Abstract
Histone deacetylase inhibitors (HDACI) are promising anti-tumor agents. Although transcriptional deregulation is thought to be the main mechanism underlying their therapeutic effects, the exact mechanism and targets by which HDAC inhibitors achieve their anti-tumor effects remains poorly understood. It is not known whether any of the HDAC members support robust tumor growth. In this report we show that HDAC6, a cytoplasmic localized and cytoskeleton-associated deacetylase, is required for efficient oncogenic transformation and tumor formation. We found that HDAC6 expression is induced upon oncogenic Ras transformation. Fibroblasts deficient in HDAC6 are more resistant to both oncogenic Ras and ErbB2-dependent transformation, indicating a critical role for HDAC6 in oncogene-induced transformation. Supporting this hypothesis, inactivation of HDAC6 in several cancer cell lines reduces anchorage-independent growth and the ability to form tumors in mice. The loss of anchorage-independent growth is associated with increased anoikis and defects in AKT and ERK kinase activation upon loss of adhesion. Lastly, HDAC6 null mice are more resistant to chemical carcinogen-induced skin tumors. Our results provide the first experimental evidence that a specific HDAC member is required for efficient oncogenic transformation and indicate that HDAC6 is an important component underlying the anti-tumor effects of HDAC inhibitors.
Keywords: HDAC6, deacetylation, anoikis, transformation, HDAC inhibitor
Introduction
HDACs are a family of enzymes that were initially characterized as histone deacetylases (thus named HDAC). Reversible histone acetylation on lysine residues is a dynamic and highly regulated post-translational modification which plays a central role in gene regulation and chromatin remodeling (1). Accordingly, HDACs have been extensively and almost exclusively studied for their roles in transcriptional regulation and chromatin remodeling. HDACs have drawn intense research interest as inhibitors for these enzymes display potent anti-tumor activities and induce cancer growth arrest or cell death (2). Many HDAC inhibitors (HDACI) are at various stages of clinical trials for cancer patients (3–5) and suberoylanilide hydroxamic acid (SAHA) has been approved for the clinical use in advanced, refractory cutaneous T-cell lymphoma (6). Despite the potent activity of these compounds, the fundamental question of how HDAC inhibitors achieve their anti-tumor effect remains poorly understood. This is due in part to the existence of at least eleven HDAC family members in the human genome (7). To date, it is not known which of the HDAC family members are the critical targets that underlie the anti-tumor activity of HDAC inhibitors. This knowledge not only would pave ways to the development of more selective and less toxic inhibitors by targeting specific HDAC member(s), but will also elucidate the biological pathway(s) critical for HDAC inhibitors to treat tumors, thereby facilitating rational design for future cancer therapy.
The well-established role for HDACs in histone acetylation and gene transcription has led to a general assumption that HDAC inhibitors achieve their therapeutic effects by affecting specific transcriptional programs important for proliferation and apoptosis (8). Indeed, HDAC2, which acts as a potent transcriptional corepressor, has been shown to play an important tumor-promoting role in a murine colon cancer model caused by the APC mutation (9). However, the model that HDAC inhibitors work solely by affecting gene transcription is likely an over-simplified one. Recent studies have clearly demonstrated that some HDAC members are localized to the cytoplasm and regulate acetylation of non-nuclear proteins (10–14). Whether the non-genomic functions regulated by HDACs are important in oncogenesis, however, remains unknown.
Among the expanding non-genomic functions regulated by HDACs, those controlled by HDAC6 are best characterized (15, 16). Unlike the extensively studied nuclear HDACs involved in gene regulation, HDAC6 is localized exclusively to the cytoplasm where it associates with microtubule and actin cytoskeleton (14, 17). Studies have shown that HDAC6 affects cytoskeleton-dependent processes, including actin remodeling, fluid phase endocytosis (macropinocytosis), dynamics of cell adhesion and motility (13, 14, 17, 18). HDAC6 has also been shown to regulate microtubule-dependent transport and processing of toxic misfolded proteins, thereby protecting cells from the toxicity of protein aggregates, a common cause of neurodegenerative diseases (10, 19). Consistent with its function in the cytosol, the activities of HDAC6 are independent of histones but instead involve cytoplasmic substrates, such as tubulin and Hsp90 (17, 20, 21). Acetylation modulated by HDAC6 has been shown to regulate the formation of chaperone complexes and maturation of Hsp90 client proteins, including glucocorticoid receptor (21) and several oncogenic kinases (12, 22). Taken together, HDAC6 appears to be involved in the regulation of several critical cellular functions intimately linked to cancer. A specific role for HDAC6 in oncogenic transformation, however, is not yet established. In this report, we present evidence that HDAC6 is required for efficient oncogenic transformation, anchorage-independent proliferation and tumor growth. Inactivation of HDAC6 by genetic ablation or specific shRNA renders cells more resistant to oncogenic transformation and markedly reduces tumor cell growth in vitro and in vivo. HDAC6 deficiency also sensitizes cancer cells to anoikis, cell death induced by loss of adhesion. Supporting these observations, we found that HDAC6 null mice are more resistant to chemical carcinogen-induced skin tumor formation. Importantly, we found that deacetylase activity is required for HDAC6 to support malignant tumorigenic growth, suggesting that pharmacological inhibition of HDAC6 enzymatic activity could potentially confer an anti-tumor effect. Our study provides the first experimental evidence that a specific HDAC member is required for autonomous cancer cell growth of solid tumors both in vitro and in vivo and indicates that HDAC6 could be a therapeutic target for cancer treatment.
Materials and Methods
Retroviral vectors
HDAC6 siRNA sequence (17) (and as an alternate: AGACCTAATCGTGGGACTGC), scramble controls (23) were cloned into pSUPER-RETRO-PURO or pSUPER-RETRO-GFP/NEO (Oligoengine). cDNAs encoding human ErbB2/HER2/neu, H-RasG12V were cloned in to pBabepuro retroviral vectors. siRNA resistant wild type HDAC6, siRNA resistant catalytically inactive HDAC6 mutants (10) were cloned into pBabeBleo retroviral vectors.
Cell lines
Mouse embryonic fibroblasts (MEF) were isolated and cultured as previously described (14). MEFs were immortalized by stably expressing the early region of SV40 encoding T-Ag and t-Ag (24). Human ovarian cancer cell lines SKVO3, human breast cancer cell lines SKBR3, MCF7 and human epidermoid carcinoma cell line A431 were obtained from Duke Cell Culture Facility and maintained in RMPI1640 or DMEM (for A431) supplemented with 10%FBS. HMECs (25, 26) and PrECs (27) were maintained in defined media as recommended. The indicated cell lines were infected with retroviruses generated from the described retroviral vectors encoding either no protein, the indicated proteins, or shRNA, followed by appropriate exposure to puromycin, zeocin, or neomycin to select for stably infected polyclonal populations.
Immunoblotting
Cells were lysed in buffer (20 mM Tris-HCl at pH 7.6, 170 mM NaCl, 1 mM EDTA, 0.5% Nonidet P40, 1% Triton X-100) supplemented with phosphatase inhibitors cocktails (Sigma) and protease inhibitors cocktails (Sigma). Frozen skin tumors from the indicated mice were Dounce-homogenized in a hypotonic tissue lysis buffer (10mM HEPES, 1.5mM MgCl2, 10mM KCl, pH 7.4) containing 5μM of TSA and a cocktail of proteases and phosphatases inhibitors. SDS was then added to a final concentration of 0.1% to further extract membrane-associated proteins. Proteins lysates were analysed in a Western blotting experiment with the following antibodies diluted according to the manufacture’s recommendation: anti-α-tubulin (Sigma), anti-acetylated-α-tubulin (Sigma, 6–11-B-1), anti-β-actin (Sigma, AC-15), anti-GAPDH (Cell Signaling Technology, 14C10), anti-HDAC6 antibody(Santa Cruz, H300), anti-mouse HDAC6 antibody (14), anti-Akt (Cell Signaling Technology, #9272 ), anti-phospho(Ser 473)-Akt (Cell Signaling Technology, #9271), anti-ERK1/2 (Santa Cruz, K-23), anti-phospho(Thr 202/Tyr 204)-p42/44 ERK (Santa Cruz, E10), anti-c-ErbB2/c-Neu (Oncogene, Ab3), anti-Pan-Ras (Oncogene, Ab4), anti-Large T antigen (Santa Cruz, pAb 101), anti-acetylated Hsp90 (a kind gift from Dr. K Bhalla), anti-Hsp90 (H1090) (28). Proteins were detected with ECL reagent (Amersham Pharmacia Biotech) in accordance with the manufacturer’s protocol. To examine activation of MAPK pathway and PI3K pathway under anchorage independent conditions, confluent cells were serum starved overnight, trypsinized, washed and transferred to polyHEMA-coated Petri dishes for one hour, then stimulated with 50 μg/ml EGF for indicated time.
Levels of endogenous activated Ras-GTP were assayed as described previously (29). Briefly, tumor cell lysates were incubated with recombinant GST-RafBD. Total level of Ras was detected by SDS-PAGE and immunoblotting with the aforementioned anti-Ras antibody.
Soft-Agar Assay
Five thousand cells per 3 cm plate were suspended in soft agar as described (30) and colonies greater than 30 cells were scored after 3–4 weeks. Assays were done in triplicate and three times independently.
Proliferation assay
Five thousand cells per 24-well plate were seeded and maintained in RPMI-1640 with 10% FBS in quadruplet. Viable trypan blue-negatives cells were counted daily with hematocytometer for 5 days.
Anoikis Assay
Anoikis were induced by culturing the cells in Petri dishes coated with Poly(hydroxyethyl methacrylic) acid, or polyHEMA as described previously (31). In brief, subconfluent cells were trypsinized, washed and transferred to polyHEMA-coated Petri dishes at 25000 cells/ml in serum free media for indicated time. Apoptotic cells were then examined using Annexin V Apoptosis Detection Kit (BD Pharmingen) and cells negative for both 7-AAD and Annexin V were considered to be resistant to anoikis.
Tumor Growth
All procedures with mice were done under an Institutional Animal Care and Use Committee-approved protocol. For xenograft tumorigenesis assay, ten million cells of the indicated cell lines were mixed with Matrigel and injected subcutaneously into both flanks of two immunocompromised SCID/Beige mice (Charles River Laboratory). Tumor volumes were determined approximately twice per week and calculated as 1/2 length2 × width in unit of mm3. For reculture of xenograft tumors, tumors were harvested, minced, and trypsinized for 2 hr at 37°C and then passed through 18G needles, washed, and plated in RPMI/10% FBS plus puromycin at least 4 days to eliminate all murine cells before immunoblot analysis was performed. For chemical carcinogenesis, the backs of 5 male control (wild type) SvEv/Black Swiss mice and 10 male experimental (HDAC6 null) (14) SvEv/Black Swiss mice were shaved, and the following day, 150μL of 125 μg/mL DMBA (Sigma) in DMSO were applied topically, followed 1 week later by twice-weekly topical applications of 150 μL of 10−4 M TPA (Sigma) in DMSO for 20 weeks. Tumor number and size were recorded weekly. Student’s t-test was used to compare tumor growth in the various models. The differences between means were considered significant if P < 0.05.
Immunohistochemistry
Excised tumors were fixed in formalin, embedded in paraffin and sectioned. Ki67 (α-Ki67 antibody; Vector Laboratories) was performed on the indicated tumor sections by Duke Pathology Core Facility using standard assays.
RT–PCR
Total RNA was isolated using RNeasy Mini Kit (Qiagen), DNase-treated using DNA-free kit (Ambion), and1 μg RNA was used for cDNA synthesis reaction using Iscript Reverse transcriptase kit (BioRad). 10% of cDNA was used per RT-PCR reaction. RT-PCRProgram was 94° C 20 s, 48° C 20 s, 72°C 20 s for 40 cycles. RT-PCR primer sequences are as follows: HDAC6: forward, 5′-TCA GGT CTA CTG TGG TCG TT, reverse, 5′-TCT TCA CAT CTA GGA GAG CC; G-actin: forward 5′-ACCCAGGCATTGCTGACAGGATGC, reverse, 5′CCATCTAGAAGCATTTGC GGTGGACG
Results
HDAC6 is required for malignant growth of transformed cells in vitro
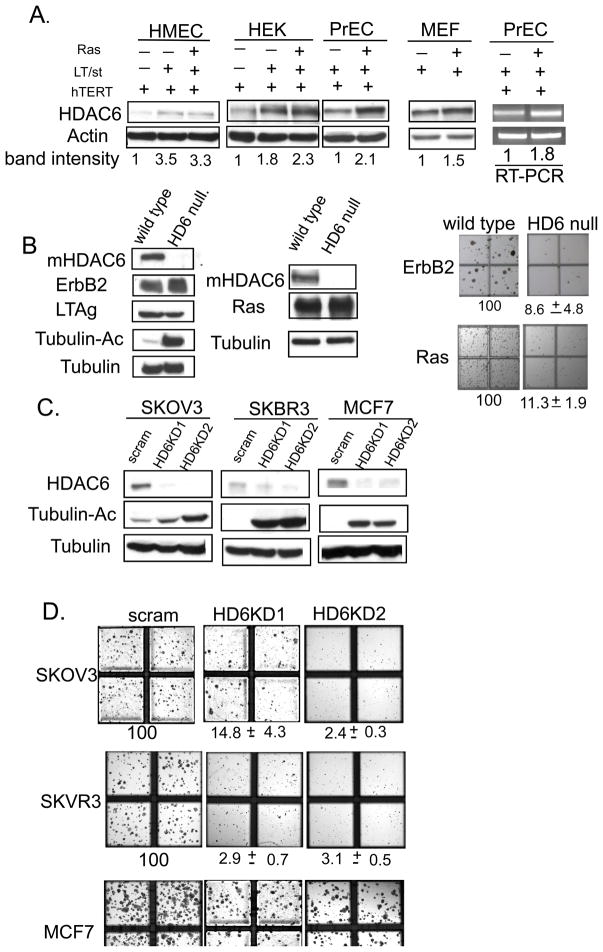
To explore a possible role for HDAC6 in malignant transformation, we examined whether HDAC6 expression is induced in transformed cells versus their non-transformed counterparts. Specifically, primary human cells from various origins were transformed into tumor cells by serial introduction of human telomerase (hTERT), SV40 early region, which contains large T antigen and small t antigen, and finally oncogenic Ras (32). As shown in Figure 1A, introduction of SV40 early region and oncogenic RasG12V induces HDAC6 proteins level by 2–4 fold in human embryonic kidney cells (HEK), prostate epithelial cells (PrECs) and mammary epithelial cells (HMEC) (data not shown). Similarly, introduction of oncogenic RasG12V mutant also modestly induces HDAC6 in mouse embryo fibroblast (MEFs), when compared to control MEFs infected with empty vector. RT-PCR analysis showed that HDAC6 mRNA was up-regulated in PrEC and HEK (Figure 1A and data not shown), indicating the oncogenic Ras can induce HDAC6. This up-regulation of HDAC6 in response to oncogenic transformation suggests a potential regulatory role of HDAC6 in the malignant transformation process.
Figure 1. HDAC6 is required for malignant growth of transformed cells.
A. Detection of HDAC6 by immunoblot and RT-PCR in transformed HEK, PrEC and MEF cells compared to their non-transformed counterparts. Actin serves as a loading control. The band intensity of the HDAC6 levels was normalized to the actin levels in each lane.
B. Detection of mouse HDAC6, Ras and tubulin by immunoblot in the transformed wild type or HDAC6 null MEFs stably expressing early region of SV40 and oncogenic RasG12V. Tubulin serves as a loading control. Photographs demonstrating anchorage independent growth of indicated polyclonal aforementioned MEFs. The bottom labels showed the average and standard deviation of the percent of the colonies growing in an anchorage independent fashioned compared to wild type transformed MEF (normalized to 100%) as calculated from triplicate plates. Data are from two independent assays
C. Detection of mouse HDAC6, ErbB2/HER2/neu, Large T antigen, acetylated tubulin and tubulin by immunoblot in the transformed wild type or HDAC6 null MEFs stably expressing early region of SV40 and human ErbB2/HER2/neu. Tubulin serves as a loading control. Photographs demonstrating anchorage independent growth of indicated polyclonal aforementioned MEFs. The bottom labels showed the average and standard deviation of the percent of the colonies growing in an anchorage independent fashioned compared to wild type transformed MEF (normalized to 100%) as calculated from triplicate plates. Data are from two independent assays
To directly assess a role of HDAC6 in oncogenic transformation, we utilized mouse embryonic fibroblasts (MEFs) derived from wild type or HDAC6 null embryos. These MEFs provide a well-established and genetically defined model to examine the requirement of HDAC6 in malignant transformation. To this end, wild type and HDAC6 null MEFs were transduced with retrovirus expressing SV40 early region and RasG12V (Figure 1B) and subsequently assayed for anchorage-independent growth in soft agar, a standard and stringent assay for malignant transformation in vitro. As shown in Figure 1B, wild type MEFs gave rise to large number of colonies after retroviral transduction of Large T, small t antigens and RasG12V oncogenes. In contrast, transduction of the same oncogenes at similar protein expression levels into HDAC6 null MEFs results in more than 10 fold fewer colonies (Figure 1B), revealing that HDAC6 is required for Ras-induced oncogenic transformation. To investigate whether HDAC6 plays a broader role in oncogene-induced transformation, we also examined ErbB2-dependent transformation in wild type and HDAC6 deficient MEFs. As shown in Figure 1C, the transforming activity of ErbB2 is also impaired in HDAC6 null MEFs as shown by a reduction in soft agar colony formation. Together, these results show that HDAC6 is required for efficient oncogene-induced transformation.
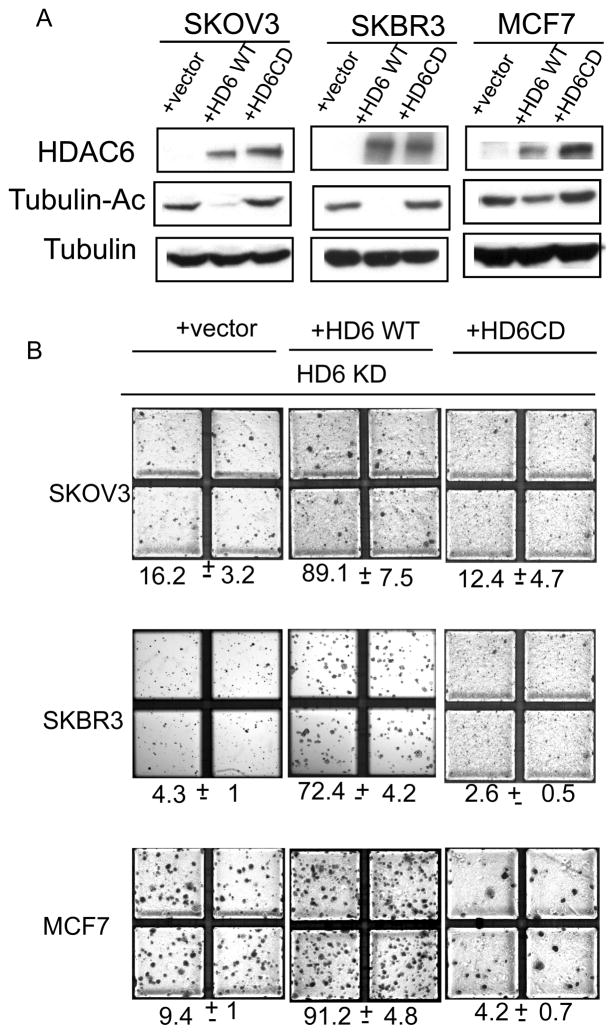
After establishing that HDAC6 is required for oncogenic transformation of primary murine fibroblasts, we asked if HDAC6 is critical to maintain the transformed phenotypes of established human cancer cell lines. To this end, we stably expressed two sets of small hairpin RNA targeting different regions of HDAC6 mRNA in three well characterized lines: ovarian cancer cell line SKOV3, and breast cancer cell lines SKBR3 and MCF-7. These specific HDAC6 shRNA but not a scramble shRNA efficiently reduced HDAC6 levels and caused an increase in the level of acetylated α-tubulin in all three cancer cell lines (Figure 2A). An increase in acetylated Hsp90 is also observed (Figure 2B), demonstrating that HDAC6 is efficiently knocked down. The control and HDAC6 knockdown tumor cell lines were then subject to soft-agar growth assay. As shown in Figure 2C, knockdown of HDAC6 by either of the two shRNA significantly inhibits the anchorage-independent growth of these cancer cells by 5 to 30-fold. Therefore, HDAC6 is not only important for oncogenic transformation of primary cell but also required for maintaining the anchorage-independent growth of established cancer cell lines.
Figure 2. Deacetylase activity of HDAC6 is required for malignant growth of malignant cells.
A. Detection of HDAC6, acetylated tubulin and tubulin by immunoblot in the indicated three different human cancer cells stably expressing a scramble control sequence, two different HDAC6 shRNA (HDAC6 KD1 and HDAC6 KD2), a HDAC6 shRNA plus a vector (+vector), HDAC6 shRNA in the presence of siRNA resistant wild type HDAC6 (+HD6 WT), HDAC6 shRNA in the presence of siRNA resistant catalytically inactive HDAC6 (+HD6CD). Tubulin serves as a loading control.
B. Detection of acetylated Hsp90 and Hsp90 by immunoblot in the SKOV3 human cancer cells stably expressing a scramble control sequence or HDAC6 shRNA (HDAC6 KD1). GAPDH serves as a loading control
C. Photographs demonstrating anchorage independent growth of indicated polyclonal human cancer cells stably expressing a scramble control sequence (scram), two different HDAC6 shRNA (HDAC6 KD1 and HDAC6 KD2), a HDAC6 shRNA plus a vector (+vector), HDAC6 shRNA in the presence of siRNA resistant wild type HDAC6 (+HD6 WT), HDAC6 shRNA in the presence of siRNA resistant catalytically inactive HDAC6 (+HD6CD). The bottom labels showed the average and standard deviation of the percent of the colonies growing in an anchorage independent fashioned compared to scramble control cells (normalized to 100%) as calculated from triplicate plates. Data are from two independent assays.
Deacetylase activity of HDAC6 is required for malignant growth of cancer cells
We next determined whether deacetylase activity of HDAC6 is required for malignant growth of cancer cells. To this end, we reconstituted the HDAC6 knockdown cancer cell lines with shRNA-resistant wild type or catalytically inactive mutant HDAC6 (Figure 2A) and determined their ability to grow in soft agar. As shown in Figure 2C, reintroduction of wild type HDAC6 effectively restored colony formation of HDAC6 knockdown cancer cells, demonstrating that the requirement of HDAC6 for anchorage-independent growth is specific. In contrast, HDAC6 knockdown cell lines reconstituted with a catalytically inactive mutant HDAC6 remain defective in forming colony in soft agar. These results show that deacetylase activity of HDAC6 is required to support malignant growth of cancer cells in vitro.
HDAC6 regulates tumor cell proliferation and survival
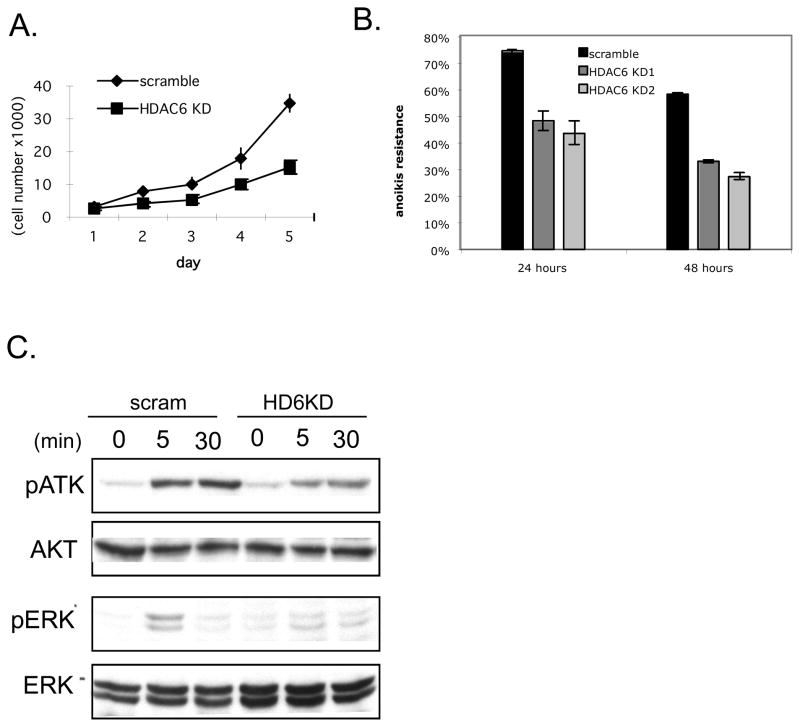
In order to form colonies in soft agar, cells need to both grow and escape anoikis, a specific form of cell death caused by the lack of proper adhesion to basement membrane or extra-cellular matrix. Resistance to anoikis is one the most important cellular mechanisms underlying anchorage-independent growth and a hallmark of malignant transformation (33). Given the possible effect of HDAC6 on cell adhesion (18), we examined whether HDAC6 status affects anoikis. To this end, we grew control and HDAC6 knockdown SKVO3 cells on culture plates coated with polyHEMA. PolyHEMA prevents cells from adhering to the plates and therefore allows one to test the ability of cells to survive in an anchorage-independent fashion (31). As shown in Figure 3A, while scramble control cells retained the resistance to anoikis induction and survived, HDAC6 knockdown SKVO3 cells were significantly more susceptible to anoikis. This result indicates that HDAC6 contributes to anoikis resistance in cancer cells.
Figure 3. The role of HDAC6 in growth and survival of cancer cells.
A. Resistance to anoikis of human ovarian cancer cells SKOV3 stably expressing a scramble control sequence (black), two different HDAC6 shRNA (gray). Each experiment was done in duplicate and results shown are representative of 2 independent experiments.
B. Detection of phospho-AKT, total AKT, phospho-ERK, and total ERK by immunoblot in the SKOV3 human ovarian cancer cells stably expressing a scramble control sequence (scram) or HDAC6 shRNA (HDAC6 KD)
It has been shown that activation of PI3K/AKT and MAPK/ERK signaling pathways are critical for anoikis resistance in cancer cells (34). To examine whether HDAC6 modulates these pathways during anchorage independent growth, we compared the phosphorylation status of AKT and ERK1/2 in scramble control cells or HDAC6 knockdown cells grown in the polyHEMA-coated plates. As shown in Figure 3B, HDAC6 knockdown cells showed a significant decrease in phosphorylation of AKT and ERK1/2 in response to growth factor stimulation, compared to scramble control cells, indicating that HDAC6 is required for growth factor induced activation of MAPK and PI3K signaling cascades that could contribute to anchorage-independent growth.
HDAC6 is required for robust tumorigenic growth of human cancer cells in vivo
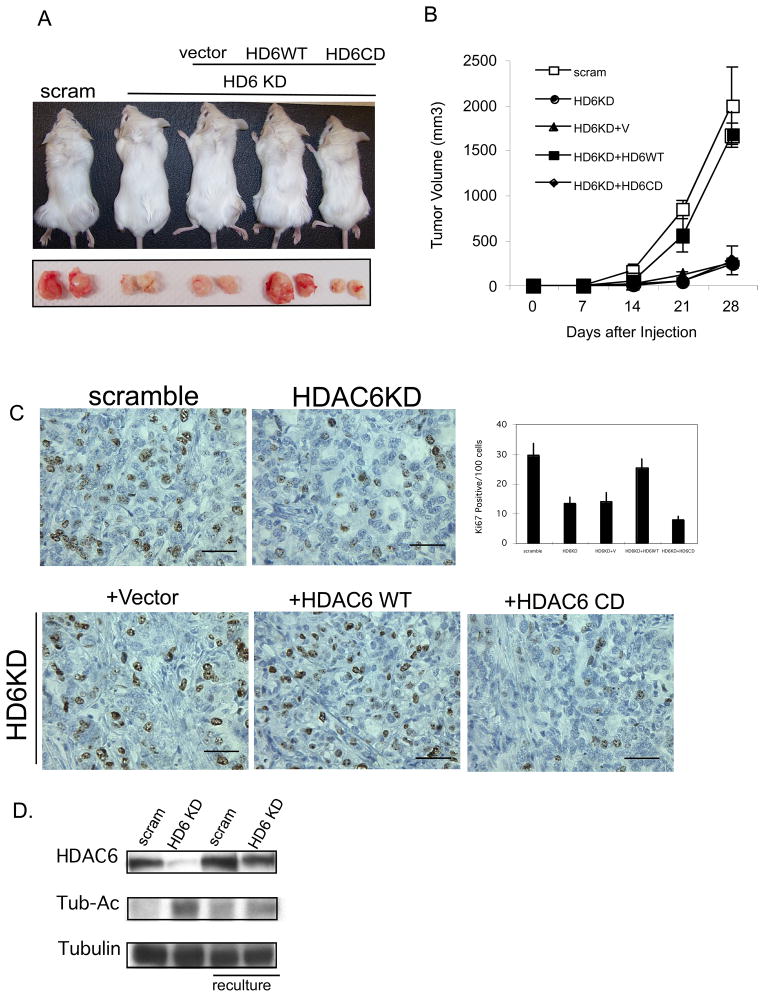
These analyses support an important role of HDAC6 in promoting oncogenic phenotypes in vitro. To determine whether HDAC6 is critical for tumorigenic growth of cancer cells in vivo, SKOV3 stably expressing either an HDAC6 specific shRNA or a scramble control were subcutaneously injected into immunocompromised SCID-Beige mice, and tumor growth was followed over time. As shown in Figure 4A, tumorigenic growth of HDAC6 knockdown cells was significantly retarded when compared to that of control cancer cells by a two fold increase in latency and significant reduction in tumor volume (~ 4 fold at 28 days). This effect is specific as HDAC6 knockdown cells reconstituted with wild type HDAC6 but not catalytically inactive mutant regained the robust tumorigenic growth comparable to the parental cell lines (Figure 4A). A similar requirement of HDAC6 for xenograft tumor growth was found in another cancer cell line A431 (Supplemental Figure 1). Importantly, tumors that eventually grew from HDAC6 knockdown cells restored HDAC6 expression (Figure 4B), suggesting a strong selective pressure against tumor cells deficient in HDAC6.
Figure 4. HDAC6 is critical for tumorigenic growth of human cancer cells in vivo.
A. (Top) Representative subcutaneous flank tumor in mice and resected tumors 28 days after SKOV3 cells expressing the described construct were injected in mice. (Bottom) Tumor volume (mm3) and standard deviation versus time (days) of SKOV3 cells stably expressing a scramble control sequence (scram), HDAC6 shRNA (HD6KD), HDAC6 shRNA in the presence vector (HD6KD+V), HDAC6 shRNA in the presence of si-RNA resistant wild type HDAC6 (HD6KD+HD6WT), HDAC6 shRNA in the presence of si-RNA resistant enzymatically inactive HDAC6 (HD6KD+HD6CD) injected into the flanks of immunocompromised mice.
B. Re-expression of HDAC6 in HDAC6 knockdown cells in xenograft. Detection of HDAC6, acetylated tubulin and tubulin by immunoblot in the SKOV3 human ovarian cancer cells stably expressing either a scramble control sequence or HDAC6 shRNA and the same cells recultured from xenograft tumors. Tubulin serves as a loading control.
C. Representative histological sections with Ki-67 staining of the aforementioned tumors from mice injected with the indicated cells. The scale bar indicates 50μm. The graph shows the average number and standard deviation of Ki-67 positive cells from 10 different fields of 2 different tumor sections.
D. Cellular growth in monolayer culture of SKOV3 cells stably expressing a scramble control sequence, or HDAC6 shRNA
Analyses of tumor samples revealed that tumors derived from HDAC6 knockdown cells showed approximately two-fold reduction in Ki-67staining, a marker for mitotic cells, indicating a reduction in tumor proliferation caused by a loss of HDAC6. Consistent with this conclusion, re-expression of wild type but not catalytically inactive HDAC6 mutant fully restored the number of Ki-67 positive cells in tumors sections (Figure 4C). To directly assess if HDAC6 affects tumor cell growth, control and HDAC6 knockdown SKOV3 cancer cells were analyzed in monolayer culture. As shown in Figure 4D, the cell number is reduced in HDAC6 knockdown cells compared to a scramble control cell lines. Together, these results show that HDAC6 is required for robust tumor cell growth.
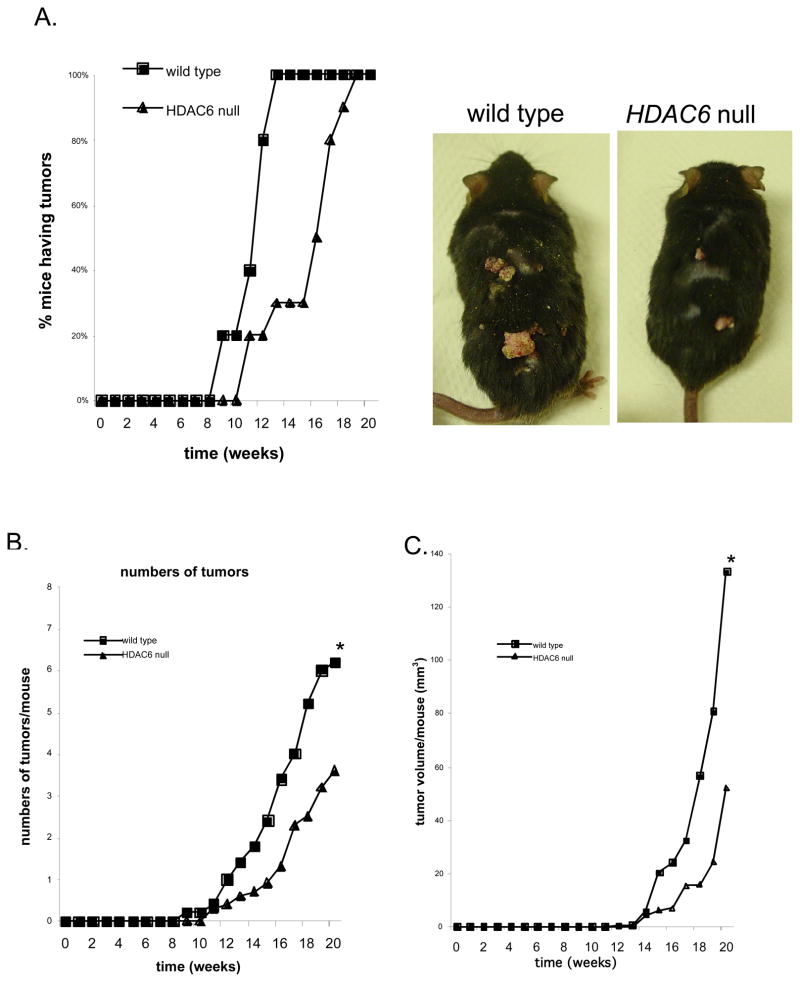
Finally, we determined whether HDAC6 is required for carcinogen-induced spontaneous tumorigenesis. Topical application of the carcinogen 7,12-Dimethylbenzanthracene (DMBA), followed by repetitive application of 12-O-tetradecanoylphorbol-13-acetate (TPA) induces papillomas with high rate of Ras mutation (35). We treated wild type and HDAC6 null mice with topical DMBA and then TPA for 20 weeks and monitored tumor growth. Consistent with previously published results (36, 37), skin tumors appeared within 9 weeks after initiation of treatment in wild type mice with an average of 6 tumors permouse by termination of the experiment (Figure 5A and 5B). Significantly, in HDAC6 null mice, the appearance of tumors was delayed by 2 weeks with an average number of 3 tumors per mouse at the termination of the experiment. Furthermore, the average volume of the tumors at week 20 was ~three-fold smaller inHDAC6 null mice when compared to the wild-type mice (P <0.05) (Figure 5C). We conclude that loss of HDAC6 impedesspontaneous formation of carcinogen-induced tumors.
Figure 5. HDAC6null mice are resistant to carcinogen-induced skin tumors.
A. Reduction of spontaneous tumors in HDAC6 null mice. Representative mice of the indicated genotype at 20 weeks (Arrow head: tumor). Percentage of wild type and HDAC6 null mice with tumors versus time after initial application of DMBA (weeks).
B. Reduction in the number of tumors in HDAC6 null mice. Mean number of tumors per wild type and HDAC6 null mouse versus time after initial application of DMBA (weeks). (*) P < 0.05
C. Reduction in tumor volume in HDAC6 null mice. Mean tumor volume per wild type and HDAC6 null mouse versus time after initial application of DMBA (weeks). (*) P < 0.05
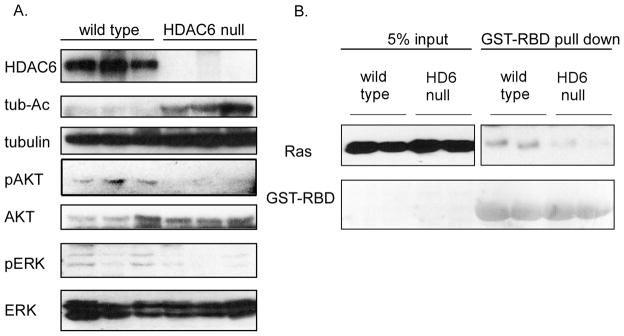
Consistent with in vitro results from established cancer cells (Figure 3B), a significant decrease in the phosphorylation of AKT and ERK1/2 was also observed in tumor derived from HDAC6 knockout mice (Figure 6A), supporting the notion that HDAC6 is required for the proper activation of MAPK and PI3K signaling cascades required for optimal tumor growth in vivo. In the light of our observation that loss of HDAC6 renders cells more resistant to Ras oncogene-driven transformation and blunting of activation of downstream effectors ERK and AKT, we further examined the activation status of Ras (29) in tumor specimens from DMBA-TPA induced skin tumors. As shown in Figure 6B, tumor lysates from HDAC6 knockout mice contain much lower levels of activated Ras than those derived from wild type littermates. Taken together, these results indicate that HDAC6 is required for efficient activation of the oncogenic Ras signaling pathway induced by DMBA-TPA.
Figure 6. The role of HDAC6 in activation of Ras signaling pathways in vivo.
A. Detection of MAPK and PI3K activation in DMBA-TPA induced skin tumors from HDAC6 null mice and wild type littermates by immunoblot analysis with phospho-specific antibodies for phosphorylated forms of ERK1/2 (p-ERK1/2) and AKT (p-AKT) respectively. Total tubulin serves as loading controls.
B. Detection of activated GTP-bound Ras via association with effector protein domains specific for activated version of Ras proteins followed by immunoblot analysis with antibodies for Ras in DMBA-TPA induced skin tumors from HDAC6 null mice and wild type littermates. GST-RafBD was visualized by Ponceau S staining.
Discussion
In this report, we present evidence that HDAC6, a cytoplasmic deacetylase, is required for efficient oncogenic tumorigenesis in both cultured cells and mouse tumor model. HDAC6 is induced during malignant transformation whereas loss of HDAC6 impedes anchorage-independent growth, resistance to anoikis, xenograft tumor growth and chemical carcinogen induced skin tumors formation. The requirement of HDAC6 in maintaining tumor phenotypes in established cancer lines reveals the potential of HDAC6 as a therapeutic target and suggests a new mechanism by which HDAC inhibitors achieve their anti-tumor activity.
Although the analysis of HDAC6 in human cancer remains scarce, HDAC6 expression has been shown to be up-regulated in primary oral squamous cell carcinoma tissue and cell lines. Elevated levels of HDAC6 were also documented in primary acute myeloid leukemia blasts, several myeloblastic cell lines and some human breast cancers (38–41). Interestingly, in breast cancer cell lines, HDAC6 was identified as an estrogen regulated gene. HDAC6 was proposed to play a role in estrogen-induced breast tumor cell motility and invasiveness (38, 40). In fact, HDAC6 is required for estrogen-induced proliferation in MCF7 cells (42). The potential importance of HDAC6 in ER-dependent tumor phenotype is supported by a clinical study of breast cancer patients, which revealed that HDAC6 levels positively correlate with a favorable response to anti-estrogen tamoxifen treatment (40). These findings are consistent with our conclusion that HDAC6 plays an important role in maintaining tumor growth and suggest a potential utility for targeting HDAC6 in breast cancer.
HDAC6 likely modulates tumor formation by several different mechanisms. We found that HDAC6 is induced in Ras oncogene-transformed cells in vitro (Figure 1). The significance of this finding is underlined by the observation that HDAC6-deficient cells and mice are more resistant to oncogenic Ras-induced oncogenesis. Indeed, in HDAC6 null mice, the Ras-dependent signaling is impaired (Figure 6). Taken together, HDAC6 likely contributes to tumorigenesis, at least in part, by facilitating the activation of Ras and downstream PI3K and MAPK pathways. The exact molecular targets mediating such a tumor promoting effect of HDAC6 remains to be established. To date, at least four HDAC6 substrates have been identified: α tubulin (17), Hsp90 (21), cortactin (13) and β-catenin (43). α–tubulin is the first HDAC6 substrate identified. While the exact function of microtubule acetylation remains uncertain, it has been correlated with a moderate enhancement in microtubule stability and altered focal adhesion turnover (18, 44). In this regard, the involvement of HDAC6 in anoikis caused by a loss of proper adhesion is of particular interest. Our results showed that HDAC6 activity is required for cancer cells to efficiently escape anoikis and grow in soft agar. Transformed cells often acquire resistance to anoikis so they can invade and metastasize to distant organs (45). Whether HDAC6 is required for efficient tumor metastasis is being investigated.
HDAC6 also regulate acetylation of Hsp90, cortactin and β-catenin (13, 14, 21, 43), which all play important roles in oncogenesis. The molecular chaperone Hsp90 is critical for the structural maturation and activity of many oncogenic proteins (46). As Hsp90 requires HDAC6 for its full activity (21), inactivation of HDAC6 in cancer cells could affect oncogenic signaling, thereby inhibiting tumor growth. Indeed, hyperacetylation of Hsp90 has been associated with a defect in oncogenic kinase stability and signaling (12). The amplification of cortactin gene, also termed EMS1, has been shown to correlate with lymph node metastasis and unfavorable clinical outcome (47). Interestingly, similar to the effect of HDAC6 shRNA, knockdown of cortactin in esophageal cancer cells also leads to susceptibility to anoikis and impairment of malignant growth in soft agar and in mice (48). In HDAC6 deficient cells, hyperacetylated cortactin showed reduced activity in stimulating actin polymerization, suggesting that HDAC6 might be required for cortactin to promote tumor formation as well (13).
Aside from oncogenic signaling, HDAC6 also plays a critical role in the disposition and clearance of toxic, misfolded protein aggregates via the aggresome-autophagy pathway (10, 49, 50). Although toxic misfolded proteins have been primarily studied in neurodegenerative disease, tumor cells are likely prone to produce excessive amounts of misfolded proteins due to their high rates of metabolism (51), protein synthesis and production of mutated proteins (52). This might be particularly true for tumors with secretory function, such as multiple myeloma, as secretory proteins are highly susceptible to misfolding. Taken together, we suggest that loss of HDAC6 inhibits cancer cell growth by causing accumulation of toxic misfolded proteins.
A tumor-promoting function for HDAC6 by clearing toxic proteins is strikingly similar to that of heat shock factor-1 (HSF-1), a transcription factor that induces heat shock proteins expression in response to the accumulation of misfolded proteins caused by heat shock or proteasome inhibition (53). Both HDAC6 and HSF-1 null MEFs showed resistance to DMBA-TPA two-stage carcinogen-induced skin tumor formation and oncogenic Ras-driven anchorage independent growth. Both HDAC6 and HSF-1 are required for maintenance of transformed phenotypes of established cancer cells and oncogenic MAPK signaling. The similar requirement of HDAC6 and HSF-1 in oncogenic transformation is consistent with the finding that HDAC6 is required for full activation of heat shock factor-1 (HSF-1) when proteasome functions are compromised (54), and strongly argues that the management of misfolded protein stress is critical for maintaining robust tumor phenotypes. Supporting this view, combined use of proteasome inhibitors and HDAC6-selective inhibitors has synergistic cytotoxic effects on cancer cell lines (10, 55).
Regardless of the mechanism, our analysis clearly demonstrates that HDAC6 is required for efficient oncogenic transformation and tumor formation. Importantly, as HDAC6 is not an essential gene and HDAC6 null mice are grossly normal (14, 56), our study further suggests that HDAC6 might be an ideal target for the design of chemotherapeutic agents for its low toxicity. Given that the deacetylase activity of HDAC6 is required to support robust tumorigenic growth (Figure 2–5), these data suggest that pharmacological inhibition of HDAC6 activity might be an effective strategy in cancer therapeutics.
For decades, protein phosphorylation/dephosphorylation controlled by oncogenic kinases has been the primary target for therapeutic manipulation in cancer treatment. The efficacy of HDAC inhibitors in model systems and clinical trials points to the potential importance of reversible protein acetylation and HDAC in oncogenic signaling. The anti-tumor activity of HDAC inhibitors has been mostly attributed to their effects on gene expression and chromatin dynamics. The identification of non-nuclear HDAC members and large number of non-nuclear acetylated proteins (57), however, has raised a critical question as to whether non-genomic mechanism might also play an important role underlying the therapeutic effect of HDACI. Our report now provides evidence that HDAC6, a cytosolic HDAC family member, is required for full and autonomous tumorigenic growth. This finding highlights the potential importance of non-genomic targets in the anti-tumor activity of HDAC inhibitors. These conclusions also strongly indicate that, similar to protein phosphorylation, reversible protein acetylation occurring outside the nucleus could play an important role in cancer biology.
Acknowledgments
We thank Dr. Phillip Febbo for kindly providing PrECs and Dr. Kapil Bhalla for kindly providing anti-acetylated Hsp90 antibody. We thank Ms. Chun-Hsiang Lai for technical assistance and Dr. Todd Cohen for comments onthe manuscript.
Grant support: Department of Defense grant W81XWH-04-01-0555 to T.-P. Y. and NIH grants CA94184 and CA126903 (to C.M.C.). Both T.P.Y and C.M.C. are Leukemia and Lymphoma Society Scholars.
References
- 1.Wade PA, Pruss D, Wolffe AP. Histone acetylation: chromatin in action. Trends Biochem Sci. 1997;22:128–32. doi: 10.1016/s0968-0004(97)01016-5. [DOI] [PubMed] [Google Scholar]
- 2.Drummond DC, Noble CO, Kirpotin DB, Guo Z, Scott GK, Benz CC. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu Rev Pharmacol Toxicol. 2005;45:495–528. doi: 10.1146/annurev.pharmtox.45.120403.095825. [DOI] [PubMed] [Google Scholar]
- 3.Byrd JC, Marcucci G, Parthun MR, et al. A phase 1 and pharmacodynamic study of depsipeptide (FK228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood. 2005;105:959–67. doi: 10.1182/blood-2004-05-1693. [DOI] [PubMed] [Google Scholar]
- 4.Gojo I, Jiemjit A, Trepel JB, et al. Phase 1 and pharmacologic study of MS-275, a histone deacetylase inhibitor, in adults with refractory and relapsed acute leukemias. Blood. 2007;109:2781–90. doi: 10.1182/blood-2006-05-021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atmaca A, Al-Batran SE, Maurer A, et al. Valproic acid (VPA) in patients with refractory advanced cancer: a dose escalating phase I clinical trial. Br J Cancer. 2007;97:177–82. doi: 10.1038/sj.bjc.6603851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallinari P, Di Marco S, Jones P, Pallaoro M, Steinkuhler C. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res. 2007;17:195–211. doi: 10.1038/sj.cr.7310149. [DOI] [PubMed] [Google Scholar]
- 7.Verdin E, Dequiedt F, Kasler HG. Class II histone deacetylases: versatile regulators. Trends Genet. 2003;19:286–93. doi: 10.1016/S0168-9525(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 8.Archer SY, Meng S, Shei A, Hodin RA. p21(WAF1) is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc Natl Acad Sci U S A. 1998;95:6791–6. doi: 10.1073/pnas.95.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu P, Martin E, Mengwasser J, Schlag P, Janssen KP, Gottlicher M. Induction of HDAC2 expression upon loss of APC in colorectal tumorigenesis. Cancer Cell. 2004;5:455–63. doi: 10.1016/s1535-6108(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 10.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115:727–38. doi: 10.1016/s0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 11.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Bali P, Pranpat M, Bradner J, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280:26729–34. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Yuan Z, Zhang Y, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Molecular cell. 2007;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao YS, Hubbert CC, Lu J, Lee YS, Lee JY, Yao TP. Histone deacetylase 6 regulates growth factor-induced actin remodeling and endocytosis. Mol Cell Biol. 2007;27:8637–47. doi: 10.1128/MCB.00393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyault C, Sadoul K, Pabion M, Khochbin S. HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination. Oncogene. 2007;26:5468–76. doi: 10.1038/sj.onc.1210614. [DOI] [PubMed] [Google Scholar]
- 16.Matthias P, Yoshida M, Khochbin S. HDAC6 a new cellular stress surveillance factor. Cell Cycle. 2008;7:7–10. doi: 10.4161/cc.7.1.5186. [DOI] [PubMed] [Google Scholar]
- 17.Hubbert C, Guardiola A, Shao R, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–8. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 18.Tran AD, Marmo TP, Salam AA, et al. HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions. J Cell Sci. 2007;120:1469–79. doi: 10.1242/jcs.03431. [DOI] [PubMed] [Google Scholar]
- 19.Kwon S, Zhang Y, Matthias P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes & development. 2007;21:3381–94. doi: 10.1101/gad.461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci U S A. 2003;100:4389–94. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacs JJ, Murphy PJ, Gaillard S, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Molecular cell. 2005;18:601–7. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Scroggins BT, Robzyk K, Wang D, et al. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Molecular cell. 2007;25:151–9. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim KH, O’Hayer K, Adam SJ, et al. Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr Biol. 2006;16:2385–94. doi: 10.1016/j.cub.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Tevethia MJ. Immortalization of primary mouse embryo fibroblasts with SV40 virions, viral DNA, and a subgenomic DNA fragment in a quantitative assay. Virology. 1984;137:414–21. doi: 10.1016/0042-6822(84)90234-4. [DOI] [PubMed] [Google Scholar]
- 25.Kendall SD, Linardic CM, Adam SJ, Counter CM. A network of genetic events sufficient to convert normal human cells to a tumorigenic state. Cancer Res. 2005;65:9824–8. doi: 10.1158/0008-5472.CAN-05-1543. [DOI] [PubMed] [Google Scholar]
- 26.Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes & development. 2007;21:1714–9. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger R, Febbo PG, Majumder PK, et al. Androgen-induced differentiation and tumorigenicity of human prostate epithelial cells. Cancer Res. 2004;64:8867–75. doi: 10.1158/0008-5472.CAN-04-2938. [DOI] [PubMed] [Google Scholar]
- 28.Johnson JL, Toft DO. A novel chaperone complex for steroid receptors involving heat shock proteins, immunophilins, and p23. J Biol Chem. 1994;269:24989–93. [PubMed] [Google Scholar]
- 29.de Rooij J, Bos JL. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene. 1997;14:623–5. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]
- 30.Cifone MA, Fidler IJ. Correlation of patterns of anchorage-independent growth with in vivo behavior of cells from a murine fibrosarcoma. Proc Natl Acad Sci U S A. 1980;77:1039–43. doi: 10.1073/pnas.77.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. The Journal of cell biology. 1994;124:619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–8. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 33.Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–9. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- 34.Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24:425–39. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- 35.Quintanilla M, Brown K, Ramsden M, Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- 36.Reiners JJ, Jr, Singh KP. Susceptibility of 129/SvEv mice in two-stage carcinogenesis protocols to 12-O-tetradecanoylphorbol-13-acetate promotion. Carcinogenesis. 1997;18:593–7. doi: 10.1093/carcin/18.3.593. [DOI] [PubMed] [Google Scholar]
- 37.Scott KA, Moore RJ, Arnott CH, et al. An anti-tumor necrosis factor-alpha antibody inhibits the development of experimental skin tumors. Mol Cancer Ther. 2003;2:445–51. [PubMed] [Google Scholar]
- 38.Yoshida N, Omoto Y, Inoue A, et al. Prediction of prognosis of estrogen receptor-positive breast cancer with combination of selected estrogen-regulated genes. Cancer Sci. 2004;95:496–502. doi: 10.1111/j.1349-7006.2004.tb03239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradbury CA, Khanim FL, Hayden R, et al. Histone deacetylases in acute myeloid leukaemia show a distinctive pattern of expression that changes selectively in response to deacetylase inhibitors. Leukemia. 2005;19:1751–9. doi: 10.1038/sj.leu.2403910. [DOI] [PubMed] [Google Scholar]
- 40.Saji S, Kawakami M, Hayashi S, et al. Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene. 2005;24:4531–9. doi: 10.1038/sj.onc.1208646. [DOI] [PubMed] [Google Scholar]
- 41.Sakuma T, Uzawa K, Onda T, et al. Aberrant expression of histone deacetylase 6 in oral squamous cell carcinoma. International journal of oncology. 2006;29:117–24. [PubMed] [Google Scholar]
- 42.Itoh Y, Suzuki T, Kouketsu A, et al. Design, synthesis, structure--selectivity relationship, and effect on human cancer cells of a novel series of histone deacetylase 6-selective inhibitors. J Med Chem. 2007;50:5425–38. doi: 10.1021/jm7009217. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Zhang X, Polakiewicz RD, Yao TP, Comb MJ. HDAC6 is required for EGF-induced beta -catenin nuclear localization. J Biol Chem. 2008 doi: 10.1074/jbc.C700185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuyama A, Shimazu T, Sumida Y, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 2002;21:6820–31. doi: 10.1093/emboj/cdf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geiger TR, Peeper DS. Critical role for TrkB kinase function in anoikis suppression, tumorigenesis, and metastasis. Cancer Res. 2007;67:6221–9. doi: 10.1158/0008-5472.CAN-07-0121. [DOI] [PubMed] [Google Scholar]
- 46.Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 2003;3:213–7. doi: 10.1016/s1535-6108(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 47.Schuuring E. The involvement of the chromosome 11q13 region in human malignancies: cyclin D1 and EMS1 are two new candidate oncogenes--a review. Gene. 1995;159:83–96. doi: 10.1016/0378-1119(94)00562-7. [DOI] [PubMed] [Google Scholar]
- 48.Luo ML, Shen XM, Zhang Y, et al. Amplification and overexpression of CTTN (EMS1) contribute to the metastasis of esophageal squamous cell carcinoma by promoting cell migration and anoikis resistance. Cancer Res. 2006;66:11690–9. doi: 10.1158/0008-5472.CAN-06-1484. [DOI] [PubMed] [Google Scholar]
- 49.Iwata A, Riley BE, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J Biol Chem. 2005;280:40282–92. doi: 10.1074/jbc.M508786200. [DOI] [PubMed] [Google Scholar]
- 50.Pandey UB, Nie Z, Batlevi Y, et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–63. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 51.Warburg O. The metabolism of tumours. Constable; London: 1930. [Google Scholar]
- 52.Neckers L, Neckers K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutic agents. Expert Opin Emerg Drugs. 2002;7:277–88. doi: 10.1517/14728214.7.2.277. [DOI] [PubMed] [Google Scholar]
- 53.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–18. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyault C, Zhang Y, Fritah S, et al. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes & development. 2007;21:2172–81. doi: 10.1101/gad.436407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hideshima T, Bradner JE, Wong J, et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci U S A. 2005;102:8567–72. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Kwon S, Yamaguchi T, et al. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol. 2008;28:1688–701. doi: 10.1128/MCB.01154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim YK, Lee EK, Kang JK, et al. Activation of NF-kappaB by HDAC inhibitor apicidin through Sp1-dependent de novo protein synthesis: its implication for resistance to apoptosis. Cell Death Differ. 2006;13:2033–41. doi: 10.1038/sj.cdd.4401915. [DOI] [PubMed] [Google Scholar]