetti FW. Gallagher RE. Gallo RC. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci USA. 1978;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohde RA. Expression of resistance in plants to nematodes. Annu Rev Phytopathol. 1972;10:233–252. [Google Scholar]
- 33.Antczak C. Kloepping C. Radu C, et al. Revisiting old drugs as novel agents for retinoblastoma: in vitro and in vivo antitumor activity of cardenolides. Invest Ophthalmol Vis Sci. 2009;50(7):3065–3073. doi: 10.1167/iovs.08-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansson S. Lindholm P. Gullbo J. Larsson R. Bohlin L. Claeson P. Cytotoxicity of digitoxin and related cardiac glycosides in human tumor cells. Anticancer Drugs. 2001;12(5):475–483. doi: 10.1097/00001813-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Reregistration Eligibility Decision for Rotenone. United States Environmental Protection Agency; Washington, DC: 2007. [Google Scholar]
- 36.Bunting KD. ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells. 2002;20(1):11–20. doi: 10.1002/stem.200011. [DOI] [PubMed] [Google Scholar]
- 37.Jaggi JS. Kappel BJ. McDevitt MR, et al. Efforts to control the errant products of a targeted in vivo generator. Cancer Res. 2005;65(11):4888–4895. doi: 10.1158/0008-5472.CAN-04-3096. [DOI] [PubMed] [Google Scholar]