Abstract
While prostate gland development is dependent on androgens, other hormones including retinoids and estrogens can influence this process. Brief exposure to high-dose estrogen during the neonatal period in rats leads to permanent, lobe-specific aberrations in the prostate gland, a phenomenon referred to as developmental estrogenization. We have previously shown that this response is mediated through alterations in steroid receptor expression; however, further downstream mechanisms remain unclear. Herein, we examined Sonic hedgehog (Shh)-patched (ptc)-gli in the developing rat prostate gland, its role in branching morphogenesis, and the effects of neonatal estrogens on its expression and localization to determine whether a disturbance in this signaling pathway is involved in mediating the estrogenized phenotype. Shh was expressed in epithelial cells at the distal tips of elongating ducts in discreet, heterogeneous foci, while ptc and gli1–3 were expressed in the adjacent mesenchymal cells in the developing gland. The addition of Shh protein to cultured neonatal prostates reduced ductal growth and branching, decreased Fgf10 transcript, and increased Bmp4 expression in the adjacent mesenchyme. Shh-induced growth suppression was reversed by exogenous Fgf10, but not noggin, indicating that Fgf10 suppression is the proximate cause of the growth inhibition. A model is proposed to show how highly localized Shh expression along with regulation of downstream morphogens participates in dichotomous branching during prostate morphogenesis. Neonatal exposure to high-dose estradiol suppressed Shh, ptc, gli1, and gli3 expressions and concomitantly blocked ductal branching in the dorsal and lateral prostate lobes specifically. In contrast, ventral lobe branching and Shh-ptc-gli expression were minimally affected by estrogen exposure. Organ culture studies with lateral prostates confirmed that estradiol suppressed Shh-ptc-gli expression directly at the prostatic level. Taken together, the present findings indicate that lobe-specific decreases in Shh-ptc-gli expression are involved in mediating estradiol-induced suppression of dorsal and lateral lobe ductal growth and branching during prostate morphogenesis.
Keywords: Sonic hedgehog, Patched, Gli, Fgf10, Bmp4, Prostate, Estrogen, Estradiol
Introduction
Prostate gland development is dependent on androgens which stimulate ductal outgrowth, branching morphogenesis, cellular differentiation, and onset of secretory activity (George and Peterson, 1988; Siiteri and Wilson, 1974). In addition, there is evidence that other hormones including estrogens and retinoids can influence these processes, although their exact role in normal prostatic development is less clear (Aboseif et al., 1997; Price, 1963; Seo et al., 1997). In humans, prostate morphogenesis occurs entirely in utero under the influence of testosterone secreted from the fetal testes (Lowsley, 1912; Shapiro, 1990). There is also clear indication that rising maternal estrogens during the third trimester have a direct effect on the human prostate since the epithelium develops marked squamous metaplasia which sloughs at birth when maternal estrogen levels abruptly fall (Brody and Goldman, 1940; Zondek and Zondek, 1975). Furthermore, maternal exposure to pharmacological levels of diethylstilbestrol (DES) has been shown to induce prostatic abnormalities in human offspring (Driscoll and Taylor, 1980). Consequently, it has been proposed that excessive estrogenization during prostatic development may contribute to the high incidence of benign prostatic hyperplasia (BPH) and prostatic carcinoma currently observed in the aging male population (Santti et al., 1994).
Prostate gland development in the rodent is initiated late in gestation as buds emerge from the urogenital sinus (UGS), and in contrast to humans, the gland undergoes extensive branching morphogenesis and cellular differentiation during the postnatal period (Hayashi et al., 1991). Thus, the neonatal rodent prostate gland has emerged as a useful model for fetal prostate development in humans. Brief exposure of rodents to estrogens during the neonatal period has been shown to have permanent, irreversible, and dose-dependent effects on the prostate gland’s morphology, cellular organization, and function (Prins, 1992; Prins and Birch, 1995; Pylkkanen et al., 1993; Rajfer and Coffey, 1979; vom Saal et al., 1997). If estrogenic exposures are high, the permanent imprints include reduced prostatic growth, epithelial differentiation defects, altered secretory function, and aging-associated dysplasia similar to prostatic intraepithelial neoplasia or PIN (Naslund and Coffey, 1986; Prins, 1992, 1997; Prins et al., 1993; Rajfer and Coffey, 1979). In the rat model, the responses are lobe-specific with differentiation abnormalities and adult-onset dysplasia occurring with the highest frequency and severity in the ventral prostate lobe (VP), while the dorsal (DP) and lateral (LP) lobes show greater inhibition of ductal branching and complexity (Prins, 1992, 1997). This process, referred to as estrogenic imprinting or developmental estrogenization, is used as a model to evaluate the role of exogenous and endogenous estrogens as a potential predisposing factor for prostate diseases later in life.
The mechanism of developmental estrogenization of the prostate gland is not well understood. Previous studies from our laboratory using estrogen receptor (ER) knockout mice for ERα and ERβ determined that the effects are mediated through stromal ERα which is transiently up-regulated following neonatal estrogen exposure (Prins and Birch, 1997). Furthermore, the expression of several steroid receptors which mediate steroid action in the developing prostate is drastically altered both temporally and quantitatively by neonatal estrogens. Most notably, androgen receptor (AR) is markedly down-regulated, while ERα, progesterone receptor (PR), and retinoic acid receptors (RARα, RARβ, and RXRα) are significantly up-regulated in a cell-specific manner (Prins and Birch, 1995, 1997; Prins et al., 1998, 2001a,b, 2002; Pu et al., 2003; Woodham et al., 2003). In addition, retinoid metabolizing enzymes and retinoid levels are affected by estrogenization in a lobe-specific manner (Prins et al., 2002; Pu et al., 2003). The net effect of these alterations is that the developing prostate is no longer under predominant androgen regulation through AR but is rather driven by estrogens, progesterone, and retinoid signals. We currently hypothesize that critical developmental genes which dictate prostate morphogenesis may be downstream targets of steroid action in the prostate gland. Furthermore, we predict that the expression levels and patterns of these steroid-regulated developmental genes will be disturbed by the prostatic steroid receptor shift as a result of neonatal estrogenic exposure. In the present study, we test the hypothesis that the Shh-ptc-gli signaling pathway may be altered in response to prostatic estrogenization and that this may, in part, mediate specific aspects of that phenotype.
Sonic hedgehog (Shh) is a secreted glycoprotein produced by epithelial cells at mesenchymal interfaces in developing tissues where it is involved in determination of cell fate, proliferation, and embryonic patterning (see review by Ingham and McMahon, 2001). Shh is expressed in a spatially defined manner in developing glands, most commonly at the apical edge of outgrowing ducts. This secreted morphogen binds to membrane-bound patched (ptc) receptors on adjacent mesenchymal cells, thus establishing epithelial–mesenchymal cross-talk. Liganding of ptc by Shh relieves its inhibition on smoothened (smo) which allows for activation of Gli transcription factors that directly mediate Shh’s effects. In vertebrates, there are three known Gli transcripts; gli1, gli2, and gli3 which have both redundant and unique actions. Importantly, gli1 and gli2 are transcriptional activators, while gli3 is believed to be a transcriptional repressor (Meyer and Roelink, 2003) which permits tight regulation of Shh actions. Both short-range and long-range actions of Shh have been described which differ as a function of concentration gradients (Gritli-Linde et al., 2001). In most structures, Shh is considered to be a critical regulatory morphogen since it regulates the expression of other secreted morphogens and homeobox genes (Chuang and McMahon, 2003; Haraguchi et al., 2001; Perriton et al., 2002; Roberts et al., 1995, 1998; Schneider et al., 2000). It has also been shown to induce ptc and gli1 expression, thus establishing an autoregulatory loop (Marigo and Babin, 1996).
Recently, the spatiotemporal expression of Shh-ptc-gli1 was characterized for the murine prostate gland (Lamm et al., 2002), while Shh-ptc mRNA expression was temporally described for the rat ventral prostate (Freestone et al., 2003). Similar to the lung, Shh was localized to the distal tips of the outgrowing murine prostatic buds, while ptc and gli1 were expressed in the adjacent mesenchyme. Expression of these signaling molecules was highest in the perinatal UGS complex and levels declined as prostate morphogenesis proceeded. Since studies with Shh null mice demonstrated that prostatic budding could be initiated by testosterone in the absence of Shh, it does not appear to be necessary for prostatic induction (Berman et al., 2004; Freestone et al., 2003). Nonetheless, inhibition of Shh action in UGS and prostate organ cultures by cyclopamine, an inhibitor of smo, demonstrated that Shh plays an important role in prostate ductal outgrowth, patterning, and epithelial differentiation during glandular morphogenesis (Berman et al., 2004; Freestone et al., 2003; Lamm et al., 2002). The exact nature of that role, however, remains unclear.
Tight regulation of developmental gene expression in specific spatiotemporal patterns is critical for normal development of all structures. However, little is known about the mechanisms involved in activating Shh gene expression in general and in the prostate specifically. There is evidence that spatiotemporal expression of Shh can be regulated by steroids in several organs. Retinoids have been shown to regulate Shh expression in the developing lungs, central nervous system, wing buds, and skeleton (Cardoso et al., 1996; Helms et al., 1994, 1997; Suzuki et al., 1999; Tamura et al., 1997). While testosterone is not required for Shh expression in the UGS, it can up-regulate transcript levels of Shh (Freestone et al., 2003; Podlasek et al., 1999). Furthermore, the localization of Shh expression in the mouse prostate is dramatically influenced by dihydrotestosterone, suggesting that androgens may regulate a critical male-specific spatial expression pattern (Lamm et al., 2002). It is therefore possible that a shift in steroidal regulation of prostate morphogenesis from androgen dominance to estrogen, progesterone, and/or retinoid dominance, as occurs following neonatal estrogen exposure, may alter the expression of Shh and/or its cognate signaling molecules.
Since a staged spatiotemporal accounting of Shh, ptc, and gli1–3 expression is not complete for the rat prostate lobes, we first present a detailed characterization of these molecules during the active phases of rat prostate branching morphogenesis. The potential role for Shh during prostate development was next examined in a neonatal prostate organ culture system using exogenous Shh or anti-Shh antibodies with subsequent analysis of branching morphogenesis, prostatic Fgf10, and Bmp4 expression. We then sought to determine whether Shh, ptc, and/or gli1, gli2, and gli3 are regulated by estrogens in the developing prostate gland and whether a disturbance of this pathway is involved in mediating the neonatal estrogenization of the prostate. Our findings indicate that Shh-ptc-gli expression is suppressed by estradiol in a lobe-specific manner and suggest that this may play a role in reduced ductal outgrowth and branching defects in the dorsolateral prostate.
Materials and methods
Animals
All rats were handled in accordance with the principles and procedures of the Guiding Principles for the Care and Use of Animal Research, and the experiments were approved by the Institutional Animal Care Committee. Timed pregnant female Sprague–Dawley rats were purchased from Zivic-Miller (Pittsburgh, PA), housed individually in a temperature-controlled (21°C) and light-controlled (14 h L–10 h D) room and fed standard Purina rat chow (Ralston-Purina, St. Louis, MO) ad libitum. The day of birth was designated as day 0. All males from a single mother were assigned to one of two groups and treated on postnatal days (pnd) 0, 3, and 5 with subcutaneous injections of either 25 µg 17β-estradiol-3-benzoate (Sigma–Aldrich Chemical Co., St. Louis, MO) in 25 µl of peanut oil (Arachis sp.) or oil alone as controls. Pups from both treatment groups were killed by decapitation on pnd 1, 3, 6, 10, 30, or 90, and the UGS-prostate complexes were dissected for subsequent analysis. Thus, pups killed on pnd 1 and 3 were exposed to a single dose of estradiol on day 0, while offspring killed on pnd 6 and later were exposed three times to estradiol. After completion of morphogenesis (pnd 30), prostatic ductal complexity was examined in each treatment group. The LPs were separated into LP1 and LP2 ductal arrays and microdissected in 0.5% collagenase IV. The number of terminal ductal tips was counted manually under the microscope for each LP1 and LP2, and data were analyzed by Student t test. Due to complexity of the VP at pnd 30, accurate ductal tip and branch point counting for this lobe were not possible.
In vitro estrogenic exposure
Since the in vivo estrogenic effects on Shh-ptc-gli expression were concentrated in the LP, in vitro experiments were performed with this lobe to determine if the effect was mediated directly at the prostatic level. LPs were isolated on pnd 0 and cultured on Millicell-CM filters (Millipore Corp., Bedford, MA) floating in 2-ml medium in a six-well plates. One side of the LP from each animal was cultured in basal organ culture medium (BOCM), while the contralateral lobe from each animal was cultured in BOCM with 20 µM 17β-estradiol (Sigma–Aldrich). Previous in vitro studies over a fivefold log dose range had established that this concentration produced strong but not complete growth inhibition of the prostate lobes (Putz and Prins, 2002). The BOCM consisted of DMEM/F-12 (Invitrogen/GIBCO) containing 10−8 M testosterone (Sigma–Aldrich), 50 µg/ml gentamycin, and 1 × insulin–transferrin–selenium (Invitrogen). The LPs (n = 10) were cultured in a 5% CO2 incubator for 6 days with medium changed every 48 h. Photographs were captured with a Burle video camera and Snappy 3.0 software to monitor growth and determine 2-D area. At pnd 6, the prostate compartment was used for RNA isolation and realtime RT–PCR.
Prostate organ culture with Shh protein, SHH beads, and anti- Shh antibodies
To examine the effects of exogenous Shh on prostate branching, VPs and LPs were isolated on pnd 0 and separately cultured as described above for 4–6 days in BOCM with 2 µg/ml mouse Shh protein (R&D Systems) or 2 µg/ml BSA (contralateral lobes). This was replicated on six separate sets of tissue. To determine the effect of exogenous Shh on gene expression, eight additional paired cultures were terminated after 18 h (i.e., before observable growth alterations), RNA was isolated, and Fgf10 and Bmp4 mRNA were quantitated by real-time RT–PCR. In other cultures, VPs from pnd 0 pups were cultured for 4–6 days in the presence of (1) BOCM alone, (2) BOCM plus 2 µg/ml Shh, (3) BOCM plus 2 µg/ml Shh and 0.5 µg/ml Fgf10 (R&D Systems), or (4) BOCM plus 2 µg/ml Shh and 1 µg/ml noggin (R&D Systems), a Bmp4 antagonist (n = 3–4 for each group). Further assessment of the effect of Shh on Fgf10 expression was made by culture of pnd 0 UGS/prostate complexes in anti-Shh antibody (5 µg/ml 5E1; Developmental Studies Hybridoma Bank, University of Iowa) or control IgG (n = 3) for 24 h followed by whole-mount in situ hybridization (ISH) for Fgf10 mRNA.
To examine the effect of localized Shh on prostatic branching and Fgf10 expression, SHH beads were employed. Affi-Gel blue beads (150-µM diameter; Bio-Rad) were washed in PBS, incubated in 1 mg/ml human SHH protein (kindly provided by Curis, Inc., Cambridge, MA) at 37°C for 1 h and stored at 4°C for up to 1 week. Control beads were incubated with an equal concentration of BSA. Paired pnd 0 VPs and LPs received SHH beads positioned in the proximal or distal mesenchyme with BSA beads placed in a corresponding region of the contralateral lobe. The lobes were cultured for 4 days in BOCM, after which three from each group were analyzed for Fgf10 mRNA by whole-mount ISH.
Whole-mount in situ hybridization (ISH)
The UGS-prostatic complexes were fixed in 4% paraformaldehyde, dehydrated, and digested with proteinase K. Following prehybridization, tissues were hybridized overnight at 60°C with 0.5–0.6 µg/ml digoxigenin-labeled RNA probes, washed at high and low stringency, incubated overnight at 4°C in antidigoxygenin alkaline phosphatase-conjugated antiserum (Roche, Indianapolis, IN), and color-reacted with NBT and BCIP (Roche). To allow for temporal and treatment comparisons, pnd 1, 3, and 6 prostatic complexes from in vivo control and estrogenized rats were processed together, and direct comparisons were made within each run. A minimum of four separate ISH assays were performed for each separate gene from in vivo experiments. For in vitro studies, all tissues from individual experiments were processed together to allow direct comparisons of signal intensity. The prostates were photographed with a Zeiss AxioCam color digital camera using AxioVision 2.0.5 software. To identify cellular localization of gene expression, ISH-stained tissues were cross-sectioned at 10 µm.
Cloned cDNA for mouse Shh (0.9 kb) was kindly provided by Dr. Liang Ma (Tulane University, LA), and cloned cDNAs for mouse gli1 (1.6 kb), mouse gli2 (1.0 kb), and mouse gli3 (2.4 kb) were kindly provided by Dr. A. Joyner (Skirball Institute of Biomolecular Medicine, NY). The rat Patched1 (ptc1) and rat Fgf10 templates were prepared by TA cloning a 660 and a 498 bp PCR fragment, respectively, into PCR II vector. Digoxigenin-labeled RNA probes were prepared by in vitro transcription using appropriate RNA polymerases (DIG RNA labeling kit, Roche).
Immunocytochemistry
Ptc protein and p63, a basal cell marker, were localized by immunocytochemistry as described (Prins et al., 1991). Briefly, frozen sections were fixed in 2% paraformaldehyde, blocked with 2% serum, incubated overnight at 4°C with anti-ptc antibody (1 µg/ml G-19; Santa Cruz Biotechnology, Santa Cruz, CA) or anti-p63 antibody (0.4 µg/ml H-137; Santa Cruz), reacted with biotinylated anti-IgG (Vector Laboratories, Inc., Burlingame, CA), and detected with avidin-biotin peroxidase (ABC-Elite, Vector Labs) using diaminobenzidine tetrachloride as a chromagen. For controls, normal goat or rabbit IgG (Vector) was substituted for primary antibody on separate sections.
Real-time RT–PCR
Two procedures were used for RNA extraction and reverse transcription (RT) depending upon tissue volume. A standard assay for pnd 6–90 VP involved RNA extraction with Trizol (Invitrogen, Carlsbad, CA), DNase I digestion (Roche), and RT with AMV at 42°C for 60 min using the RT System (Promega, Madison, WI). A microassay for smaller tissues (pnd 1–6 VP, pnd 6–10 LP and DP) used RNeasy Kit (Qiagen, Valencia, CA) for RNA extraction, On-Column DNase I Digestion, and RT with MMLV at 37°C for 60 min using First Strand cDNA Synthesis Kit (Fermentas Inc., Hanover, MD). Random primers were used for reverse transcription.
The exon spanning primers and dual-labeled probe sets used for PCR are shown in Table 1. For dual-labeled probes, the 5′-reporters were FAM for Shh, gli1, Fgf10, and Bmp4; Hex for ptc, gli2, and RPL19; and Texas red for gli3, and the 3′ was labeled with black hole quencher. Plasmids containing each DNA sequence (Shh, ptc1, gli1, gli2, gli3, Fgf10, Bmp4, and RPL19) were cloned with TOPO TA cloning kit (Invitrogen) and used for standard curves in each reaction to directly quantitate target DNA levels. Ribosomal protein L19 (RPL19) was quantitated and served as an internal reference for normalization. Direct comparisons of RPL19 per unit total RNA revealed no effect of estrogen treatment in developing prostates. Real-time PCR was performed in duplex with Platinum qPCR Supermixture-UDG (Invitrogen) using the iCycler (Biorad, Hercules, CA). Reaction conditions were optimized for single gene and multiplex PCR, and the cycle conditions were 95°C for 3 min and 40 cycles of 95°C for 15 s and 60°C for 30 s. Optical data obtained by real-time PCR were analyzed with the manufacturer’s software (iCycle Optical System Interface Version 3.0). Each assay was repeated three to ten times using different tissues. Statistical analysis involved ANOVA and two-tailed Student t test (Sigma Plot, Version 8.02, SPSS Inc., Chicago, IL).
Table 1.
Primers and Taqman probes used for RT–PCR
| Gene | Sequence | Gene bank GI # |
Amplicon size (bp) |
|---|---|---|---|
| Shh | |||
| Forward primer | caattacaaccccgacatca | 8394266 | 142 |
| Reverse primer | agtcactcgaagcttcactcc | ||
| Probe | ctctgagtcatcagccggtctgctc | ||
| Ptc1 | |||
| Forward primer | tcacagagacagggtacatgg | 4092049 | 104 |
| Reverse primer | cccggactgtagctttgc | ||
| Probe | ccttcccagaagcagtccaaaggtg | ||
| Gli1 | |||
| Forward primer | cagggaagagagcagactgac | 16555894 | 72 |
| Reverse primer | caggaggattgtgctcca | ||
| Probe | caccatgcctcagcagagccc | ||
| Gli2 | |||
| Forward primer | atccccgcttggactgac | 1707589 | 84 |
| Reverse primer | acctcggcctcctgctta | ||
| Probe | ccaggtcttccttgagatcggcc | ||
| Gli3 | |||
| Forward primer | ggcctccagtaccacttcaa | 16555896 | 105 |
| Reverse primer | ctgagaccctgcacactctg | ||
| Probe | tcaccgagagagaagaaacgcaatca | ||
| Fgf10 | |||
| Forward primer | cgtcaaagccattaacagca | 6978836 | 107 |
| Reverse primer | cctctatcctctctttcagtttacagt | ||
| Probe | tgagccatagagtttccccttcttgttc | ||
| Bmp4 | |||
| Forward primer | gattggctcccaagaatcat | 6978570 | 114 |
| Reverse primer | cctagcaggacttggcataa | ||
| Probe | cgaccatcagcattcggttaccag | ||
| RPL19 | |||
| Forward primer | ggaagcctgtgactgtccat | 14389296 | 101 |
| Reverse primer | ggcagtacccttcctcttcc | ||
| Probe | aagggcaggcatatgggcat |
Designed using the following Web sites: http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi and http://www.bioinfo.rpi.edu/applications/mfold/old/dna/.
Results
Shh-ptc-gli expression in the developing rat prostate
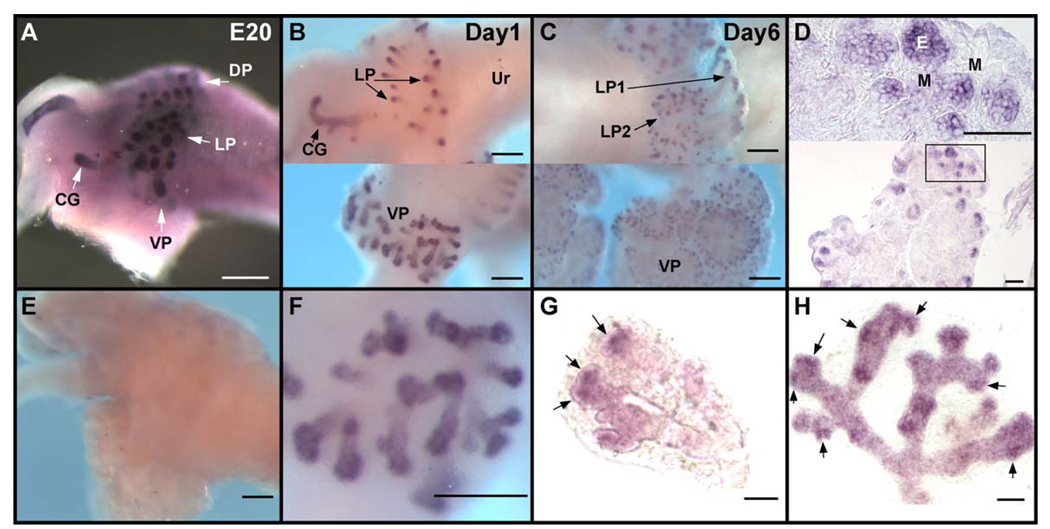
Whole-mount ISH combined with real-time RT–PCR was used to determine the spatiotemporal expression patterns of Shh, ptc, and gli1, 2, and 3 in the rat prostate gland during the active period of branching morphogenesis. There were no noticeable differences between the VP, DP, or LP with regards to expression patterns of these hedgehog signaling molecules. Prostatic budding is initiated at fetal day 18.5 of a 21-day gestation in the rat when UGS epithelial cells penetrate into the surrounding UGS mesenchyme in the dorsal, lateral, and ventral directions. At fetal day 20 (E20), the earliest time point examined in the present study, there was robust Shh mRNA expression in the prostatic buds in all three lobes with the greatest concentration in the central-to-distal aspects of these protruding ducts (Fig. 1A). By pnd 1 (i.e., 2 days later), Shh transcript levels had declined relative to the intense expression observed at fetal day 20, and expression was now restricted to the distal region of the elongating ducts (Figs. 1B, F) where it remained for the remainder of morphogenesis (Fig. 1C). Cross-sectional analysis demonstrated that Shh mRNA was confined to the epithelial cells at the leading edge of the prostatic buds and was absent in the proximal-to-central ducts (Fig. 1D). Importantly, the Shh expression pattern at the distal tips was not evenly distributed among all epithelial cells but rather was heterogeneous as early as pnd 1, a pattern which persisted as ducts elongated and branched. This is shown in Figs. 1F – H where distinct Shh expression foci are observed with adjacent regions of lower expression levels. By pnd 6, there was a noticeable decline in overall Shh signal intensity by whole-mount ISH (Fig. 1C), and this was confirmed by RT–PCR which showed a significant reduction in Shh transcript levels at pnd 6 as compared to pnd 1 (Fig. 2A, oil controls in hatched bars). By day 30, when prostatic branching is complete, Shh transcript levels reached a nadir where they remained through adulthood (Fig. 2B, hatched bars).
Fig. 1.
Whole-mount ISH of Shh transcript in the developing prostate of control rats. Examples shown in A–C were processed together to allow direct comparison of signal intensity. (A) E20 UGS; Shh is expressed along the central to distal regions of the emerging ducts. Arrows denote the budding ventral (VP), dorsal (DP), and lateral (LP) prostate lobes and the coagulating gland (CG). (B) Postnatal day 1 LP (top) and VP (bottom) from the same UGS-prostate complex at separate focal planes. Shh is localized to the distal tips of the emerging epithelial ducts. (C) Postnatal day 6 LP (top) and VP (bottom) from the same UGS-prostate complex at separate focal planes. Shh transcript remains at the distal tips of the lateral LP1 and LP2 ducts and the VP. The signal intensity in each prostatic region is reduced as compared to pnd1. (D) Cross section of pnd 6 VP from whole-mount ISH at low (bottom) and high (top) power confirms that Shh is expressed exclusively in the epithelial cells at the distal tips of the ducts. (E) Sense Shh probe on pnd 3 UGS-prostatic complex shows no signal and demonstrates antisense Shh probe specificity. (F) High-power view of pnd1 VP shown in B shows the strongest Shh mRNA intensity at the distal tips as well as regional heterogeneity of the Shh signal. (G) The distal tip of a pnd1 VP following treatment with dispase to remove surrounding mesenchyme. Expression foci of Shh are observed at the distal tips (arrows) with adjacent regions of lower signal intensity. (H) The distal aspect of a pnd3 VP duct displays heterogeneous Shh signal with focal patches of high expression (arrows) at the distal tips. Ur indicates urethra; E, epithelium; M, mesenchyme. Bars in A, B, C, E, F = 200 µm; in D, G, H = 50 µm.
Fig. 2.
Real-time RT–PCR for Shh ptc gli1, gli2, and gli3 in the developing VP of control rats (hatched bars) and rats exposed neonatally to estradiol (solid bars). To accommodate differing tissue volumes, a microassay was performed for pnd 1, 3, and 6 VP (panel A), while a standard assay was performed for pnd 6 through 90 (panel B). Day 6 was performed in both assays to allow direct comparisons. The level of transcripts for all five molecules was high at birth, significantly declined by pnd 6 and reached a nadir by day 30 where it remained through adulthood. Exposure to neonatal estrogens did not significantly alter expression of these genes when compared to oil-treated control rats. Bars represent the mean with SEM for six samples per treatment and time point for microassay and three samples per treatment and time point for standard assay. *P < 0.05; **P< 0.01; ***P < 0.001 versus day 1 oil. + P < 0.05; + + P < 0.01; + + + P < 0.001 versus day 6 oil. #P < 0.05; ##P < 0.01; ###P < 0.001 versus day 10 oil.
Ptc, the transmembrane receptor for Shh, was localized to the distal periductal mesenchymal cells in the developing prostate gland of the newborn rat, thus establishing an epithelial to stromal cell signaling pathway (Fig. 3A). As the ducts elongated and branched, the highest concentration of ptc mRNA was visualized in the distal ducts, and an expression boundary was observed between the proximal and central ducts (Fig. 3C). Cross-sectional analysis of ISH stained tissues revealed the strongest ptc mRNA signal in mesenchymal cells immediately adjacent to outgrowing ducts (Fig. 3E). A reduced intensity signal was also noted in distal epithelial cells (Fig. 3E); however, there was no epithelial signal for ptc mRNA in the proximocentral ducts (Fig. 3F). This localization pattern was confirmed by immunocytochemistry which revealed ptc protein in periductal mesenchymal and epithelial cells in the distal ducts (Fig. 3G), whereas in the proximocentral regions, ptc protein was present at low levels in the mesenchyme alone (Fig. 3H). By pnd 6, there was a significant decline in ptc mRNA levels observed both by whole-mount ISH as well as by RT–PCR (Fig. 2A, hatched bars) and levels continued to decline to a nadir in adulthood (Fig. 2B).
Fig. 3.
Whole-mount ISH of ptc transcript in the developing prostate of control rats. Examples shown in B–D were processed together to allow direct comparison of signal intensity. (A) Postnatal day 1 UGS-prostatic complex; ptc is expressed in the condensed mesenchymal cells surrounding the outgrowing epithelial buds of the VP, DP, and LP. (B) The entire VP on pnd 1 shows intense ptc expression in the periductal mesenchymal cells immediately adjacent to the emerging ducts. (C) A microdissected VP ductal region at pnd 3 allows visualization of ptc signal along the ductal length to reveal an increasing gradient from the proximal duct out towards the distal tips. (D) A microdissected VP ductal region at pnd 6 shows a noticeable decline in ptc signal intensity as compared to pnd 1 and 3. (E) Cross-section of pnd 3 VP from whole-mount ISH confirms strong ptc expression in the mesenchymal cells immediately adjacent to the ducts (arrowheads). In addition, weaker ptc signal is present in epithelial cells (E) of this distal–central ductal region, while interductal mesenchyme (M) is negative. (F) Cross-section of proximal ducts from pnd 3 LP whole-mount ISH reveals ptc transcript within the periductal mesenchymal cells (arrowhead) but no ptc expression in the proximal epithelial cells. (G) Immunolocalization of ptc protein in pnd 3 VP distal ducts and counterstained with hematoxylin. Ptc protein is localized in both mesenchymal and epithelial cells in the distal region. (Inset) Negative ICC control with IgG substituted for primary antibody. (H) Immunolocalization of ptc protein in pnd 3 VP proximal ducts shows only mesenchymal stain with no epithelial ptc protein. E indicates epithelium; M, mesenchyme. Bars in A–D = 200 µm; in E–H = 50 µm.
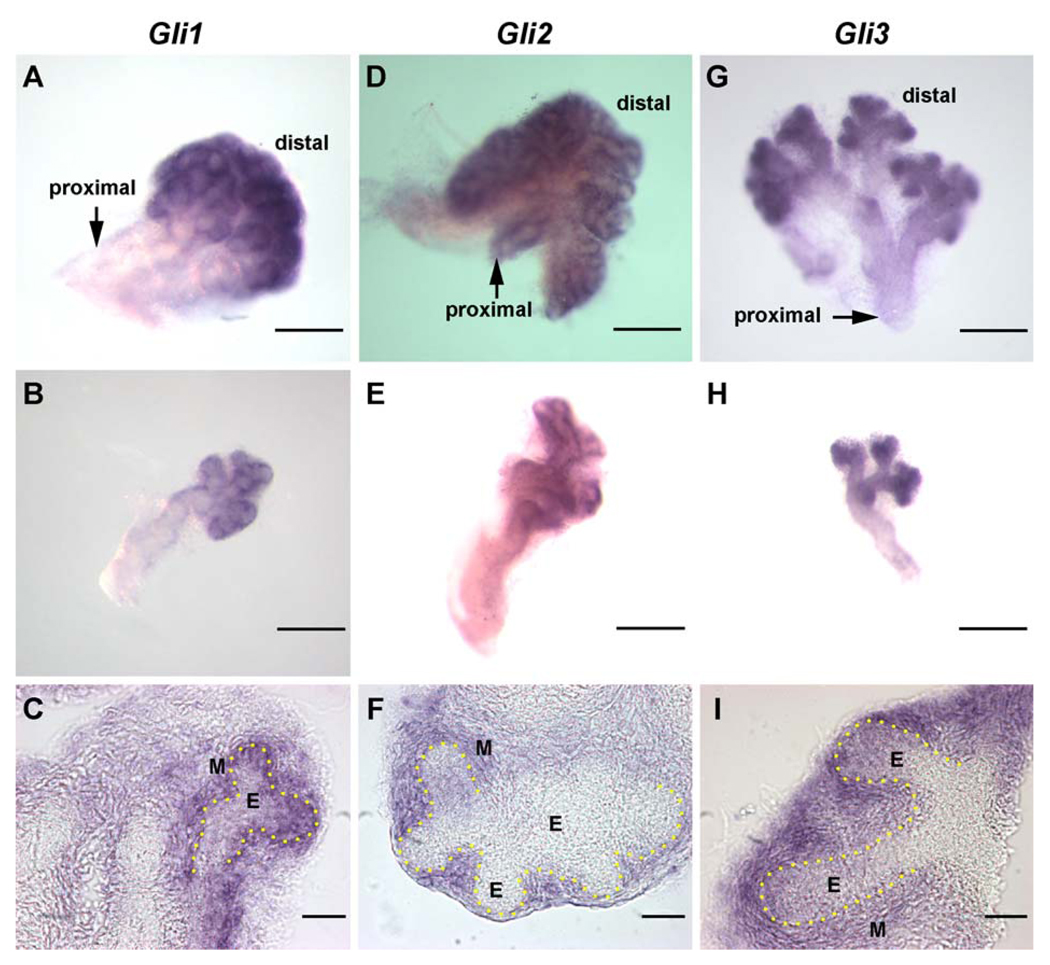
The three downstream gli transcription factors exhibited similar spatiotemporal expression patterns during prostatic development with some subtle differences (Fig. 4). Strong expression of gli1, 2, and 3 mRNA by periductal mesenchymal cells was observed on the day of birth. As the ducts elongated and branched, strong gli1, 2, and 3 expression was retained at the leading distal tips (Fig. 4); however, proximodistal variations were observed between them. Gli1 was expressed in the central and distal periductal mesenchyme (Figs. 4A and B), gli2 expression was retained along the entire ductal length with an increasing proximodistal gradient (Figs. 4D and E), and gli3 localized to the distal tip exclusively with a broader mesenchymal expression than observed for gli1 and gli2 (Figs. 4G and H). While cross-sectional analysis of the ISH-stained tissues revealed strong mesenchymal localization for all three glis, weaker stain was also observed in the distal tip epithelial cells for gli1 and gli3, but not for gli2 (Figs. 4C, F, and I). These subtle differences may have physiologic relevance, since gli1 and 2 are believed to be activators of Shh targets whereas gli3 is generally believed to be a repressor of downstream genes (Ingham and McMahon, 2001). Similar to Shh and ptc expression, levels of gli1, 2, and 3 mRNA significantly declined by pnd 6 and reached nadirs at day 30 where they remained through adulthood (Fig. 2, hatched bars).
Fig. 4.
Whole-mount ISH of gli1 (A–C), gli2 (D–F), and gli3 (G–I) transcripts in the VP of pnd 3 control rats. Examples shown in A, D, and G are from dissected sublobes of the VP, while B, E, and H are microdissected individual ducts. C, F, and I are cross-sections from whole-mount ISH for the three glis. (A) Gli1 transcript is localized to the central to distal region of the developing VP. (B) A microdissected VP duct shows strong gli1 expression in the periductal mesenchyme in the central to distal ductal region and no expression in the proximal ductal region. (C) Cross-section shows the greatest concentration of gli1 in the condensed mesenchyme immediately adjacent to the distal epithelial duct with weaker signal in the distal tip epithelium. The yellow dots outline the basement membrane. (D) Gli2 transcript is localized to the mesenchyme along the entire ductal length of the VP. (E) A microdissected VP duct localizes the gli2 signal to the periductal mesenchyme. (F) Cross-section shows gli2 signal in the mesenchyme but not in the epithelium. (G) Gli3 transcript is localized to the distal tip region of the developing VP. (H) A microdissected VP duct reveals a broad mesenchymal expression of gli3 at the distal tips of the duct, while the proximal and central ducts are negative. (I) Cross-section shows that gli3 localizes to the distal mesenchyme in a broader region than observed for gli1 and gli2 as well as a weak epithelial stain. E indicates epithelium; M, mesenchyme. Bars in A, B, D, E, G, H = 200 µm; in C, F, I = 50 µm.
Shh inhibits ductal elongation and branching through suppression of Fgf10 expression
In contrast to a stimulatory role for Shh in branching morphogenesis of other structures (Ingham and McMahon, 2001; Pepicelli et al., 1998), several recent studies have shown that Shh protein addition to cultured prostates inhibits ductal growth, while Shh antagonists increase branching, suggesting an inhibitory role for Shh in prostate morphogenesis (Berman et al., 2004; Freestone et al., 2003; Wang et al., 2003). To understand the mechanism of this effect, experiments were undertaken using the cultured neonatal rat prostate as a model system. Shh was applied either globally to the medium of cultured VPs or LPs or locally via placement of SHH-soaked beads in proximal or distal mesenchyme, and growth was monitored over several days. As was previously observed, the addition of Shh protein to culture medium markedly suppressed ductal elongation and branching morphogenesis throughout the prostate lobes (Fig. 5A). Immunocytochemistry for p63, a basal cell marker, revealed that despite the reduction in growth and branching, differentiation of the epithelial cells into a bilayer of luminal and basal cells was not affected by exogenous Shh (Figs. 5D – E). Local application of SHH beads within the distal mesenchyme of either the VP or LP resulted in inhibition of ductal elongation and branching in the immediate vicinity of the bead, while distant ducts remained unaffected (Fig. 5B). In contrast, SHH beads in the proximal mesenchyme did not influence subsequent duct elongation or branching (Fig. 5C) which suggested that the ability of Shh to affect branching required a distinct local environment.
Fig. 5.
Cultured VP and LP following application of Shh or BSA. Paired lobes from a single rat were treated with either BSA or Shh to allow direct comparisons of Shh treatment. (A) VPs (top) cultured for 3 days and LPs (bottom) cultured for 2 days in the presence of BSA (left) or Shh protein added directly to the culture medium. Shh addition suppressed ductal outgrowth and branching in both lobes. (B) BSA beads (left arrowheads) and SHH beads (right arrowheads) were placed in the distal mesenchyme of VP (top) and LP (bottom) paired lobes on pnd 0 and cultured for 4 days (VP) or 3 days (LP). Local application of SHH resulted in localized ductal growth and branching inhibition in the immediate vicinity of the bead. (C) BSA beads (left) and SHH beads (right) implanted in the VP proximal mesenchyme (arrowheads) on pnd 0 and cultured for 4 days showed no inhibition of ductal growth and branching by SHH. (D) Immunocytochem-istry for p63 (basal cell marker) in a VP grown for 6 days in basal medium with BSA shows a bilayer of stained basal cells along the basement membrane and unstained luminal cells above the basal layer which indicates differentiation of the epithelial cells. Basal cells lie adjacent to the basement membrane in an intermittent pattern in the proximal ducts and a continuous pattern in the distal tips which follows the differentiation wave of luminal epithelial cells in a proximal-to-distal fashion during prostate morphogenesis. (E) p63 Immunostain of a VP cultured for 6 days in Shh protein. Although the branching pattern is inhibited as compared to the contralateral lobe in BSA (D), the differentiation of the epithelial cells as revealed by the basal cell pattern is the same as the BSA-control cultures. Bars in A–C = 500 µm; in D–E = 50 µm.
One possibility is that Shh affects the expression of local growth factors necessary for prostatic growth and branching. To address this, the expression of Fgf10 and Bmp4 mRNA was assessed as a function of Shh manipulation since (1) both of these mesenchymal genes are known downstream targets of Shh in other systems (Haraguchi et al., 2001), (2) Fgf10 is expressed in the distal, but not proximal mesenchyme in the developing prostate (Thomson and Cunha, 1999), (3) Fgf10 plays an stimulatory role in prostatic branching morphogenesis (Donjacour et al., 2003), and (4) Bmp4 plays an inhibitory role in prostatic ductal outgrowth and branching (Lamm et al., 2001). Using RT–PCR, Fgf10 and Bmp4 transcripts were quantitated in VPs and LPs cultured with Shh protein in the medium. Since this culture system results in a marked shift in the prostatic epithelial–stromal ratio, gene expression was assessed before observable growth alterations. In both lobes, Shh protein significantly down-regulated Fgf10 expression and increased Bmp4 expression within 18 h, suggesting that the growth inhibitory effects of Shh may be mediated through alterations in the expression of these morphogens (Fig. 6A). To address this directly, replacement experiments were conducted with exogenous Fgf10 protein or noggin, a Bmp4 antagonist, added to prostates cultured with Shh protein for 4–6 days. As shown in Fig. 6B, Shh protein suppressed ductal elongation and branching of the VP, and this was largely overridden by exogenous Fgf10. In contrast, antagonism of Bmp4 with noggin was unable to reverse the growth suppression by Shh protein (Fig. 6C). Prostates cultured with either Fgf10 or noggin alone in the presence of 10 nM testosterone exhibited a modest increase in prostate size and branching (data not shown); however, this was not significant when measured by 2-D analysis. As an alternate approach to demonstrate Fgf10 regulation by Shh, UGS-prostatic complexes were removed on pnd 0 and cultured for 24 h with anti-Shh or control antibody (n = 3). The expression of ptc, which is directly stimulated by Shh, was repressed which confirms that the antibodies functionally blocked Shh action (Fig. 7A). Importantly, Fgf10 expression was markedly increased in Shh-inhibited prostates when compared to controls as determined by wmISH (Fig. 7B). Taken together, these data indicate that the prostatic growth suppression by Shh is mediated through suppression of mesenchymal Fgf10.
Fig. 6.
(A) Real-time RT–PCR data showing the effect of Shh protein on VP and LP expression of Fgf10 and Bmp4 mRNA after 18 h of culture. In both lobes, Shh protein (solid bars) inhibited Fgf10 expression and increased prostatic Bmp4 expression as compared to BSA controls (hatched bars). Bars represent the mean ± SEM for eight samples per treatment. *P < 0.05; ***P < 0.001 Shh versus BSA. (B) VP cultured for 6 days in basal medium with BSA (left), Shh protein (center) or Shh + Fgf10 (right). The Shh cultures with or without Fgf10 are contralateral lobes to allow direct comparisons. Ductal growth and branching inhibition due to Shh protein was reversed by exogenous Fgf10. (C) VP cultured for 4 days in basal medium with BSA (left), Shh protein (center), or Shh + noggin (right). The Shh cultures with or without noggin are contralateral lobes. Ductal growth and branching inhibition due to Shh protein was not affected by the Bmp4 antagonist. Bar in B–C = 500 µm.
Fig. 7.
The effects of Shh on prostatic Fgf10 expression in the developing prostate gland. (A) Whole-mount ISH for ptc transcript in the pnd 0 UGS-prostate complex following culture for 24 h in the absence (left) or presence (right) of anti-Shh antibodies. Ptc expression was lost following treatment with anti-Shh antibodies, indicating that they functionally blocked Shh action. (B) Whole-mount ISH for Fgf10 transcript in the pnd 0 UGS-prostate complex following culture for 24 h in the absence (left) or presence (right) of anti-Shh antibodies. Blockade of Shh action markedly increased Fgf10 expression in all prostate lobes. Identical results were obtained with three separate sets of tissues. (C) Fgf10 expression by wmISH in the pnd1 LP and VP 1 day after placement of BSA beads on the prostate surface. (D) Fgf10 expression was reduced in the LP and VP 1 day after placement of SHH beads along the prostate surface. The tissue in D was processed together with the tissue in C to allow comparison of stain intensity. Similar results were obtained from three separate tissue sets. (E) Fgf10 expression by wmISH in the VP 3 days after implantation of a BSA bead (top) or a SHH bead (bottom) in the distal mesenchyme. Fgf10 expression was reduced in the immediate vicinity of the SHH bead but was unaffected by the BSA bead. (F) The VP shown in E (bottom) before wmISH shows inhibition of ductal growth and branching in the immediate vicinity of the SHH bead. Bar in A–F = 200 µm.
Since Shh is exclusively expressed in distal ductal tips of the branching prostate, we examined the local regulation of Fgf10 expression by SHH beads implanted along the distal mesenchyme. Prostatic-UGS complexes were removed on pnd 0; SHH or BSA beads were placed along the LP and VP surfaces and cultured for 24 h. Whole-mount ISH for Fgf10 revealed a strong reduction in mesenchymal Fgf10 mRNA expression in explants with SHH beads as compared to the BSA controls (Figs. 7C – D). Similarly, in VPs cultured for 3 days with SHH beads implanted in the distal mesenchyme, Fgf10 expression was reduced in the immediate vicinity of the SHH beads where ductal growth and branching were inhibited (Figs. 7E – F). In contrast, Fgf10 expression was unaffected at a distance from the beads where branching was normal as well as in the immediate vicinity of BSA beads (Fig. 7E). These data indicate that Shh at distinct distal sites causes local down-regulation of Fgf10 expression in the adjacent mesenchyme.
Neonatal estradiol exposure suppresses Shh-ptc-gli expression in a lobe-specific manner
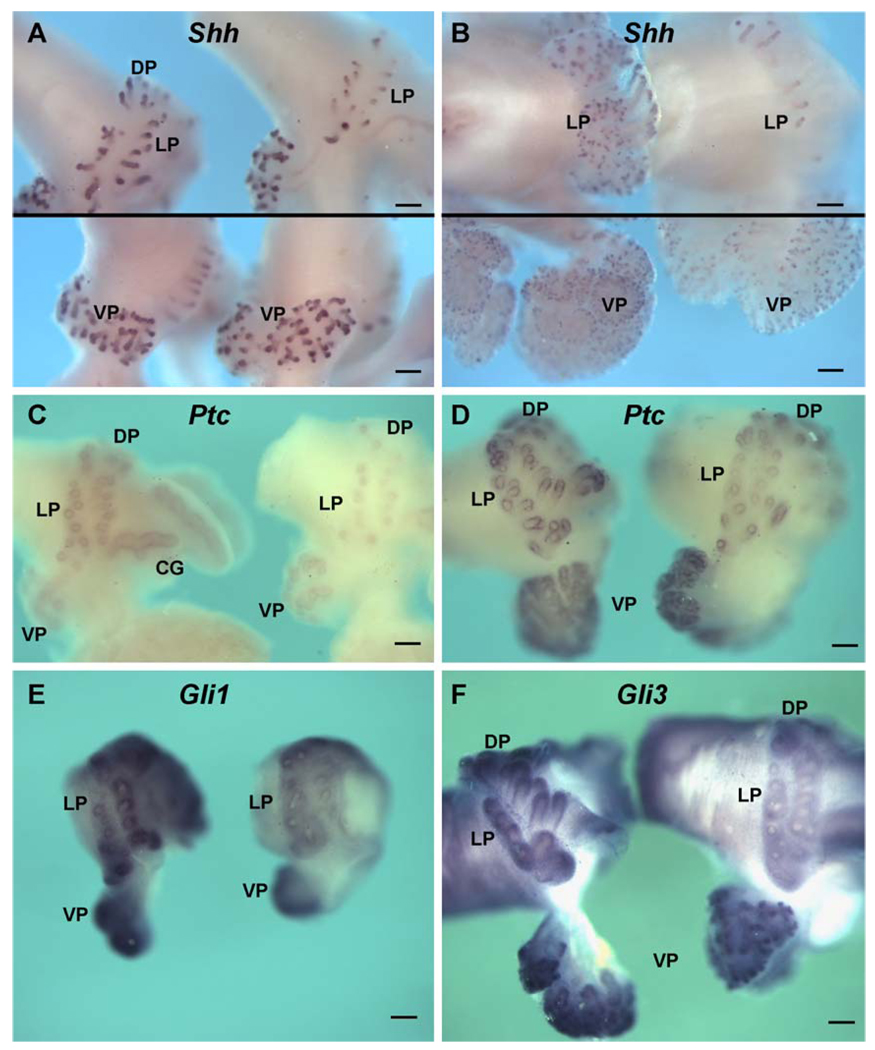
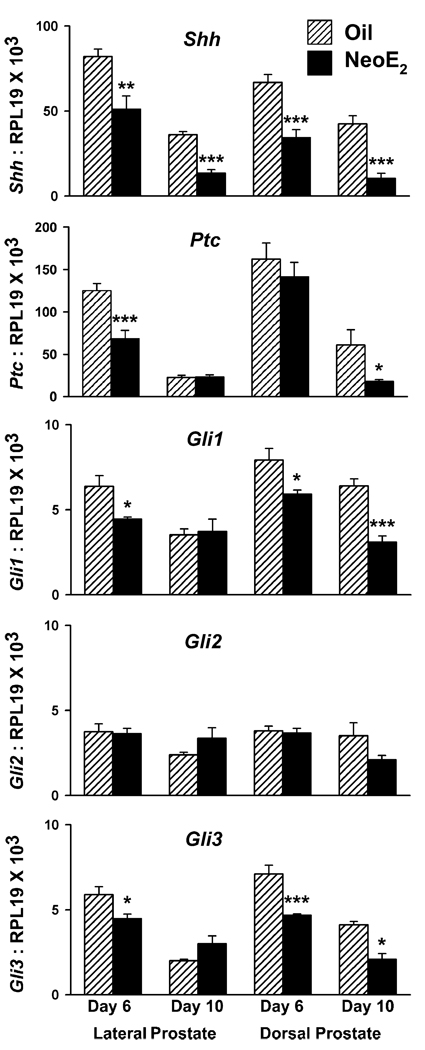
Exposure to estradiol in vivo on pnd 0, 3, and 5 reduced Shh-ptc and gli mRNA expression in the DP and LP. In contrast, expression of these signaling molecules in the VP was not significantly affected. As early as pnd 1, wmISH consistently revealed strong suppression of Shh transcript in the LP and DP regions when tissues from control and estrogen-treated rats were processed together (Fig. 8A, top), while Shh expression was similar between the two groups in the VP (Fig. 8A, bottom). This suppression of Shh expression in the dorsolateral prostate persisted through pnd 6 (Fig. 8B). Furthermore, real-time RT–PCR of Shh mRNA levels in day 6 and 10 LP and DP revealed a significant reduction following estradiol exposure in those lobes specifically (Fig. 9), while no statistical difference was noted in the VP between the treatment groups at any time point (Fig. 2). Similar to Shh, estradiol reduced ptc expression in the LP and DP as early as pnd 1 (Fig. 8C), and this effect persisted through pnd 6 for the LP and pnd 10 for the DP (Figs. 8D and 9). In contrast, VP ptc expression was not affected by estradiol exposure at any time point (Figs. 2A and B; 8C and D). Likewise, gli1 and gli3 expressions were suppressed in the LP and DP through pnd 10 following neonatal estradiol exposure (Figs. 8E and F; 9), while expression in the VP was not changed (Figs. 2 and 8E and F). Notably, gli2 expression was not affected by this steroid in any of the prostate lobes (Figs. 2 and 9). It is noteworthy that estrogen did not affect the localization of the hedgehog signaling molecules in the prostate lobes.
Fig. 8.
Whole-mount ISH for Shh ptc gli1, and gli3 expression in the UGS-prostatic complexes from control and estrogen-exposed rats. Treated tissues for each probe were processed and photographed together to allow direct comparisons of signal intensity between the treatment groups. (A) Shh message in pnd 1 oil (left) and estradiol-treated (right) rats. A focused image of the dorsolateral region is shown in the top panel, while the VPs from the same tissues are shown in the bottom panel at separate focal planes. Epithelial Shh expression in the distal tips of outgrowing ducts is markedly suppressed in the estrogen-exposed LP and DP as compared to oil-treated controls, while expression in the VP is unaffected. (B) Shh transcript in pnd 6 prostates from rats treated with oil (left) or estradiol (right). Shh expression remains suppressed in the LP of estrogen-treated rats as compared to oil controls (top panel), while levels in the VP remain unchanged. (C and D) Ptc ISH in pnd 1 (C) and pnd 3 (D) rats from oil (left) and estradiol (right) treatment groups. LP and DP ptc expression is markedly suppressed in estrogen-treated animals as compared to oil controls while VP expression remains unaffected. (E) Gli1 ISH in pnd1 complexes from control (left) and estrogen-treated rats (right). Gli1 expression is markedly reduced in estrogen-exposed LPs as compared to controls, while VP levels remained unaffected. (F) Gli3 ISH in pnd 3 complexes from control (left) and estrogen-exposed rats (right). Ductal growth and gli3 expression are markedly reduced in the LPs of rats exposed to estradiol as compared to controls, while VP expression remains unaffected. Bars = 200 µm.
Fig. 9.
Real-time RT–PCR for Shh ptc gli1, gli2, and gli3 in the LP and DP of control (hatched bars) and estrogen-exposed rats (NeoE2; solid bars) on pnd 6 and 10. Bars represent the mean with SEM for four to ten samples per treatment and time point. *P < 0.05; **P < 0.01; ***P < 0.001 for NeoE2 versus oil at specific time points.
Organ culture of lateral lobes indicates direct effects of estradiol on Shh-ptc-gli expression
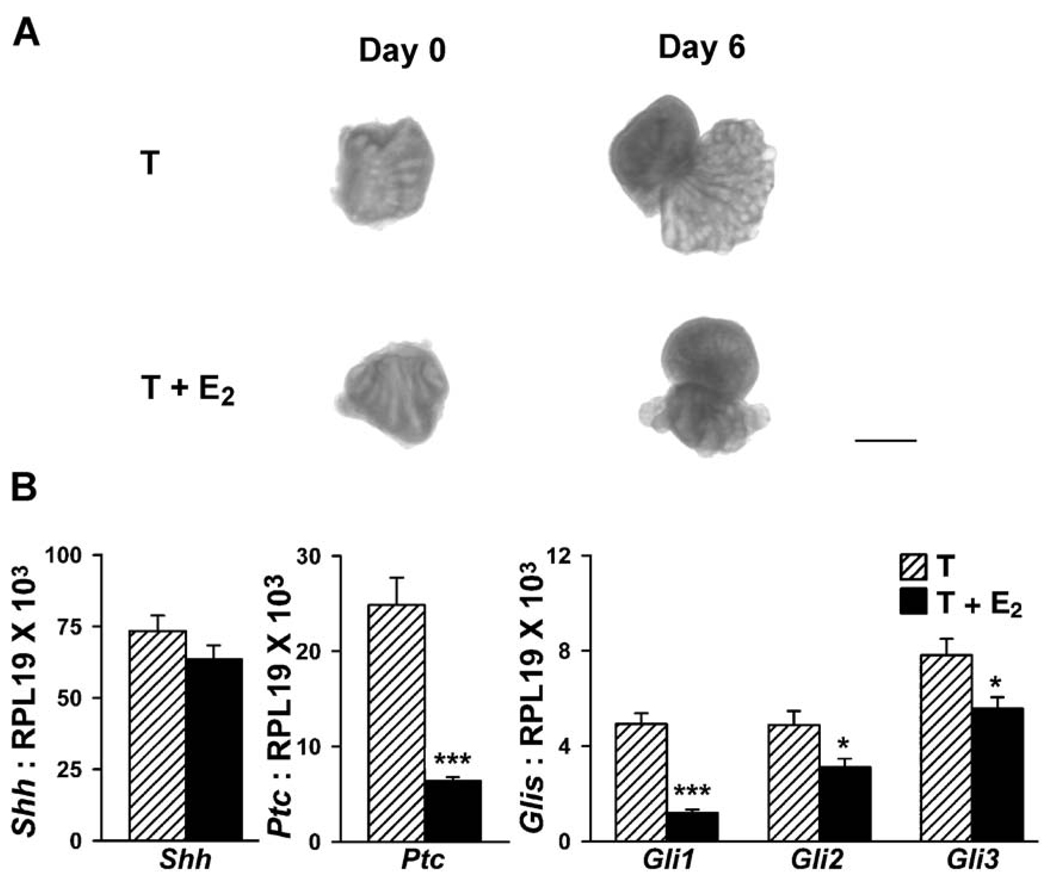
To determine whether the effects of estrogen on prostatic Shh-ptc-gli expression were direct prostatic effects or indirectly mediated through systemic alterations following estrogen treatment, an organ culture system was employed for the developing LP. Previous in vitro studies with VPs have shown a recapitulation of several, but not all, of the estrogen effects, indicating that estrogen has direct effects at the level of the prostate gland (Jarred et al., 2000). In the present study, LPs were removed on pnd 0 and grown in vitro with testosterone or testosterone plus 20 µM estradiol (contralateral lobe). As shown in Fig. 10A, LP growth and branching in vitro were markedly suppressed by estradiol. Measurement of Shh transcript levels in the cultured LPs by real-time RT–PCR revealed lower levels of Shh mRNA following in vitro estradiol exposure (Fig. 10B); however, this trend was not statistically significant (P = 0.09). In contrast, ptc, gli1, gli2, and gli3 mRNA were all significantly reduced by estradiol in vitro to the full extent as observed in vivo (Fig. 10B). Together, these findings indicate that the estrogenic effects are mediated directly at the prostatic level.
Fig. 10.
(A) LPs collected on pnd 0 and cultured for 6 days in basal medium with 10−8 M testosterone (T) or with testosterone plus 20 µM estradiol (T + E2). Ductal elongation and branching observed in day 6 T cultures were suppressed when LPs were cultured in T + E2. Bar = 500 µm. (B) Real-time RT–PCR for Shh, ptc gli1, gli2, and gli3 in the LP after 6 days in culture with T (hatched bars) or T + E2 (solid bars). Bars represent the mean and SEM for 10 replicates. *P < 0.05; ***P < 0.0001, T versus T + E2.
Neonatal estradiol exposure suppresses prostatic ductal branching in a lobe-specific manner
Our previous studies have shown that neonatal exposure to estrogens interrupts branching morphogenesis and cellular differentiation in the developing prostate and that these phenomena have a degree of lobe specificity to them (Prins, 1992, 1997). While all lobes show a similar overall reduction in growth and adult size, branching is most restricted in the LP. In light of the above lobe-specific findings with regards to Shh-ptc-gli expression, we further characterized the estrogen-induced branching deficits by microdissecting the lobes in collagenase on pnd 30 (when branching is normally completed) and counting the number of terminal ductal tips in the lateral LP1 and LP2 ductal systems. Fig. 11A shows a single LP from a control animal where the elongated LP1 ducts and bushy LP2 ducts are visualized with multiple branch points and terminal ductal tips or acini. In contrast, the dorsolateral-UGS complex from an estrogenized rat clearly shows the lack of branching and absence of acini in the dorsal and lateral ducts (Fig. 11B). Comparison of the number of terminal ductal tips or acini in the lateral LP1 and LP2 lobes of the control and estrogenized rats revealed a significant difference between the groups (Fig. 11E). Furthermore, the number of LP1 tips in estrogenized rats was 6.7 ± 0.3 which is the exact number of main LP1 ducts found in a control LP, indicating a complete inhibition of branching in that lateral region as a result of high-dose estrogen exposure. While the number of tips was greatly reduced in the estrogenized LP2, there was visual and numerical evidence that some branching occurred in that region, albeit minimal. In contrast, the VP of the estrogenized prostate (Fig. 11D), although significantly reduced in size as compared to the control ventral lobe (Fig. 11C), exhibited a complex branching pattern, so much so that an accurate counting of the branch points and terminal tips was not possible.
Fig. 11.
Day 30 prostate lobes from control and estrogenized rats following microdissection and collagenase treatment to visualize ductal branching and terminal acini. (A) A single LP (one side) from a control rat reveals the presence of two branched structures, the elongated LP1 ducts, and the bushy LP2 ducts. (Bottom left) A single microdissected LP1 duct reveals the extended proximal duct which gives the duct a palm tree appearance. (Bottom right) A single microdissected LP2 duct reveals a shorter, more highly branched structure. (B) The entire dorsolateral-urethral complex from an estrogenized rat reveals the short ducts in the DP and LP which have failed to branch and form distal acini. Ur indicates urethra. (C) One ventral lobe from a control animal without collagenase treatment reveals a complex, highly branched structure. (D) One ventral lobe form of an estrogenized rat reveals a reduction in overall size but similar complexity and branching as observed in the oil control rats. Bars = 500 µm. (E) Number of terminal ductal tips (acini) in the LP1 and LP2 ducts of control (hatched bars) and estradiol-exposed rats (solid bars). Bars represent the mean ± SEM. *P < 0.05; **P < 0.0001 versus control (n = 3).
Discussion
Expression and localization of Shh-ptc-gli1–3 in the developing rat prostate lobes
The present study provides a clear spatiotemporal picture of the expression patterns of the Shh-ptc-gli signaling molecules in the separate rat prostate lobes during development. The patterns described are in close agreement with similar findings for the mouse prostate gland (Lamm et al., 2002; Podlasek et al., 1999) and the developing rat ventral lobe (Freestone et al., 2003). Overall, the expression of the hedgehog signaling molecules is high at birth when budding is underway and rapidly declines over the next several days when morphogenesis is complete. Whole-mount ISH of the UGS-prostatic complex from fetal day 20 to pnd 6 showed that, during the initial budding phase, Shh has a broad epithelial expression along the ductal length which rapidly transitions into a distal tip epithelial expression pattern as the ducts elongate and branch. Furthermore, the expression of Shh at the tips is not uniform but rather appears heterogeneous with foci of higher Shh expression at specific sites which may allow for highly localized Shh actions at those sites. The highest concentration of ptc and gli1–3 transcripts was localized to the condensed mesenchymal cells adjacent to the elongating epithelial ducts in the distal regions, which in total provides for the greatest Shh signal being transmitted at the distal aspect of the developing gland. Similar patterning for Shh-ptc has been observed in several developing structures with axis polarity including the lungs (Helms et al., 1997; Pepicelli et al., 1998), external genitalia (Haraguchi et al., 2001), and limbs, (Yang et al., 1997) suggesting a common mechanistic role for this paracrine signal. It is noteworthy that ptc receptor and gli1 and gli3 transcription factors were also localized, albeit at lower expression levels, in the distal tip epithelial cells but were not expressed in the proximal or central ductal epithelial cells. A low level of ptc was previously detected by RT– PCR in isolated epithelial cells of the mouse prostate (Lamm et al., 2002). Thus, in addition to paracrine signaling, Shh may have a role in directly activating genes in the distal epithelial cells in an autocrine manner.
Distal signaling center in the developing prostate
The highly localized expression of Shh at the leading edge of the outgrowing ducts is undoubtedly of critical importance in its functional role. Several other developmental genes, including Nkx3.1 (Bhatia-Gaur et al., 1999; Prins et al., 2001a,b), Hoxb13 (Sreenath et al., 1999), and Bmp7 (Huang et al., 2003), have been localized to the prostatic distal tip epithelium, while Fgf10 and Bmp4 have been localized to the distal mesenchyme (Huang et al., 2004; Thomson and Cunha, 1999) making this region reminiscent of the distal signaling center described for lungs (Cardoso, 2000; Weaver et al., 1999). The lung distal signaling center is believed to be involved in cell fate specification along the proximal–distal axis of the respiratory tract, and it is proposed that cells at the distal tip, exposed to high concentrations of morphogens, assume a distal phenotype, whereas cells at a distance assume or retain a proximal character. It is well recognized that prostatic ducts have morphologic and functional differences along the proximal–distal axis, although the molecular determinants of this polarity have been unclear (Prins et al., 1992). We propose that a distal signaling center exists within the rodent prostate gland with Shh-ptc-gli playing a central role in determining polarity along the proximal–distal ductal axis.
Unlike Shh whose expression is strictly limited to the distal epithelium, ptc, gli2, and, to a degree, gli1 are expressed along the ductal length with decreasing expression towards the proximal duct. This continued proximal–central duct expression as the ducts elongate and branch may allow for long-range Shh actions along the ductal length. Recent studies (Gritli-Linde et al., 2001) demonstrated that Shh protein associates with GAGs and can be detected over long distances in the basement membrane, raising the possibility of graded Shh effects along the ductal length. Long-range as well as short-range actions of Shh have been described in other systems, and these differ based on its concentration gradient as well as the presence or absence of specific transcription factors in the target cells (Ingham and McMahon, 2001). Thus, it is noteworthy that the activating gli1 and gli2 are expressed along the ductal length, while gli3, a repressor of Shh targets, is expressed only in the distal mesenchyme. These differences may lead to distinctive molecular responses to Shh along the ductal length as opposed to the distal signaling center.
Role for Shh in prostatic branching morphogenesis
The precise roles of Shh in prostatic development are not well clarified at the present time. Although initially thought to be essential for prostatic budding (Podlasek et al., 1999), recent studies with Shh null mice demonstrated that Shh is not required for prostatic initiation (Berman et al., 2004; Freestone et al., 2003). Experiments to address the role of Shh in prostatic ductal growth and branching have produced conflicting data. Implantation of anti-SHH antibody beads in the UGS inhibited prostatic ductal outgrowth (Podlasek et al., 1999), while cyclopamine, an Shh antagonist, reduced distal epithelial cell proliferation (Freestone et al., 2003) which suggests a stimulatory role for Shh in prostatic growth. In contrast, addition of Shh to the culture medium reduced prostatic ductal length and branching, while cyclopamine had the opposite effect, suggesting an inhibitory role for Shh in these processes (Berman et al., 2004; Freestone et al., 2003; Wang et al., 2003). The present findings help to explain the growth suppressive effects of exogenous Shh in the prostate by demonstrating that decreased Fgf10 expression following Shh application is the proximate cause of growth and branching suppression. Ductal elongation and branching are known to be driven by Fgf10 in the prostate gland as well as other structures (Bellusci et al., 1997; Donjacour et al., 2003; Hoffman et al., 2002; Thomson and Cunha, 1999). In the present study, the addition of Shh to prostate cultures rapidly down-regulated prostatic Fgf10 transcript levels, while anti-Shh antibodies increased Fgf10 expression throughout the distal mesenchyme which indicates that Fgf10 is downstream of Shh in the prostate gland. Importantly, the Shh-induced growth suppression was reversed by concomitant addition of Fgf10 to the prostate cultures. Shh addition to the cultures also resulted in increased expression of prostatic Bmp4, a secreted morphogen known to restrict ductal outgrowth (Lamm et al., 2001). However, the addition of noggin, a Bmp4 antagonist, did not override the Shh-induced growth inhibition which indicates that the elevation in Bmp4 does not by itself mediate the Shh effects. Overexpression of Shh has been shown to suppress hair follicle morphogenesis as opposed to its normal stimulatory role which emphasizes that the exact timing, location, and concentration of Shh affect the resultant phenotype (Ellis et al., 2003). Taken together, we propose that the reported effects of ubiquitous administration of Shh or Shh antagonists to cultured prostates are a result of global alterations in Fgf10 expression throughout the prostatic mesenchyme.
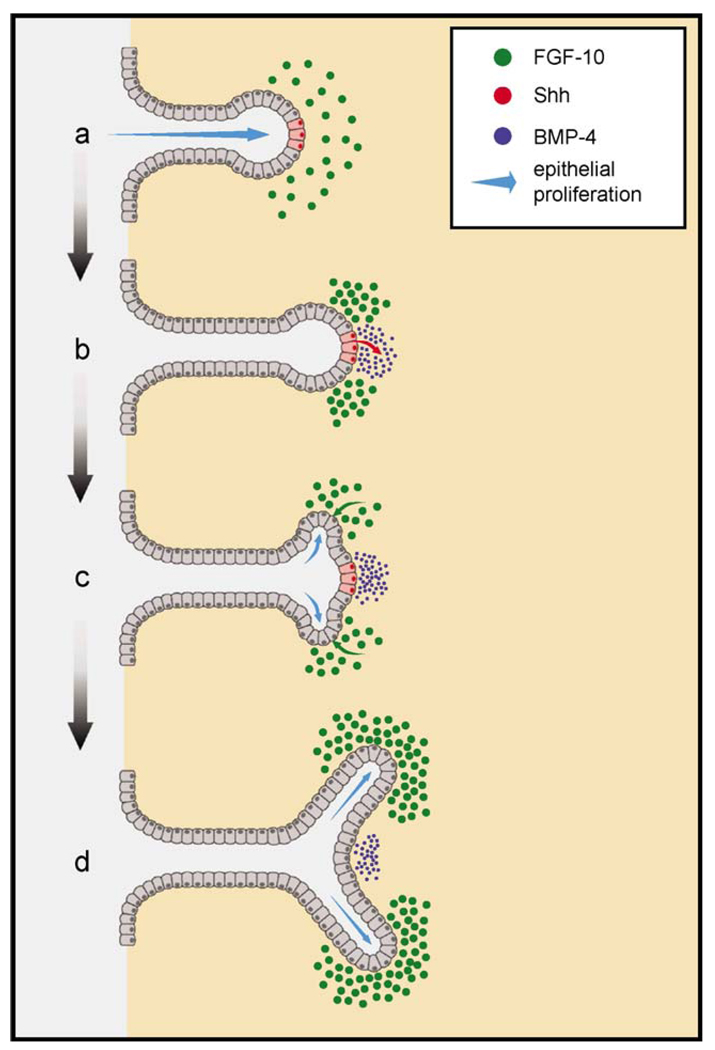
In normal developing tissues including the prostate, the spatial expression of developmental genes is critical in dictating the final phenotype. While evaluating the effects of regional Shh application using SHH beads, we observed that growth and branching inhibition was dependent on the location of the beads. Thus, SHH beads implanted proximally, where Fgf10 is not expressed, had little effect, while similar beads implanted in the distal mesenchyme locally down-regulated mesenchymal Fgf10 and concomitantly inhibited ductal elongation and branching in the immediate vicinity. This is significant in light of our present results on the regionalized expression pattern of prostatic Shh. Not only is Shh highly restricted to the distal tip of elongating ducts, its expression appears heterogeneous within the distal signaling center with focal areas of higher and lower expression. We offer a model in Fig. 12 for the potential involvement of Shh in dichotomous branching of the prostate ducts which incorporates the highly localized, focal Shh expression pattern along with the findings that Shh suppresses Fgf10 and enhances Bmp4 expression in adjacent mesenchymal cells in the distal prostate gland. We propose that as the prostatic ducts elongate and make contact with Fgf10-expressing mesenchymal cells, focal expression of Shh at the distal tips will locally down-regulate Fgf10 expression in the mesenchyme immediately adjacent to the Shh foci. This localized reduction in Fgf10 will create expression domains with relatively higher Fgf10 levels lateral to the Shh foci (Fig. 12b). Higher Fgf10 activation of FgfRiiib on adjacent epithelial cells will increase epithelial proliferation at the site of each lateral Fgf10 subdomain (Fig. 12c). Disparate epithelial proliferation rates will result in the sprouting of two buds on each side of the Shh foci, thus initiating a branchpoint (Fig. 12d). Contributing to this, mesenchymal Bmp4 expression will be locally up-regulated adjacent to the Shh foci, and this will inhibit ductal outgrowth at that restricted Shh/Bmp4 domain. In this manner, Shh, Fgf10, and Bmp4 may work in synchrony to initiate and maintain dichotomous branching at the distal aspects of elongating ducts. Importantly, interruption of this signaling network by altered expression of Shh and/or Fgf10 or their cognate receptors would result in growth and branching abnormalities. It is noteworthy that a similar hypothesis has been ventured for the role of Shh with Fgf10 in branching morphogenesis of the lung (Acosta et al., 2001; Weaver et al., 2000).
Fig. 12.
A schematic representation of Shh Fgf10, and Bmp4 interactions during dichotomous branching of the developing rat prostate ducts. Shh is expressed in highly localized, discreet foci (red cells in a) within the distal signaling center as the ducts elongate. When these cells contact the distal mesenchymal cells which express Fgf10 (green circles), the secreted Shh (b, red arrow) activates ptc on mesenchymal cells and down-regulates Fgf10 expression (b, loss of green). The focal Fgf10 down-regulation results in lateral subdomains of higher Fgf10 expression adjacent to the Shh foci which will, in turn, result in higher activation (c, green arrow) and proliferation (c, blue arrows) of adjacent epithelial cells. In addition, the secreted Shh up-regulates Bmp4 expression (b and c, blue circles) in mesenchymal cells immediately adjacent to the Shh foci which suppress epithelial cell proliferation at the central domain. Together, the disparate epithelial proliferation rates result in the sprouting of two buds on each side of the Shh foci (c), initiating a branchpoint (d).
Neonatal estrogen suppresses Shh-ptc-gli and ductal growth and branching in a lobe-specific manner
A major aim of the present study was to determine if neonatal estrogenic exposure affected prostatic Shh-ptc-gli expression and whether this contributed to the estrogenized phenotype in the developing and adult prostate. The estrogenized rat prostate can be described as a proximalized phenotype in which the morphologic and differentiation characteristics of the proximal ducts extend far into the glandular structure at the expense of a central–distal phenotype, which is suppressed (Prins et al., 2001b). For the dorsolateral prostate, this proximalized phenotype includes branching deficiencies where the proximal ducts fail to branch in the distal regions. Since it has been demonstrated that disruption of the lung distal signaling center disrupts distal pattern formation and leads to proximalized phenotypes in the pulmonary system (Cardoso, 2000; Weaver et al., 1999), we suspected that the proximalized DLP phenotype was related to disruption of distal signaling center genes that are specifically involved in distal branching. The present results support this hypothesis by showing a lobe-specific effect of estrogens on both Shh-ptc-gli expression and ductal outgrowth and branching. Following in vivo exposure to estrogens, Shh, ptc, gli1, and gli3 were significantly reduced in the DP and LP within 24 h, while the VP appeared unaffected. Furthermore, we observed that the main ducts of the DLP were stunted and exhibited marked branching deficiencies, while the VP exhibited similar branching complexity in the estrogenized and control rats. It is important to recognize that other factors are affected by estrogens besides the Shh-ptc-gli system which could account for the reduced branching independent of Shh. However, taken together with the model proposed above for the role of Shh in dichotomous branching, the present results strongly implicate the lobe-specific suppression of the Shh-ptc-gli pathway in mediating the suppression of ductal outgrowth and branching in the dorsal and lateral lobes.
It is of note that estradiol does not affect the localization of Shh expression in the prostate. This is relevant since Lamm et al. (2002) have shown that DHT shifts Shh expression in the female UGS from the cranial margin to the tips of the nascent buds; that is, DHT activates a male-specific spatial pattern. Apparently, estradiol does not counteract this effect. It is also important to point out that estrogenization of the prostate inhibits epithelial proliferation and stimulates mesenchymal proliferation leading to a relative increase in prostatic stromal mass (Prins, 1992). However, this shift in cell ratios cannot account for the decreased ptc and gli expression in the present study since the relative number of stromal cells increases following estrogenization yet mesenchymal ptc and gli decrease.
The organ culture studies demonstrated that estradiol is capable of suppressing LP Shh-ptc-gli expression in vitro which indicates that the estrogenic effects are mediated directly at the prostatic level. However, it is noteworthy that the estrogenic suppression of Shh mRNA was not as marked in vitro as observed in vivo which implicates the additional involvement of a systemic factor in lowering prostatic Shh levels. One potential candidate is the decreased circulating androgens as a result of neonatal estrogen exposure (Corbier et al., 1992; Haavisto et al., 2001; Prins, 1992). Although androgens are not required for Shh expression in the UGS, they have been shown to increase levels of Shh transcript (Freestone et al., 2003; Lamm et al., 2002) which raises the possibility that androgens have a supplemental role in maintaining normal Shh expression. We propose that a combination of reduced circulating androgens together with direct effects of estrogen may result in the in vivo suppression of DP and LP Shh levels. We also predict that the direct prostatic effects of estrogen on epithelial Shh expression are mediated through paracrine factors since ERα, which initiates the events, is expressed in periductal mesenchymal cells (Prins and Birch, 1997; Prins et al., 2001a,b). In contrast to Shh, estrogenic regulation of ptc and gli1–3 was fully recapitulated in vitro, indicating that their suppression is mediated entirely at the prostatic level. It is of interest that ptc and glis are markedly down-regulated by estradiol in vitro when Shh levels are only marginally suppressed which suggests that their reduced levels are not secondary to reduced Shh expression but may be direct targets of estrogen action through the up-regulated ERα in the mesenchyme. Alternatively, estrogens may indirectly function at the prostatic level through retinoids via RAR/RXRs (Prins et al., 2002; Pu et al., 2003) or through the ERα-induced progesterone receptor in the mesenchymal cells (Sabharwal et al., 2000).
The molecular basis for lobe-specific effects of estrogen on Shh and its downstream signaling molecules is not clear at present. Our previous studies have shown similar ERα localization and up-regulation following neonatal estrogen exposure in all prostate lobes; thus, the presence or absence of the initiating receptor is not a factor in lobe specificity (Prins and Birch, 1997). Likewise, AR is immediately down-regulated to a similar degree in all lobes following neonatal estrogenization (Prins and Birch, 1995). Recently, we observed a lobe-specific expression pattern for RXRγ, retinaldehyde dehydrogenase 2 (RALDH2), and CYP 26 which form and degrade intracellular retinoic acid, respectively, as well as cellular retinoic acid binding proteins, CRABP 1 and 2 which sequester and regulate retinoic acid availability within cells (Pu et al., 2003). Transcript levels for each of these molecules were high in the developing DP and LP but were minimally expressed in the VP, suggesting a greater developmental role for retinoids in the DLP. Furthermore, neonatal estrogens drastically reduced RALDH2 and CYP26 transcripts and suppressed CRABP 1 and 2 levels in the DP and LP specifically which may compromise retinoid availability. This is highly significant since retinoic acids have been shown to be critical regulators of Shh expression in multiple developing structures (Cardoso et al., 1996; Goyette et al., 2000; Helms et al., 1994, 1997; Niederreither et al., 2002). Future studies are aimed at examining the potential lobe-specific retinoid regulation of Shh-ptc-gli in the DLP.
In closing, lobe-specific responses to neonatal estrogens have been previously described, but their molecular basis has been unclear. While the VP is the site of epithelial differentiation defects and adult-onset dysplasia, the LP epithelium shows minimal differentiation abnormalities but major branching deficiencies (Prins, 1997). These distinctive regional responses to the same initial compound suggest that they are mediated through separate pathways. Previous studies have shown that permanent reductions in AR and ERβ are specific to the VP and are thought to contribute to adult dysplasia in that lobe (Prins, 1992; Prins et al., 1993, 1998). Since the present data indicate that the Shh-ptc-gli pathway participates in prostatic branching morphogenesis through localized regulation of other critical morphogens, it is likely that the regional suppression of the Shh-ptc-gli signaling system in the DP and LP contributes to the lobe-specific growth deficits in that prostatic region.
Acknowledgments
The authors would like to thank Dr. Liang Ma at Tulane University for assistance in establishing whole-mount ISH and for generously providing the Shh cDNA plasmid, Dr. Alexander Joyner at New York University for generously providing the mouse cDNA plasmids for gli1, gli2, and gli3, Lynn Birch for technical assistance, and Dr. Oliver Putz for graphic renditions of our data. Work was supported by NIH grant DK-40890.
References
- Aboseif SR, Dahiya R, Narayan P, Cunha GR. Effect of retinoic acid on prostatic development. Prostate. 1997;31:161–167. doi: 10.1002/(sici)1097-0045(19970515)31:3<161::aid-pros3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Acosta JM, Thebaud B, Castillo C, Mailleu A, Tefft D, Wuenschell C, Anderson KD, Bourbon J, Thiery J-P, Bellusci S, Warburton D. Novel mechanisms in murine nitrofen-induced pulmonary hypoplasia: FGF-10 rescue in culture. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2001;281:L250–L257. doi: 10.1152/ajplung.2001.281.1.L250. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan B. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- Berman DM, Desai N, Wang X, Karhadkar SS, Reynon M, Abate-Shen C, Beachy PA, Shen MM. Roles for hedgehog signaling in androgen production and prostate ductal morphogenesis. Dev. Biol. 2004;267:387–398. doi: 10.1016/j.ydbio.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, Kim M, Desai N, Young P, Norton CR, Gridley T, Cardiff RD, Cunha GR, Abate-Shen C, Shen MM. Roles for Nkx3.1 in prostate development and cancer. Genes Dev. 1999;13:966–977. doi: 10.1101/gad.13.8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody H, Goldman SF. Metaplasia of the epithelium of the prostatic glands, utricle and urethra of the fetus and newborn infant. Arch. Pathol. Lab. Med. 1940;29:494. [Google Scholar]
- Cardoso WV. Lung morphogenesis revisited: old facts, current ideas. Dev. Dyn. 2000;219:121–130. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1053>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Mitsialis SA, Brody JS, Williams MC. Retinoic acid alters the expression of pattern-related genes in the developing rat lung. Dev. Dyn. 1996;207:47–59. doi: 10.1002/(SICI)1097-0177(199609)207:1<47::AID-AJA6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Chuang P-T, McMahon AP. Branching morphogenesis of the lung: new molecular insights into an old problem. Trends Cell Biol. 2003;13:86–91. doi: 10.1016/s0962-8924(02)00031-4. [DOI] [PubMed] [Google Scholar]
- Corbier P, Allioli N, Roffi J. Variations de la reponse testiculaire a hCG au cours de la periode perinatale chez le Rat: infulence des oestrogenes. Arch. Int. Physiol., Biochim. Biophys. 1992;100:389–397. doi: 10.3109/13813459209000731. [DOI] [PubMed] [Google Scholar]
- Donjacour AA, Thomson AA, Cunha GR. FGF-10 plays an essential role in the growth of the fetal prostate. Dev. Biol. 2003;261:39–54. doi: 10.1016/s0012-1606(03)00250-1. [DOI] [PubMed] [Google Scholar]
- Driscoll SG, Taylor SH. Effects of prenatal maternal estrogen on the male urogenital system. Obstet. Gynecol. 1980;56:537–542. [PubMed] [Google Scholar]
- Ellis T, Smyth I, Riley E, Bowles J, Adolphe C, Rothnagel JA, Wicking C, Wainwright BJ. Overexpression of Sonic hedgehog suppresses embryonic hair follicle morphogenesis. Dev. Biol. 2003;263:203–215. doi: 10.1016/s0012-1606(03)00394-4. [DOI] [PubMed] [Google Scholar]
- Freestone SH, Marker P, Grace OC, Tomlinson DC, Cunha GR, Harnden P, Thomson AA. Sonic hedgehog regulates prostatic growth and epithelial differentiation. Dev. Biol. 2003;264:352–362. doi: 10.1016/j.ydbio.2003.08.018. [DOI] [PubMed] [Google Scholar]
- George FW, Peterson KG. 5α-dihydrotestosterone formation is necessary for embryogenesis of the rat prostate. Endocrinology. 1988;122:1159–1164. doi: 10.1210/endo-122-3-1159. [DOI] [PubMed] [Google Scholar]
- Goyette P, Allan D, Peschard P, Chen CF, Wang W, Lohnes D. Regulation of Gli activity by all-trans retinoic acid in mouse keratinocytes. Cancer Res. 2000;60:5386–5389. [PubMed] [Google Scholar]
- Gritli-Linde A, Lewis P, McMahon AP, Linde A. The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev. Biol. 2001;236:364–386. doi: 10.1006/dbio.2001.0336. [DOI] [PubMed] [Google Scholar]
- Haavisto T, Nurmela K, Pohjanvirta R, Huuskonen H, El-Gehani F, Paranko J. Prenatal testosterone and luteinizing hormone levels in male rats exposed during pregnancy to 2,3,7,8-tetrachlorodibenzo-p-dioxin and diethylstilbestrol. Mol. Cell. Endocrinol. 2001;178:169–179. doi: 10.1016/s0303-7207(01)00425-7. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G. Unique functions of Sonic hedgehog signaling during external genitalia development. Development. 2001;128:4241–4250. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Sugimura Y, Kawamura J, Donjacour AA, Cunha GR. Morphological and functional heterogeneity in the rat prostatic gland. Biol. Reprod. 1991;45:308–321. doi: 10.1095/biolreprod45.2.308. [DOI] [PubMed] [Google Scholar]
- Helms JA, Thaller C, Eichele G. Relationship between retinoic acid and Sonic hedgehog, two polarizing signals in the chick wing bud. Development. 1994;120:3267–3274. doi: 10.1242/dev.120.11.3267. [DOI] [PubMed] [Google Scholar]
- Helms JA, Kim CH, Hu D, Minkoff R, Thaller C, Eichele G. Sonic hedgehog participates in craniofacial morphogenesis and is down-regulated by teratogenic doses of retinoic acid. Dev. Biol. 1997;187:25–35. doi: 10.1006/dbio.1997.8589. [DOI] [PubMed] [Google Scholar]
- Hoffman MP, Kidder BL, Steinberg ZL, Lakhani S, Ho SM, Klein-man HK, Larsen M. Gene expression profiles of mouse sub-mandibular gland development: FGFR1 regulates branching morphogenesis in vitro through BMP- and FGF-dependent mechanisms. Development. 2002;129:5767–5778. doi: 10.1242/dev.00172. [DOI] [PubMed] [Google Scholar]
- Huang L, Pu Y, Alam S, Prins GS. Neonatal estrogen exposure disrupts prostate morphogenesis through alterations in BMP-4, BMP-7 and FGF-10 expression; The Endocrine Society’s 85th Annual Meeting; Philadelphia, PA. Chevy Chase, MD: The Endocrine Society; 2003. p. 334. (Abstract # P332-113) [Google Scholar]
- Huang L, Pu Y, Alam S, Birch L, Prins GS. Estrogenic regulation of signaling pathways and homeobox genes during rat prostate development. J. Androl. 2004;25:330–337. doi: 10.1002/j.1939-4640.2004.tb02796.x. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Review: hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Jarred R, Cancilla B, Prins GS, Thayer K, Cunha GR, Risbridger G. Evidence that estrogens directly alter androgen-regulated prostate development. Endocrinology. 2000;141:3471–3477. doi: 10.1210/endo.141.9.7648. [DOI] [PubMed] [Google Scholar]
- Lamm ML, Podlasek CA, Barnett DH, Lee J, Clemens JQ, Hebner CM, Bushman W. Mesenchymal factor bone morphogenetic protein 4 restricts ductal budding and branching morphogenesis in the developing prostate. Dev. Biol. 2001;232:301–314. doi: 10.1006/dbio.2001.0187. [DOI] [PubMed] [Google Scholar]
- Lamm ML, Catbagan WS, Laciak RJ, Barnett DH, Hebner CM, Gaffield W, Walterhouse D, Iannaccone P, Bushman W. Sonic hedgehog activates mesenchymal Gli1 expression during prostate ductal bud formation. Dev. Biol. 2002;249:349–366. doi: 10.1006/dbio.2002.0774. [DOI] [PubMed] [Google Scholar]
- Lowsley OS. The development of the human prostate gland with reference to the development of other structures at the neck of the urinary bladder. Am. J. Anat. 1912;13:299–348. [Google Scholar]
- Marigo V, Babin CJ. Regulation of patched by Sonic hedgehog in the developing neural tube. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9347–9351. doi: 10.1073/pnas.93.18.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer NP, Roelink H. The amino-terminal region of Gli3 antagonizes the Shh response and acts in dorsoventral fate specification in the developing spinal cord. Dev. Biol. 2003;257:343–355. doi: 10.1016/s0012-1606(03)00065-4. [DOI] [PubMed] [Google Scholar]
- Naslund MJ, Coffey DS. The differential effects of neonatal androgen, estrogen and progesterone on adult rat prostate growth. J. Urol. 1986;136:1136–1140. doi: 10.1016/s0022-5347(17)45239-6. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Schugbaur B, Chanbon P, Dolle P. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development. 2002;129:3563–3574. doi: 10.1242/dev.129.15.3563. [DOI] [PubMed] [Google Scholar]
- Pepicelli C, Lewis P, McMahon A. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr. Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev. Biol. 2002;247:26–46. doi: 10.1006/dbio.2002.0668. [DOI] [PubMed] [Google Scholar]
- Podlasek CA, Barnett DH, Clemens JQ, Bak PM, Bushman W. Prostate development requires Sonic hedgehog expressed by the urogenital sinus epithelium. Dev. Biol. 1999;209:28–39. doi: 10.1006/dbio.1999.9229. [DOI] [PubMed] [Google Scholar]
- Price D. Comparative aspects of development and structure in the prostate. In: Vollmer EP, editor. Biology of the Prostate and Related Tissues. vol. 12. Washington, DC: National Cancer Institute; 1963. pp. 1–27. [PubMed] [Google Scholar]
- Prins GS. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology. 1992;130:3703–3714. doi: 10.1210/endo.130.6.1597166. [DOI] [PubMed] [Google Scholar]
- Prins GS. Developmental estrogenization of the prostate gland. (Chapter 10) In: Naz RK, editor. Prostate: Basic and Clinical Aspects. Boca Raton: C.R.C. Press; 1997. pp. 247–265. [Google Scholar]
- Prins GS, Birch L. The developmental pattern of androgen receptor expression in rat prostate lobes is altered after neonatal exposure to estrogen. Endocrinology. 1995;136:1303–1314. doi: 10.1210/endo.136.3.7867585. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology. 1997;138:1801–1809. doi: 10.1210/endo.138.5.5106. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Greene GL. Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology. 1991;129:3187–3199. doi: 10.1210/endo-129-6-3187. [DOI] [PubMed] [Google Scholar]
- Prins GS, Cooke PS, Birch L, Donjacour AA, Yalcinkaya TM, Siiteri PK, Cunha GR. Androgen receptor expression and 5α-reductase activity along the proximal-distal axis of the rat prostatic duct. Endocrinology. 1992;130:3066–3073. doi: 10.1210/endo.130.5.1572313. [DOI] [PubMed] [Google Scholar]
- Prins GS, Woodham C, Lepinske M, Birch L. Effects of neonatal estrogen exposure on prostatic secretory genes and their correlation with androgen receptor expression in the separate prostate lobes of the adult rat. Endocrinology. 1993;132:2387–2398. doi: 10.1210/endo.132.6.8504743. [DOI] [PubMed] [Google Scholar]
- Prins GS, Marmer M, Woodham C, Chang WY, Kuiper G, Gustafsson JA, Birch L. Estrogen receptor-β messenger ribonucleic acid ontogeny in the prostate of normal and neonatally estrogenized rats. Endocrinology. 1998;139:874–883. doi: 10.1210/endo.139.3.5827. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland in mediated through stromal estrogen receptor α: studies with αERKO and βERKO mice. Cancer Res. 2001a;61:6089–6097. [PubMed] [Google Scholar]
- Prins GS, Birch L, Habermann H, Chang WY, Tebeau C, Putz O, Bieberich C. Influence of neonatal estrogens on rat prostate development. Reprod. Fertil. Dev. 2001b;13:241–252. doi: 10.1071/rd00107. [DOI] [PubMed] [Google Scholar]
- Prins GS, Chang WY, Wang Y, van Breemen RB. Retinoic acid receptors and retinoids are up-regulated in the developing and adult rat prostate by neonatal estrogen exposure. Endocrinology. 2002;143:3628–3640. doi: 10.1210/en.2002-220184. [DOI] [PubMed] [Google Scholar]
- Pu Y, Deng L, Davies PJP, Prins GS. Retinoic acid metabolizing enzymes, binding proteins and RXRs are differentially expressed in the developing and adult rat prostate lobes and are altered by neonatal estrogens in a lobe-specific manner; The Endocrine Society’s 85th Annual Meeting; Philadelphia, PA. Chevy Chase, MD: The Endocrine Society; 2003. p. 530. (Abstract # P533-236) [Google Scholar]
- Putz O, Prins GS. Prostate gland development and estrogenic imprinting. In: Burnstein KL, editor. Steroid Hormones and Cell Cycle Regulation. Boston, MA: Kluwer Academic Publishers; 2002. pp. 73–89. [Google Scholar]
- Pylkkanen L, Makela S, Valve E, Harkonen P, Toikkanen S, Santti R. Prostatic dysplasia associated with increased expression of C-myc in neonatally estrogenized mice. J. Urol. 1993;149:1593–1601. doi: 10.1016/s0022-5347(17)36458-3. [DOI] [PubMed] [Google Scholar]
- Rajfer J, Coffey DS. Effects of neonatal steroids on male sex tissues. Invest. Urol. 1979;17:3–8. [PubMed] [Google Scholar]
- Roberts DJ, Johnson RL, Burke AC, Nelson CE, Morgan BA, Tabin C. Sonic hedgehog is an endodermal signal inducing Bmp4 and Hox gene during induction and regulation of the chick hind-gut. Development. 1995;121:3163–3174. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Smith DM, Goff DJ, Tabin C. Epithelial-mesenchymal signaling during the regionalization of the chick gut. Development. 1998;125:2791–2801. doi: 10.1242/dev.125.15.2791. [DOI] [PubMed] [Google Scholar]
- Sabharwal V, Putz O, Prins GS. Neonatal estrogen exposure induces progesterone receptor expression in the developing prostate gland; 95th Annual Meeting of the American Urologic Association; Atlanta. Chevy Chase, MD: The Endocrine Society; 2000. p. 97. [Google Scholar]
- Santti R, Newbold RR, Makela S, Pylkkanen L, McLachlan JA. Developmental estrogenization and prostatic neoplasia. Prostate. 1994;24:67–78. doi: 10.1002/pros.2990240204. [DOI] [PubMed] [Google Scholar]
- Schneider A, Brand T, Zweigerdt R, Arnold H. Targeted disruption of the Nkx3.1 gene in mice results in morphogenetic defects of minor salivary glands: parallels to glandular duct morphogenesis in prostate. Mech. Dev. 2000;95:163–174. doi: 10.1016/s0925-4773(00)00355-5. [DOI] [PubMed] [Google Scholar]
- Seo R, McGuire M, Chung M, Bushman W. Inhibition of prostate ductal morphogenesis by retinoic acid. J. Urol. 1997;158:931–935. doi: 10.1097/00005392-199709000-00074. [DOI] [PubMed] [Google Scholar]
- Shapiro E. Embryologic development of the prostate. Urol. Clin. North Am. 1990;17:487–493. [PubMed] [Google Scholar]
- Siiteri PK, Wilson JD. Testosterone formation and metabolism during male sexual differentiation in the human embryo. JCEM. 1974;38:113–125. doi: 10.1210/jcem-38-1-113. [DOI] [PubMed] [Google Scholar]
- Sreenath T, Orosz A, Fujita K, Bieberich CJ. Androgen-independent expression of hoxb-13 in the mouse prostate. Prostate. 1999;41:203–207. doi: 10.1002/(sici)1097-0045(19991101)41:3<203::aid-pros8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Oohara I, Kurokawa T. Retinoic acid given at late embryonic stage depresses Sonic hedgehog and Hoxd-4 expression in the pharyngeal area and induces skeletal malformation in flounder (Parralichthys olivaceus) embryos. Dev. Growth Differ. 1999;41:143–152. doi: 10.1046/j.1440-169x.1999.00420.x. [DOI] [PubMed] [Google Scholar]
- Tamura K, Yokouchi Y, Kuroiwa A, Ide H. Retinoic acid changes the proximodistal development competence and affinity of distal cells in the developing chick limb bud. Dev. Biol. 1997;188:224–234. doi: 10.1006/dbio.1997.8627. [DOI] [PubMed] [Google Scholar]
- Thomson AA, Cunha GR. Prostatic growth and development are regulated by FGF10. Development. 1999;126:3693–3701. doi: 10.1242/dev.126.16.3693. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, Dhar MD, Ganjam VK, Parmigiani S, Welshons WV. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc. Natl. Acad. Sci. U. S. A. 1997;94:2056–2061. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shou J, Ross S, Koeppen H, de Sauvage FJ, Gao W-Q. Inhibition of epithelial ductal branching in the prostate by Sonic hedgehog is indirectly mediated by stromal cells. J. Biol. Chem. 2003;278:18506–18513. doi: 10.1074/jbc.M300968200. [DOI] [PubMed] [Google Scholar]
- Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BLM. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development. 1999;126:4005–4015. doi: 10.1242/dev.126.18.4005. [DOI] [PubMed] [Google Scholar]
- Weaver M, Dunn NR, Hogan BL. BMP4 and FGF10 play opposing roles during lung bud morphogenesis. Development. 2000;127:2695–2704. doi: 10.1242/dev.127.12.2695. [DOI] [PubMed] [Google Scholar]
- Woodham C, Birch L, Prins GS. Neonatal estrogens down regulate prostatic androgen receptor levels through a proteosome-mediated protein degradation pathway. Endocrinology. 2003;144:4841–4850. doi: 10.1210/en.2003-0035. [DOI] [PubMed] [Google Scholar]
- Yang Y, Drossopoulou G, Chuang PT, Duprez D, Marti E, Buncrot N, Vargession N, Clarke J, Niswander L, McMahon A, Tickle C. Relationship between dose, distance and time in Sonic hedgehog-mediated regulation of anteroposterior polarity in the chick limb. Development. 1997;124:4393–4404. doi: 10.1242/dev.124.21.4393. [DOI] [PubMed] [Google Scholar]
- Zondek T, Zondek LH. The fetal and neonatal prostate. In: Goland M, editor. Normal and Abnormal Growth of the Prostate. Springfield, IL: Thomas C. Thomas; 1975. pp. 5–28. [Google Scholar]