Abstract
Importance of the field
The treatment of Hodgkin lymphoma (HL) with the use of radiotherapy and systemic chemotherapy has been one of the success stories of modern oncology. HL therapy has been the paradigm for the systematic evaluation of different curative modalities resulting in cure for the majority of patients. The current focus is on designing initial therapeutic strategies which retain efficacy and minimize long-term toxicity. Appropriate use of pathologic, clinical, biologic and radiologic prognostic factors in identification of aggressive HL is paramount in designing a successful therapeutic strategy.
Areas covered in this review
This review addresses the current and future use of prognostic tools, including PET scanning and other biomarkers, in identifying patients with aggressive HL with reference to publications from the last two decades. The current standard approaches with the use of combined modality therapy and systemic chemotherapy as well as the promising role of future response-adapted strategies is reviewed.
What the reader will gain
The reader will obtain a comprehensive review of risk assessment strategies as well as current and investigational therapeutic approaches in the management of HL.
Take home message
In HL, appropriate utilization of risk assessment strategies is required to maximize therapeutic outcomes while minimizing toxicity, especially long-term toxicity. Response-adapted therapy utilizing PET has the potential to profoundly improve the therapeutic landscape in HL.
Keywords: Aggressive, Hodgkin lymphoma, prognostic factors, chemotherapy, combined modality therapy
1. Introduction
In 2009, an estimated 8,510 new cases of Hodgkin’s lymphoma (HL) and 1,290 deaths from HL were expected in the United States.1 HL is a highly curable disease and the reported trends in 5-year relative survival for the time periods of 1975-77, 1984-86, and 1996-2004 were continuously improved at 74%, 79%, and 86%, respectively. 1
Cure rates greater than 90% for early HL and more than 70% for those with advanced HL are expected. Identifying high-risk patients who will relapse after initial therapy is of paramount importance in the development of intensified and/or improved therapeutic approaches for this unfavorable-risk group of patients. The risk-adapted approaches in current clinical utilize pathologic, biologic and clinical prognostic factors to identify these patients and design appropriately tailored treatment strategies. The major emphasis of ongoing randomized trials in HL is development and validation of response-adapted strategies utilizing functional imaging with Positron Emission Tomography (PET). Response assessment-based algorithms hopefully will lead to improved overall outcomes with escalation/de-escalation of therapy.
2. Staging
The stage for an individual HL patient is the most important determinant of prognosis and appropriate therapy. The four-stage Ann Arbor system, first introduced in 1971, is generally used for classifying the anatomic extent of HL.2 In 1990, the Cotswold modification was proposed and introduced modifications such as designations for bulky disease and anatomic substages for stage III disease.3 Accurate staging is thus crucial and should incorporate pathologic, clinical, and radiologic data including presence or absence of systemic (B) symptoms (e.g. fevers, night sweats, and weight loss), bulky disease or extranodal involvement (Table 1).
Table 1.
Cotswold Modification of the Ann Arbor Staging for HL
| Stage | Description |
|---|---|
| I | Involvement of a single lymph node region or lymphoid structure |
| II | Involvement of ≥ two lymph node regions on the same side of the diaphragm |
| III | Involvement of lymph node regions on both sides of the diaphragm |
| III1 With or without involvement of splenic, hilar, celiac, or portal nodes | |
| III2 With involvement of para-aortic, iliac, and mesenteric nodes | |
| IV | Diffuse or disseminated involvement of one or more extranodal organs or tissues, with or without associated lymph node involvement |
| A | No symptoms |
| B | Fever (temperature, >38°C [100.4°F]), drenching night sweats, unexplained loss of >10% of body weight within the preceding 6 months |
| X | Bulky disease (a widening of the mediastinum by more than one-third due to presence of a nodal mass with a maximal dimension greater than 10 cm) |
| E | Involvement of a single extranodal site that is contiguous or proximal to the known nodal site |
| CS | Clinical stage |
| PS | Pathologic stage |
An initial radiologic examination consisting of computerized tomography (CT) scans of the neck, chest, abdomen, pelvis and PET is often obtained. PET imaging is utilized to improve initial staging assessment compared to CT scanning and is able to detect additional sites of disease compared to conventional staging. This results in modification of disease stage (usually upstaging) in 15-20% and has an impact on management in 5-15% of patients.4 Although most studies usually do not provide universal pathological verification of the additional PET findings not revealed by conventional staging methods, it is assumed that the additional PET findings truly represent lymphoma. Additionally, PET is widely utilized to assess response to therapy while on treatment and to evaluate residual tumor tissue upon completion of therapy.
3. Treatment Groups
Prognostic factors in HL are important in determining likely outcomes of patients and allow for selection of appropriate initial therapy. The most important determinants of prognosis in routine use are the clinical stage and presence of systemic symptoms. Additional well established prognostic factors in assigning risk-based treatment groups include bulky disease (> 10 cm in diameter), presence of multiple sites of disease, elevated ESR, extranodal disease, and advanced age.
Using these factors in combination with clinical staging, three treatment groups have been established by the leading clinical trials group (Table 2). These groups include:
Early favorable (Stages I- II, no B symptoms)
Early unfavorable (Stages I-II with at least one risk factor), and
Advanced ( Stages III- IV or any stage with bulky disease or intra-abdominal disease)
Table 2.
HL Treatment Group Stratifications in Current Use by Cooperative Groups
| Treatment Group |
EORTC/ GELA | GHSG | NCIC/ECOG |
|---|---|---|---|
| Early-stage Favorable |
Stage I-II, No risk factors (supradiaphragmatic) |
Stage I-II, No risk factors | Favorable Stage I-II (No risk factors) |
| Early-stage Unfavorable |
Stage I-II, ≥1 risk factors (supradiaphragmatic) |
Stage I,IIA & ≥ 1 risk factors; Stage IIB with C/D & without A/B |
Unfavorable Stage I-II (≥ 1 risk factor) |
| Advanced Stage |
Stages III-IV | Stage IIB with A/B; Stage Stages III-IV |
Stages I-II, bulky or intra- abdominal sites; Stages III- IV |
| Risk Factors |
A: large mediastinal mass B: age ≥ 50 years C: elevated ESR D: > 4 involved regions |
A: large mediastinal mass B: extranodal disease C: elevated ESR D: ≥ 3 involved areas |
A: age ≥ 40 years B: not LPHL or NS histology C: ESR ≥ 50 mm/hr D: ≥ involved nodal regions |
Radiotherapy alone has been used traditionally for early stage favorable disease. Current treatment guidelines favor the routine use of combined modality radiotherapy for early favorable disease or using limited duration chemotherapy alone in this setting. The treatment of early stage unfavorable disease is reliant on systemic chemotherapy regimens as in advanced disease with subsequent involved field radiotherapy. Advanced disease is treated primarily with multi-agent chemotherapy regimens with a role for consolidative radiotherapy in cases of partial responses or bulky disease.
The different assignments for sub-classifications result in challenging interpretations and comparisons of trial results involving these sub-groups but it is generally accepted that clinical stages III-IV and early stage patients with bulk disease or systemic symptoms constitute the category of unfavorable-risk patients. For this category, prognostic models have been developed incorporating laboratory and clinical parameters in an effort to predict which patients are likely to respond poorly to initial anthracycline-based chemotherapy.
4. Prognostic Factors
With the high rates of long term survival seen in HL as well as the significant long-term morbidity due to treatment-related toxicity, the urgent need is for prognostic models which can identify patients with extremely poor prognosis whom can be prospectively identified. A variety of clinical, biological, and laboratory factors have been evaluated for their prognostic utility. A number of clinical prognostic scoring systems have been proposed but the International Prognostic Score (IPS) proposed by Vasenclever and Diehl is the most widely accepted model in clinical usage.5
4.1. International Prognostic Score (IPS)
The International Prognostic Factors Project on Advanced Hodgkin’s Disease published their analysis of prognostic factors for 5,141 patients with advanced HL in a landmark publication in 1998.
Using freedom from disease progression as the end-point, a total of seven clinical factors were employed to construct the prognostic score. Complete data from 1,618 patients were incorporated in the model and an additional 2,643 patients were used for validation. The IPS was defined as the number of prognostic factors available at diagnosis. All these risk factors were associated with similar relative risks ranging from 1.26.to 1.49 upon multivariate analysis. These independent identified risk factors were:
serum albumin < 4 g/dl,
hemoglobin < 10.5 g/dl,
male sex,
age ≥ 45 years,
WBC ≥ 15,000/ mm3,
lymphocytopenia (total lymphocytes ≤ 600/mm3 or lymphocytes < 8% of total WBC),
stage 4 disease.
The IPS predicted the rate of freedom from progression of disease at 5 years of follow-up as: no factor – 84%; 1 factor- 77%; 2 factors- 67%; 3 factors- 60%; 4 factors- 51%; and 5 or more factors- 42%. (Table 3) The percentages of patients falling in these categories were 7%, 22%, 29%, 23%, and 12% respectively. Though this prognostic model was developed to identify sub-groups of advanced disease at very high-risk of recurrence with initial anthracycline-based therapy, even the highest-risk category (IPS > 4) still enjoyed 5-year failure free survival (FFS) of 42% and overall survival (OS) of 59%. Also, the approximately 80% of patients with 0-3 risk factors retain a 5-year FFS of 71% and OS of greater than 80%. Thus there was no distinct sub-group of patients at very high-risk of recurrence which could be identified on the basis of clinical characteristics.
Table 3.
Freedom from Progression (FFP) and Overall Survival (OS) in the IPS according to Individual and Grouped factors.
| Prognostic Score | 5- year FFP | 5-year OS |
|---|---|---|
| Individual | ||
| 0 | 84 | 89 |
| 1 | 77 | 90 |
| 2 | 67 | 81 |
| 3 | 60 | 78 |
| 4 | 51 | 61 |
| ≥ 5 | 42 | 56 |
| Grouped | ||
| 0 or 1 | 79 | 90 |
| 0-2 | 74 | 86 |
| 0-3 | 70 | 83 |
| ≥ 4 | 47 | 59 |
The German Hodgkin Lymphoma Study Group (GHSG) undertook an evaluation of the applicability of the IPS for earlier stage patients in 1424 adult patients with clinical stages I-IIIA treated on successive trials.6 The IPS could be determined in 961 (70%) patients and identified 40% of the early unfavorable patients who had a 8% lower FFS at 6-years follow-up. Only a low serum albumin (in the IPS) was a factor with significant individual contribution while extranodal involvement was an additional factor associated with worse prognosis. It was concluded that the IPS has a modest predictive value for early stage unfavorable HL patients.
The applicability of the IPS in predicting the outcome of patients with relapsed or refractory HL patients has also been evaluated. In a retrospective analysis of 379 patients who underwent autologous stem cell transplant (ASCT), the event-free survival (EFS) and OS were correlated with the IPS.7 The estimated 10-year event-free survival rates for patients with IPS 0-1, 2-3, or ≥ 4 risk factors were 38%, 23%, and 7%, respectively. Likewise, the IPS was predictive of 10-year OS with corresponding survival rates of 48%, 30%, and 15%, respectively for these risk groups.
4.2. Prognostic Role of PET
PET has become widely used in the management of HL and is applicable in the areas of staging, restaging, therapy monitoring and surveillance. PET is able to detect an additional number of presumed HL sites compared with conventional staging methods. As a result, there is a modification of disease stage (usually upstaging) in about 15-20% of patients with an impact on management in about 5-15%.4 Accuracy of PET for assessment of patients with known relapse is similar to pretreatment staging.4
Post-therapy response assessment with PET is well established in HL and has been incorporated into the consensus criteria of the International Harmonization Project in Lymphoma to determine remission status of HL.8 In a recent meta-analysis, the pooled sensitivity and specificity of PET after completion of therapy were 84% and 90% respectively.9 The value of PET in this setting is its ability to distinguish between viable tumor and necrosis or fibrosis in residual masses often present after treatment in patients without any other clinical or biochemical evidence of disease.
The prognostic utility of interim response assessment during therapy of advanced HL was evaluated in a recent meta-analysis.10 For patients with low to intermediate risk advanced HL, PET had a sensitivity of 81% and specificity of 97% and seemed to be a reliable prognostic test to identify poor responders. The positive predictive value (PPV) of PET in HL is much lower at only 65% though still higher than that of CT.4 Approximately 30-40% of patients with PET-positive residual masses do not progress or relapse and thus biopsy confirmation of positive PET findings prior to administering salvage treatment is required. In contrast, in a report of PET evaluation in 311 HL patients treated with BEACOPP on the GHSG HD15 trial, the negative predictive value (NPV) at 1-year follow-up was assessed at 94%.11 In another report, interim-PET assessment was more effective than the IPS in predicting outcome in 260 patients with advanced HL who were treated with ABVD.12 A risk-adapted approach in 108 advanced HL patients with standard or high risk groups (IPS 0-2 or > 3) using interim PET revealed that adjustment of BEACOPP chemotherapy (escalated vs. standard dose) was feasible and resulted in a reduction of the cumulative chemotherapy dose with no impairment of EFS or OS.13
In the setting of salvage therapy and ASCT, the utility of interim-PET was evaluated in a pooled analysis of 7 studies.14 In this analysis, the presence of a positive pre-ASCT PET scan correlated with an increased risk of progression (hazard ratio 3.23) and decreased OS (hazard ratio 4.53). Two large series of functional imaging (PET and gallium scans) have revealed markedly improved progression free and overall survival in patients with a negative pre-transplant functional imaging study.15,16
5. Biologic factors
5.1 Tumor infiltration by macrophages
An adverse prognosis has been associated with the presence of macrophage-histiocytes in lymph node biopsy specimens of patients with HL.17,18 Steidl et al analyzed 130 frozen samples from patients with classic HL during diagnostic lymph node biopsy using gene expression profiling. This identified a gene signature of tumor associated macrophages which was associated with primary treatment failure.19 Further, using immunohistochemical analysis in an independent cohort of 166 patients, an increased number of CD68+ macrophages was correlated with reduced progression free survival (PFS), increased relapse after ASCT, and shortened HL-specific survival. Interestingly, in patients with limited-stage HL, the absence of CD-68+ macrophages was correlated with 10-year disease-specific survival of 100% with current standard therapies.
5.2 Apoptosis Markers
Biologic markers associated with apoptosis have been studied in HL for assessment of prognostic importance. Altered expression of bcl-2 and bcl-2 family of proteins such as bcl-Xl, BAX in Reed-Sternberg cells may prevent apoptosis and explain resistance to treatment induced apoptosis.20,21 Several reports have indicated that expression of bcl-2 is associated with an unfavorable outcome.22,23 Similarly, p53 expression detected by immunohistochemistry has been associated with unfavorable prognosis in some reports.21,24 High expression of bcl-Xl and a high apoptotic index as measured by terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate-biotin nick-end labeling (TUNEL) has been independently associated with a worse outcome.21
6. Cytokine Markers
6.1 Soluble CD30
Elevated levels of the soluble form of CD30 antigen (sCD30) can be detected in the serum of patients with HL.25 Elevated sCD30 has been associated with a poor outcome with poor FFS and OS and may improve the prognostic value of the IPS.26,27 In a large study of 321 patients, sCD30 levels were independently predictive of a 5-year FFS rate of less than 50%.28
6.2 Serum Interleukin-10 (IL-10)
IL-10 is a pleiotropic cytokine which protects hematopoeitic cells from apoptosis induced by glucocorticoids and doxorubicin. Hodgkin and Reed Sternberg (HRS) cells express functional IL-10 receptors and elevated IL-10 levels may inhibit apoptosis of HRS cells. Elevated serum IL-10 levels have been found in up to 50% of HL patients and have been associated with inferior FFS and OS in patients treated with ABVD or BEACOPP chemotherapy.29-32 Elevated serum IL-10 levels confer a poor survival and may add to the prognostic value of the IPS in prediction of outcomes in HL.26
6.3 CCL17/thymus and activation related chemokine (TARC)
CCL17/TARC is a chemokine secreted by RS cells and its chemotactic properties may explain the infiltration of reactive T lymphocytes in HL.33 Elevated CCl7/TARC levels have been seen in the majority of patients with HL.34 Persistent elevation of TARC after completion of treatment has been associated with poorer survival and could be important for treatment monitoring.35,36
7 Combined Modality Therapy In Early HL
For decades, radiotherapy had been the mainstay in the treatment of early stage HL. With an emphasis on improving outcomes, the use of limited radiotherapy and limited chemotherapy has been extensively evaluated in early stage HL. The rationale for this combined approach has been the recognition that radiotherapy will miss occult HL outside radiotherapy ports while systemic chemotherapy is less likely to provide cures in cases of bulky disease.
The effect of more extensive radiotherapy and chemotherapy on long-term outcome was studied in a meta-analysis of 3,888 patients in 23 rando mized clinical trials.37 More extensive radiotherapy reduced the 10-year treatment failure rate by more than one third (31% vs. 43% failures), but there was no apparent improvement in OS (77% in both). The addition of chemotherapy to radiotherapy halved the 10-year risk of failure (16% v 33%), with a non-significant improvement in OS. There was a non-significant reduction of deaths due to HL by combined modality therapy which was counterbalanced by a non-significant increase of deaths from other causes including secondary malignancies. More extensive radiotherapy fields or the use of combined modality therapy had a large effect on disease control, but only a small effect on OS. These early studies of combined modality therapy used the MOPP regimen while more recent studies have utilized the ABVD regimen in concert with its established role in advanced HL.
The Milan group has published the mature results of their prospective trial of ABVD × 4 cycles with either subtotal nodal irradiation (STNI) or involved field radiotherapy (IFRT).38 The 12-year FFP and OS rates were not different. The toxicity profile was favorable for the combined modality arm with no cytokine support requirements, hospitalizations or secondary malignancies and absence of severe cardiopulmonary toxicity.
The GHSG HD7 trial evaluated the use of ABVD × 2 cycles followed by extended-field radiotherapy (EFRT) vs. EFRT alone in 650 patients with stage I-IIB favorable HL.39 At a median follow-up of 87 months, though the CR rate and OS were similar, the 7-year freedom from treatment failure (FFTF) rates were significantly superior in the combined modality arm (88% vs.67%) due to a higher relapse rate in the EFRT arm. The GHSG HD8 trial evaluated EFRT vs. involved-field radiotherapy (IFRT) in 1064 evaluable patients with early stage unfavorable HL.40 After receiving COPP-ABVD × 4 cycles, patients received either EFRT or IFRT (total dose 30 Gy plus 10 Gy to bulky sites in both arms). At 5-year follow-up, the complete response rate, freedom from treatment failure rate and overall survival were not different between the two arms. In contrast, EFRT was associated with more acute hematologic and gastrointestinal toxicities.
The EORTC-GELA H8 trial evaluated reductions in chemotherapy duration and radiotherapy fields in 1538 patients with stage I-II favorable (H8-F) and unfavorable (H8-U) HL.41 In the H8-F part, patients were randomized to MOPP-ABV × 3 cycles vs. STNI. With a median follow-up of 92 months, patients in the combined modality arm had improved 5-year EFS (98% vs. 74%) and improved 10-year OS (97% vs. 92%). In the H8-U trial, patients were randomized to MOPP-ABV × 6 cycles plus IFRT, MOPP-ABV × 4 cycles plus IFRT, or MOPP-ABV × 4 cycles plus STNI. With a median follow-up of 92 months there were no differences in EFS between the three arms. The investigators concluded that chemotherapy plus IFRT was the standard of care for early-stage HL.
More recently, the GHSG HD10 trial in early-stage favorable HL compared 2 and 4 cycles of ABVD as well as 20Gy or 30Gy IFRT.42 With a median follow-up of 79–91 months, the final results reveal no significant difference between the chemotherapy arms in terms of OS, FFTP, and PFS. There were also no significant differences in terms of the same survival parameters between the radiotherapy arms or when all four arms were compared. Based on these results, ABVD × 2 plus 20 Gy IFRT has been proposed as the new standard in early stage favorable HL. The HD13 trial investigated omission of bleomycin, or dacarbazine from HD10 regimen and interim results have shown that efficacy is reduced with the omission of dacarbazine from the regimen; results for the arm with bleomycin omitted are awaited. 43
In the GHSG HD11 trial in early-stage unfavorable HL, 1395 patients were randomized to 4 cycles ABVD vs. baseline BEACOPP and also IFRT 20 Gy vs. 30 Gy.44 Toxicities were increased in the intensive therapy arms though CR rates were similar in all arms at the end of therapy. OS was similar in all arms though FFTP was inferior in the ABVD/20 Gy arm suggesting that ABVD × 4/ RT 30 Gy remains the standard.
Mature results of the evaluations of an abbreviated version of the Stanford V regimen (8 weeks) plus 30Gy IFRT in stage I-IIA non-bulky HL have been presented recently.45 At a median follow-up of 9 years, OS did not differ among patients with favorable or unfavorable risk though freedom from progression was better in the favorable risk group (100% vs.89%). Although cytokine support was required in nearly one-half the patients, neutropenic infection rates were very low. This approach had low non-hematologic toxicity and no secondary leukemias or pulmonary toxicity was noted.
A slightly different approach involving comparison of radiotherapy/ combined modality therapy vs. ABVD chemotherapy alone in stage I-IIA, non-bulky HL has been evaluated by the National Cancer Institute Canada-Eastern Cooperative Oncology Group.46 Among 399 patients, 5-year PFS was superior in the radiotherapy containing arm (93% vs.87%) though OS was not different. The superior PFS for the radiotherapy containing arm was due to more relapses in the unfavorable subset of patients treated with ABVD alone but was offset by mortality from other causes and acute treatment related toxicities resulting in no differences in overall survival.
In the setting of combined-modality therapy, the use of conformal techniques during RT treatment planning reduces the irradiated volume to include only the macroscopic lymphoma. Accuracy is improved by using PET co-registered with the planning CT scans. 47 Pre-RT PET for treatment planning may lead to significant modifications of RT treatment strategy including clinical target volume and total dose.48
8 Systemic Chemotherapy In Advanced HL
Advanced HL historically has been treated with systemic chemotherapy approaches followed by radiotherapy to bulky disease sites. The development of successive generations of effective multi-agent regimens through prospective randomized trials has culminated in the current generation of extremely effective regimens. Though the successes with continued investigations have been heartening the realization of long-term severe and fatal toxicities have resulted in the current ongoing evaluation of response-adapted strategies in this setting.
DeVita et al first reported results of combination chemotherapy with the MOPP regimen (nitrogen mustard, vincristine, procarbazine, prednisone) in 1970. In stage III-IV HL patients, MOPP achieved a high complete remission rate (CR), durable complete remissions (29–42 months), and long-term disease free survival DFS (47% at 4 years).49 Long-term follow-up results of 188 patients treated at the National Cancer Institute revealed an 84% CR rate with 66% of complete responders remaining disease free more than 10 years.50 Overall, the 10-year disease-free survival rate was 54%. MOPP is associated with hematologic toxicity, neurotoxicity, and gonadal toxicity in men and women.50,51 The development of secondary myelodysplastia and acute leukemia contributes further to the toxicity profile of this regimen.52
In order to improve treatment outcomes, Bonadonna et al reported in 1975 results with the ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) regimen.53 In the initial report, 60 patients with untreated stage IIB–IVB HL were randomly assigned to 6 cycles of ABVD or MOPP. Both regimens had similar CR rates and showed absence of cross-resistance. The long-term follow-up of 232 untreated stage IIB-IIIB patients randomized to 6 cycles of MOPP or ABVD with radiotherapy given midway have been reported.54 Seven-year FFP and OS were higher with the ABVD regimen. Gonadal dysfunction and secondary leukemia were reported with MOPP patients only while there were no significant abnormalities in cardiopulmonary function with either regimen.
The next generation of clinical trials incorporated multiple non-cross-resistant agents and then alternated regimens, sequenced regimens, or integrated multiple drugs into hybrid regimens.55-57 The Cancer and Leukemia Group B (CALGB) compared MOPP alone, MOPP alternating with ABVD, and ABVD alone in stage III-IV or relapsed HL patients.58 In this trial, the CR and 5-year FFS rates were inferior in the MOPP arm though OS was not significantly different among the three arms. ABVD therapy was clearly as effective as MOPP alternating with ABVD, and both were superior to MOPP alone; ABVD was also found to be less myelosuppressive than MOPP.
A randomized trial conducted by the National Cancer Institute of Canada showed that MOPP/ABV hybrid regimen or MOPP alternating with ABVD had similar 5-year OS and FFS.59 The hybrid regimen had significantly more febrile neutropenia and stomatitis. A U.S. Intergroup trial showed that the MOPP/ABV hybrid was as effective as ABVD with equivalent CR rates, as well as 5-year FFS and OS in patients with advanced HL.60 Again the hybrid regimen was associated with increased acute toxicity (pulmonary and hematologic) and also an increased incidence of secondary myelodysplasia and leukemia. Based on these results and favorable toxicity profile, ABVD was established as the standard regimen for the treatment of advanced HL.
The German Hodgkin’s Lymphoma Study Group (GHSG) developed a mathematical model of tumor growth and chemotherapy effects which predicted increased tumor control with chemotherapy dose escalation and intensification.61 On this basis the BEACOPP regimen was introduced which involved a shortened treatment duration (24 vs. 32 weeks) and higher dose intensity than COPP/ABVD by increasing doses of doxorubicin and cyclophosphamide and adding etoposide.62 In the pilot study, in patients with stage IIB–IV HL the CR rate was 93% and the FFTF rate was 89% at 40 month median follow-up. The escalated-BEACOPP regimen involved increased doses of doxorubicin, cyclophosphamide, and etoposide with Filgrastim support.63 In stage IIB–IVA patients, the FFTF rate was 90% at 32 months. There was an increase in severe hematologic toxicity and four patients developed secondary malignancies.
Results of some recent important randomized trials of chemotherapy in HL are summarized in Table 5. In the GHSG HD9 study, 1196 patients with unfavorable stage IIB-IV disease were randomized to COPP-ABVD, BEACOPP, or escalated BEACOPP, followed by radiotherapy to sites of initial bulky disease and to any residual tumor.64,65 The 5-year FFTF rates for COPP-ABVD, BEACOPP, and escalated BEACOPP were 69%, 76%, and 87%, respectively while the 5-year OS rates were 83%, 88%, and 91% respectively. Improvements in survival with escalated BEACOPP were accompanied by a markedly increased incidence of acute grade 3/4 hematologic toxicity as well as grade 3/4 infections (22%). Recently, updated results at a median follow-up of 111 months have revealed the persistence of the survival benefit with escalated BEACOPP.65 The rates of second malignancies do not differ among the 3 regimens though escalated BEACOPP had a markedly higher rate of secondary leukemias. Results from the HD2000 Italian trial comparing BEACOPP vs. ABVD vs. COPP-EBV-CAD (CEC) revealed a superior 5-year progression free survival rate for BEACOPP vs. ABVD (81% vs. 68%) though overall survival was not different.66 Preliminary results from an Italian randomized study in advanced HL suggest that a BEACOPP vs. ABVD strategy produced superior PFS (87% vs. 71%) but equivalent OS at 3 years.67 Salvage approaches with ASCT for both arms contributed to these results and reveal overtreatment in 71% patients who did not relapse with upfront ABVD. Results from the similarly designed EORTC 20012 trial are awaited. BEACOPP has improved PFS compared to ABVD but universal acceptance is hampered by lack of superior OS data as well as an increased incidence of acute and late toxicities; for example in the HD2000 trial BEACOPP resulted in improved PFS in the IPS 3-7 subset but at the price of 3% treatment-related mortality as well as near-universal infertility .
Table 5.
Results of major recent randomized chemotherapy trials in Advanced HL.
| Regimen | Patients | CR (%) | FFS (%) | OS (%) |
|---|---|---|---|---|
| GHSG HD9 Trial | ||||
| COPP/ABVD | 260 | 85 | 69 | 83 |
| BEACOPP | 469 | 88 | 76 | 88 |
| Esc-BEACOPP | 466 | 96 | 87 | 91* |
| GISL HD2000 Trial | ||||
| ABVD | 103 | 84 | 68 | 84 |
| CEC | 102 | 83 | 78 | 91 |
| BEACOPP | 102 | 91 | 81** | 92 |
| IIL Trial | ||||
| ABVD | 122 | 89 | 78 | 90 |
| MOPPEBVCAD | 106 | 94 | 81 | 89 |
| Stanford V | 107 | 76 | 54 | 82 |
| UK NCRI Trial | ||||
| ABVD | 261 | 67 | 74 | 90 |
| Stanford V | 259 | 67 | 76 | 92 |
OS significantly improved with Esc-BEACOPP vs. COPP-ABVD
FFS significantly improved with BEACOPP vs. ABVD
The Stanford V regimen was developed to maintain or improve the cure rate seen with earlier regimens while improving the toxicity profile.68 This shortened 12 week regimen involves reduced cumulative doses of bleomycin, doxorubicin, and nitrogen mustard with omission of procarbazine. The protocol relies on post-chemotherapy irradiation for areas of bulky disease (5 cm or greater at diagnosis) and macroscopic splenic involvement. In a prospective trial, the 5-year FFP was 89% and OS 96% among 142 patients with stage III–IV or locally extensive mediastinal stage I–II HL.69 The FFP was superior in patients with an IPS 0-2 and no secondary leukemias or cardiopulmonary toxicity was observed.
In a trial evaluating ABVD, Stanford V, and MOPP-EBV-CAD in 365 patients with stage IIB-IV HL, the CR and 5-year FFS rates were inferior with the Stanford V regimen with no significant differences in OS among the three regimens.70 These results may have been influenced by radiotherapy limitations (2 sites only), delay in start of radiotherapy, and a reduced number of patients receiving radiotherapy compared to the original report (66% vs. 90%). Results of a second trial comparing Stanford V with ABVD by the United Kingdom NCRI Lymphoma Group in 520 patients with advanced HL revealed equivalent response rates as well as 5-year progression-free survival and overall survival rates.71 Stanford V was associated with less pulmonary toxicity and less G-CSF requirement; however, more severe non-pulmonary toxicity was seen with the Stanford V regimen. Again, the reduced use of radiotherapy with Stanford V (73% patients) may have contributed to the results observed in this trial.
The role of upfront high-dose chemotherapy and ASCT has been prospectively evaluated in two European studies. 163 unfavorable-risk patients achieving complete or partial remission were randomized to ASCT or continuing 4 more cycles of doxorubicin-containing chemotherapy. With a median follow-up of 48 months, the relapse-free and overall survival rates were not significantly different.72 In another randomized study in poor-risk HL, 65 of 107 patients in complete remission accepted randomization to ASCT vs. continuing hybrid chemotherapy for 5 more cycles. At a median follow-up of 6 years, the results of this study showed no significant difference in time to treatment failure among both arms.73
In summary, incremental gains in reduced disease specific mortality from advanced HL have been made by modification of chemotherapy regimens together with radiotherapy, and supportive measures. However, these gains have been made at the expense of increased toxicity and long-term effects. Thus, there remains a need for innovative approaches which complement or replace current therapies while minimizing toxicity and late effects.
9 Therapeutic Recommendations
9.1. Early Favorable HL
Combined modality therapy with ABVD × 2 cycles followed by 20 Gy IFRT based on the GHSG HD10 results is the standard approach. ABVD × 2 alone followed by interim PET restaging and additional 2-4 cycles ABVD is an option with IFRT only in PET-positive patients. The National Comprehensive Cancer Network (NCCN) guidelines also include Stanford V × 2 cycles (8 weeks) followed by 30 Gy IFRT as an alternative.
9.2. Early Unfavorable HL
Initial therapy with ABVD × 2 cycles is followed by interim PET restaging. An additional 2 cycles for responding patients is followed by IFRT 30 Gy to areas of bulk disease. Alternatively, ABVD × 6 cycles alone can be used. The NCCN guidelines also include Stanford V × 3 cycles (12 weeks) followed by IFRT 30 Gy to areas of bulk disease (> 5 cm) as an alternative.
9.3. Advanced HL
The primary treatment strategy is ABVD × 4 cycles followed by interim PET restaging and further ABVD to a total of 6-8 cycles followed by IFRT 30 Gy) to sites of bulky disease. For patients with an IPS 4-7, escalated BEACOPP (4 cycles) is usually recommended as initial therapy followed by restaging. Patients with PET-negative status usually receive baseline BEACOPP for 4 cycles whereas patients with a less than CR continue for 4 more cycles of escalated BEACOPP. The NCCN guidelines also include Stanford V × 3 cycles (12 weeks) followed by IFRT 36 Gy to sites of bulky disease (> 5 cm) as an option.
With the widespread integration of PET in clinical practice early interim and post-therapy response assessment has become more common. Published reports have demonstrated the high negative predictive value of PET in these settings. Thus the majority of patients with interim negative PET are likely to have extremely low treatment failure rates and therapy de-escalation can be considered (e.g. ABVD × 6 cycles total for a patient with advanced HL and a negative interim PET after ABVD × 2 cycles). Close monitoring and surveillance of patients to detect early relapses and institution of effective salvage therapy has to be a component of any de-escalation strategy. It should be stressed that therapeutic de-escalation/escalation strategies are presently ongoing investigation in multiple randomized trials.
10. Nodular Lymphocyte Predominant HL (LPHL)
NLPHL is a subtype of HL with clinicopathologic features different from classic HL. The hallmark of NLPHL is the presence of CD20+, CD15−, CD30− lymphocyte predominant (LP) cells embedded within a vaguely nodular pattern of infiltrating lymphocytes. In comparison to classic HL, NLPHL has a higher age of onset (30-40 years), a higher incidence in males, a tendency for peripheral distribution, lack of B symptoms in majority, and mostly early stage disease.74,75 Standard therapy options include IFRT or regional RT (stages I-IIA), combined modality therapy (stages I-IIB), or chemotherapy (stages III-IV) followed by RT to residual/bulk disease. Most reports have evaluated MOPP or ABVD-like regimens in advanced LPHL. Salvage therapy for relapsed disease typically has involved regimens typically used in classic HL. Recently, the use of rituximab in the relapsed setting has demonstrated extremely high and durable responses.
11. Relapsed and Refractory HL
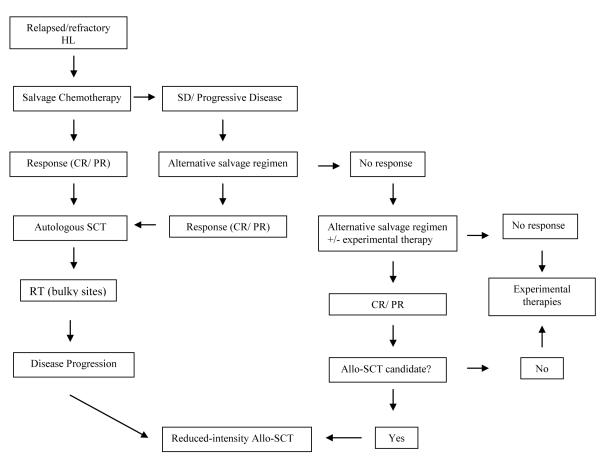
In HL, up to 5-10% patients are refractory to initial therapy and 10-30% will relapse after achieving initial remission.76 Currently, the standard approach in this setting is the use of salvage chemotherapy followed by ASCT; radiotherapy may also be utilized in a combined modality approach. An algorithm for therapy in the relapsed and refractory setting is depicted in Figure 1.
Figure 1. Approach to the HL patient with relapsed/refractory HL.
Abbreviations: Allo-SCT, allogeneic stem cell transplantation; HDCT/ASCT, high-dose chemotherapy and autologous stem cell transplant; HL, Hodgkin’s lymphoma; RT, radiotherapy.
• Prognostic factors
Duration of remission after initial therapy remains one of the most important prognostic factors; long-term survival was not reported in patients with primary progressive disease in the updated National Cancer Institute report.77 The GHSG undertook a retrospective analysis of 422 relapsed patients and developed a prognostic score comprised of 3 factors: time to relapse (≤ or > 12 months), clinical stage at relapse (III/IV), and anemia at relapse.78 In a retrospective study of 214 patients in first relapse, the presence of extranodal relapse and time to relapse < 12 months was associated with poor 4-year survival while patients with a low-risk relapse in this model (i.e. no risk factors) enjoyed 93% 4-year overall survival.79 Response to salvage DHAP chemotherapy, anemia, and duration of initial remission were prognostic factors in a prospective 102 patient German study.80 A prognostic model including B symptoms, extranodal disease, and duration of remission < 1 year was reported to predict for outcome after salvage ICE chemotherapy and provides a basis for a risk-adapted approach to high-dose therapy.81
• Salvage chemotherapy regimens
The choice of salvage regimens is determined by the type of prior therapy and repeated use of initial chemotherapy can be considered for initial remission duration >1 year. As most patients are currently treated with upfront ABVD, retreatment is limited by cumulative doxorubicin dosing. Various salvage regimens have been evaluated but no randomized trials are available to prove the superiority of any particular regimen.
Responsiveness to salvage chemotherapy is a major determinant of outcome after ASCT.82 The majority of regimens are associated with response rates in the 60-80% range with varying complete response rates (Table 6). Long-term results of ASCT in 195 patients have revealed that patients who were in complete, partial or unresponsive status at the time of ASCT had 10-year overall survival rates of 72%, 54%, and 11%, respectively.83 Additionally, the effectiveness of a salvage regimen must be complemented with a favorable toxicity profile, especially to avoid toxicity to hematopoietic stem cells in anticipation of ASCT. Ultimately, the choice of salvage regimen is dependent on a combination of the above factors.
Table 6.
Salvage chemotherapy regimens commonly utilized in Relapsed & Refractory HL.
The more commonly used regimens include mini-BEAM, dexa-BEAM, DHAP, ASHAP, ICE, IGEV, GVD, and GDP. Older regimens such as mini-BEAM, dexa-BEAM, and DHAP are associated with a significant toxicity profile. The newer gemcitabine-based regimens have the potential advantages of a more favorable toxicity profile as well as outpatient delivery with maintained effectiveness. In 65 patients receiving ICE chemotherapy, salvage IFRT prior to ASCT was associated with in-field relapses in only 3 of 17 patients suggesting a benefit with consolidative RT.81
• Autologous Stem Cell Transplant
ASCT is now the standard of care for patients with refractory or relapsed HL based on the results of two randomized trials and multiple large case series with long-term follow-up. In a small randomized prospective trial, salvage mini-BEAM chemotherapy was compared to ASCT.84 At 3-years of follow-up, the EFS was markedly superior in the BEAM/ASCT group (53% vs. 10%). A more recent prospective trial involved 163 patients with relapsed HL who were randomized to salvage chemotherapy versus ASCT. Patients who had at least a partial remission after two cycles of Dexa-BEAM received either two more cycles of Dexa-BEAM or BEAM/ASCT.85 At 3 years of follow-up, the rate of FFTP was superior in the BEAM/ASCT group (55% vs. 34%). The results of these two studies demonstrate improvement in disease control with ASCT in the relapsed and refractory HL setting. An OS benefit with ASCT was not demonstrated in either study, likely reflecting the relatively short follow-up, patient crossover to receive the ASCT arm, and the relatively responsive nature of relapsed HL to salvage therapeutic regimens.
Multiple single-arm studies in the relapsed and refractory HL setting have demonstrated that a salvage approach utilizing ASCT results in long-term PFS and OS rates in the range of 40–50% and 50–60%, respectively.83,86,87 Patients with chemotherapy refractory disease prior to ASCT generally have poor outcomes.88,89 A significant proportion of chemotherapy resistant patients can have significant 5-year survival (33%) with ASCT suggesting a beneficial dose escalation effect.90 Additional poor prognostic factors that have been reported to be predictive of inferior outcomes with ASCT include extranodal disease, relapse in multiple sites, poor performance status, multiple prior relapses, relapse in radiotherapy fields, and presence of B symptoms.79,86,91
• Reduced Intensity Conditioning (RIC) Allogeneic SCT
In the setting of post-ASCT relapse, allogeneic SCT has been investigated as salvage therapy especially with its potential advantage of a graft-versus-lymphoma (GVL) effect.92 Early studies demonstrated that myelo-ablative allogeneic SCT in relapsed HL was associated with an unacceptably high rate of transplant related mortality in the 40-50% range.93,94 More recently, the focus has been to minimize early toxicity with RIC regimens. Published reports using this approach have demonstrated reduced transplant related mortality and improved overall survival rates in comparison to myelo-ablative allogeneic SCT.95 RIC allogeneic SCT has been shown to have superior 4 year median OS in comparison to post-salvage therapy in a large retrospective analysis.96 Similarly, results using unrelated donors in the setting of RIC allogeneic SCT have been associated with reduced toxicity but persistent problems with disease relapse.97 RIC allogeneic SCT is a potential treatment option for the post-ASCT young HL patient with a suitable donor, either related or unrelated.
• Immunotherapy
In HL, CD30 is expressed on the surface of RS cells and signaling through this receptor is thought to promote the survival and proliferation of these cells.98,99 Clinical trials of first generation anti-CD30 monoclonal antibodies in heavily pretreated patients with HL demonstrated them to be largely well tolerated but with poor anti-tumor activity.100 SGN-30, a chimeric anti-CD30 product, demonstrated no responses but had a 29% stable disease rate in a phase II study in heavily pretreated HL.101 In a phase I-II study with MDX-060, a humanized anti-CD30 antibody, clinical responses as well as stable disease were observed.102 Severe pulmonary toxicity led to the closure of a study combining SGN-30 with the GVD regimen.103
In order to improve their therapeutic profile, anti-CD30 antibody constructs with cytotoxics or radioimmunoconjugates have been developed.100,104 SGN-35, an antibody-drug conjugate consisting of the tubulin inhibitor monomethyl auristatin E conjugated to the chimeric anti-CD30 monoclonal antibody SGN-30, has been tested in heavily pretreated HL patients.105 This conjugate has been generally well tolerated, with 46% response rates (25% complete) in this phase I study.
Targeting CD20 with the anti-CD20 monoclonal antibody rituximab has been studied in heavily pretreated patients with classical HL. Rituximab has single-agent activity (22% response rate) and as well as in combination with gemcitabine (48% response rate).106,107 Even though CD20 expression is absent on most RS cells in classical HL, CD20+ B cells comprise much of the microenvironment and rituximab likely exerts its effects through depletion of these cells. In contrast, in nodular lymphocyte predominant HL (NLPHL), a GHSG study in 14 relapsed refractory patients had a 100% response rate with a median PFS of 33 months.108 The Stanford group investigated extended dosing rituximab (4 weekly doses every 6 months for 2 years) in untreated and relapsed NLPHL and demonstrated a CR rate of 88% with a median PFS of 88% at 30 months.109 This responsiveness to anti-CD20 therapy is likely due to the consistent expression of CD20 by NLPHL variant HRS cells.
Epstein Barr virus (EBV)-positive HL HRS cells express tumor antigens consisting primarily of the latent membrane protein LMP1 and LMP2 antigens which are targets for adoptive immunotherapy using EBV-specific cytotoxic T lymphocytes (CTL).110 EBV-CTL administered to 14 patients with relapsed EBV+ HL were well tolerated, controlled B symptoms, and demonstrated significant antitumor activity (5 patients with CR, 1 patient with PR, and 5 patients with stable disease).
12 Expert Opinion
The successful integration of two curative therapies- systemic chemotherapy and radiotherapy- results in cure for the large majority of patients with untreated classical HL and for a significant number of relapsing or refractory patients (with ASCT). For a disease with such favorable outcomes, the objective of cure has to be appropriately balanced with that of achieving minimal long-term toxicity. Mortality due to complications of therapy approaches that of disease-related mortality in HL; in fact mortality from secondary malignancies and cardiopulmonary events exceeded that of disease-related mortality in limited stage HL.111,112 Consequently, the choice of initial therapy should be dictated by integration of up-front prognostic risk assessment, regimen effectiveness, long-term toxicity, and potential for salvage therapy.
The clinical utility of current prognostic models like the IPS is limited by inability to discriminate patients with extremely poor prognosis. In aggressive HL, the development of prognostic systems modeled on the integration of biologic prognostic markers is essential for more appropriate risk stratification. Tissue biomarkers, such as the detection of increased infiltrating macrophages in lymph node specimens are associated with B symptoms and worse outcomes in HL.17,18 The identification of an increased number of CD68+ macrophages was correlated with shortened HL-specific survival and, in patients with limited-stage HL, the absence of CD-68+ macrophages correlated with 10-year disease-specific survival of 100%.19 Persistent elevation of the chemokine CCL17/TARC after completion of treatment has been associated with poorer survival and could be an important marker for monitoring response to treatment.35,36 Markers of resistance to apoptosis determined by bcl-2 expression, p53 expression, and bcl-Xl expression have been associated with an unfavorable outcome.21-25 Likewise, prognostic serum biomarkers such as elevated serum IL-2 receptor (CD25) and sCD30 are associated with poor outcomes and should undergo prospective evaluations for prognostic impact.26,27,28,31,32
Incremental gains have resulted in the development of very effective chemotherapy regimens (ABVD, escalated BEACOPP) with a resultant departure from radiotherapy as a mainstay for limited stage HL and improved outcomes in advanced HL. The focus has consequently shifted from incremental cure gains to limitation of acute and late toxicity (and consequently mortality). In this regard, the results of the GHSG HD10 trial provide compelling evidence that reduced therapy (ABVD × 2 cycles plus IFRT 20 Gy) is as effective as current treatment approaches in early favorable HL. Likewise, interim results of an Italian randomized study in advanced HL suggest that a BEACOPP vs. ABVD strategy produced superior PFS but equivalent OS at three years.67 Salvage approaches with ASCT for both arms contributed to these results and reveal the overtreatment in the majority of patients in comparison to up-front ABVD.
One of the most intensive areas of investigation in HL is that of risk-adapted therapy as determined by interim-PET restaging.113 This strategy involves initial use of standard chemotherapy followed by interim PET after 2 cycles and subsequent tailoring of therapy based on results of functional imaging. Interim PET out-performed the IPS in a recent report of 260 patients in a joint Danish-Italian study with specificity and sensitivity at 2 years of 96% and 77%, respectively.11 Likewise, in 311 HL patients treated with BEACOPP on the GHSG HD15 trial, the negative predictive value of interim PET at 1-year follow-up was assessed at 94%.12 Based on these data, multiple trials are investigating the utility of response-adapted therapy in all stages of HL.113
Finally, further gains in HL therapy are likely to accrue from development of novel approaches such as antigen-targeted therapy as well as novel drug delivery systems rather than the development of newer multi-agent chemotherapy regimens. The development of target-driven immunotherapy such as targeting CD30 with SGN-35 (auristatin-linked anti-CD30 antibody) hold great promise for the future not just for relapsing/refractory patients but potentially for initial therapy as well.109 The use of adoptive immunotherapy targeting EBV-associated antigens LMP-1 and LMP-2 with cytotoxic T lymphocytes holds the promise of tailoring therapy to specific tumor profiles in this subset of patients.110 Delivery of biotoxins incorporated within liposomes to tumor-associated macrophages is another example of a potentially more tumor-bioprofile specific approach.114
Article Highlights.
Current risk-adapted therapy relies on staging and prognostic models such as the International Prognostic Score (IPS)
PET is valuable in staging, interim response assessment and restaging of HL
Randomized trials have established ABVD as the preferred regimen in HL
Combined modality therapy and systemic chemotherapy are the standard of care in early and advanced HL, respectively.
Salvage therapy and Autologous Stem Cell Transplant results in long-term survival in 50-60% patients with relapsed/refractory HL
Current research emphasis is on use of biomarkers, response-adapted therapy, and novel immunotherapy approaches.
Table 4.
Results of major recent randomized comnibed modality trials in Early HL.
| Regimen | Patients | CR (%) | FFP (%) | OS (%) |
|---|---|---|---|---|
| GHSG HD7 Trial | ||||
| EFRT | 311 | 95 | 67 | 92 |
| ABVD × 2/EFRT | 316 | 94 | 88* | 94 |
| GHSG HD8 Trial | ||||
| COPP-ABVD × 4/EFRT | 532 | 98 | 86 | 91 |
| COPP-ABVD × 4/IFRT | 532 | 97 | 84 | 92 |
| EORTC-GELA H8U Trial | ||||
| MOPP-ABV × 6/IFRT | 336 | 83 | 82 | 88 |
| MOPP-ABV × 4/IFRT | 333 | 85 | 80 | 85 |
| MOPP-ABV × 4/STNI | 327 | 86 | 80 | 84 |
| EORTC-GELA H8F Trial | ||||
| MOPP-ABV × 3/IFRT | 270 | 93 | 93** | 97** |
| STNI | 272 | 95 | 68 | 92 |
| GHSG HD10 Trial | ||||
| ABVD × 4 | 433 | 97 | 93 | 97.1 |
| ABVD × 2 | 414 | 97 | 91 | 96.6 |
| IFRT 30 Gy | 218 | 97 | 94 | 97.6 |
| IFRT 20 Gy | 215 | 97 | 93 | 97.5 |
| NCIC-ECOG Trial | ||||
| ABVD | 196 | - | 87 | 94 |
| ABVD/STNI | 203 | - | 93*** | 96 |
FFP significantly superior with ABVD/EFRT vs. EFRT
FFP and OS significantly superior with MOPP-ABV/IFRT vs. STNI
FFP significantly superior with ABVD/STNI vs. ABVD alone
Acknowledgments
Dr. Kadin is supported by NIH grant 5P20 RR018757.
Footnotes
Declaration of interest: The authors state no conflict of interest and have received no payment in preparation of this manuscript.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971;31(11):1860–1. [PubMed] [Google Scholar]
- 3.Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7(11):1630–6. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 4.Juweid ME. Utility of Positron Emission Tomography (PET) scanning in managing patients with Hodgkin Lymphoma. Hematology Am Soc Hematol Educ Program. 2006:259–65. doi: 10.1182/asheducation-2006.1.259. [DOI] [PubMed] [Google Scholar]
- 5.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s Disease. International Prognostic Factors Project on Advanced Hodgkin’s disease. N Engl J Med. 1998;339(21):1506–14. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 6.Franklin J, Paulus U, Lieberz D, Breuer K, Tesch H, Diehl V. Is the international prognostic score for advanced stage Hodgkin’s disease applicable to early stage patients? German Hodgkin Lymphoma Study Group. Ann Oncol. 2000;11(5):617–23. doi: 10.1023/a:1008325627670. [DOI] [PubMed] [Google Scholar]
- 7.Bierman PJ, Lynch JC, Bociek RG, et al. The International Prognostic Factors Project score for advanced Hodgkin’s disease is useful for predicting outcome of autologous hematopoietic stem cell transplantation. Ann Oncol. 2002;13(9):1370–7. doi: 10.1093/annonc/mdf228. [DOI] [PubMed] [Google Scholar]
- 8.Juweid ME, Stroobants S, Hoekstra OS, et al. Imaging Subcommittee of International Harmonization Project in Lymphoma. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25(5):571–8. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 9.Zijlstra JM, Lindauer-van der Werf G, Hoekstra OS, Hooft L, Riphagen II, Huijgens PC. 18F-fluoro-deoxyglucose positron emission tomography for post-treatment evaluation of malignant lymphoma: a systematic review. Haematologica. 2006;91(4):522–9. [PubMed] [Google Scholar]
- 10.Terasawa T, Lau J, Bardet S, et al. Fluorine-18-fluorodeoxyglucose positron emission tomography for interim response assessment of advanced-stage Hodgkin’s lymphoma and diffuse large B-cell lymphoma: a systematic review. J Clin Oncol. 2009;27(11):1906–14. doi: 10.1200/JCO.2008.16.0861. [DOI] [PubMed] [Google Scholar]
- 11.Kobe C, Dietlein M, Franklin J, et al. Positron emission tomography has a high negative predictive value for progression or early relapse for patients with residual disease after first-line chemotherapy in advanced-stage Hodgkin lymphoma. Blood. 2008;112(10):3989–94. doi: 10.1182/blood-2008-06-155820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallamini A, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25(24):3746–52. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 13.Dann EJ, Bar-Shalom R, Tamir A, et al. Risk-adapted BEACOPP regimen can reduce the cumulative dose of chemotherapy for standard and high-risk Hodgkin lymphoma with no impairment of outcome. Blood. 2007;109(3):905–9. doi: 10.1182/blood-2006-04-019901. [DOI] [PubMed] [Google Scholar]
- 14.Poulou LS, Thanos L, Ziakas PD. Unifying the predictive value of pretransplant FDG PET in patients with lymphoma: a review and meta-analysis of published trials. Eur J Nucl Med Mol Imaging. 2010;37(1):156–62. doi: 10.1007/s00259-009-1258-y. [DOI] [PubMed] [Google Scholar]
- 15.Jabbour E, Hosing C, Ayers G, et al. Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer. 2007;109(12):2481–9. doi: 10.1002/cncr.22714. [DOI] [PubMed] [Google Scholar]
- 16.Moskowitz CH, Nimer SD, Zelenetz AD, et al. Normalization of FDG-PET Pre-ASCT with Additional Non-Cross Resistant Chemotherapy Improves EFS in Patients with Relapsed and Primary Refractory Hodgkin Lymphoma-Memorial Sloan Kettering Protocol 04-047. Blood (ASH Annual Meeting Abstracts) 2008;112 Abstract 775. [Google Scholar]
- 17.Coppleson LW, Rappaport H, Strum SB, Rose J. Analysis of the Rye classification of Hodgkin’s disease. The prognostic significance of cellular composition. J Natl Cancer Inst. 1973;51(2):379–90. [PubMed] [Google Scholar]
- 18.Ree HJ, Kadin ME. Macrophage-histiocytes in Hodgkin’s disease. The relation of peanut-agglutinin-binding macrophage-histiocytes to clinicopathologic presentation and course of disease. Cancer. 1985;56(2):333–8. doi: 10.1002/1097-0142(19850715)56:2<333::aid-cncr2820560222>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Steidl C, Lee T, Shah SP, Farinha P, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362(10):875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rassidakis GZ, Medeiros LJ, McDonnell TJ, et al. BAX expression in Hodgkin and Reed-Sternberg cells of Hodgkin’s disease: correlation with clinical outcome. Clin Cancer Res. 2002;8(2):488–93. [PubMed] [Google Scholar]
- 21.Montalbán C, García JF, Abraira V, et al. Spanish Hodgkin’s Lymphoma Study Group. Influence of biologic markers on the outcome of Hodgkin’s lymphoma: a study by the Spanish Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2004;22(9):1664–73. doi: 10.1200/JCO.2004.06.105. [DOI] [PubMed] [Google Scholar]
- 22.Rassidakis GZ, Medeiros LJ, Vassilakopoulos TP, et al. BCL-2 expression in Hodgkin and Reed-Sternberg cells of classical Hodgkin disease predicts a poorer prognosis in patients treated with ABVD or equivalent regimens. Blood. 2002;100(12):3935–41. doi: 10.1182/blood.V100.12.3935. [DOI] [PubMed] [Google Scholar]
- 23.Sup SJ, Alemañy CA, Pohlman B, et al. Expression of bcl-2 in classical Hodgkin’s lymphoma: an independent predictor of poor outcome. J Clin Oncol. 2005;23(16):3773–9. doi: 10.1200/JCO.2005.04.358. [DOI] [PubMed] [Google Scholar]
- 24.Smolewski P, Robak T, Krykowski E, et al. Prognostic factors in Hodgkin’s disease: multivariate analysis of 327 patients from a single institution. Clin Cancer Res. 2000;6(3):1150–60. [PubMed] [Google Scholar]
- 25.Gause A, Pohl C, Tschiersch A, et al. Clinical significance of soluble CD30 antigen in the sera of patients with untreated Hodgkin’s disease. Blood. 1991;77(9):1983–8. [PubMed] [Google Scholar]
- 26.Axdorph U, Sjöberg J, Grimfors G, et al. Biological markers may add to prediction of outcome achieved by the International Prognostic Score in Hodgkin’s disease. Ann Oncol. 2000;11(11):1405–11. doi: 10.1023/a:1026551727795. [DOI] [PubMed] [Google Scholar]
- 27.Zanotti R, Trolese A, Ambrosetti A, et al. Serum levels of soluble CD30 improve International Prognostic Score in predicting the outcome of advanced Hodgkin’s lymphoma. Ann Oncol. 2002;13(12):1908–14. doi: 10.1093/annonc/mdf333. [DOI] [PubMed] [Google Scholar]
- 28.Visco C, Nadali G, Vassilakopoulos TP, et al. Very high levels of soluble CD30 recognize the patients with classical Hodgkin’s lymphoma retaining a very poor prognosis. Eur J Haematol. 2006;77(5):387–94. doi: 10.1111/j.1600-0609.2006.00725.x. [DOI] [PubMed] [Google Scholar]
- 29.Sarris AH, Kliche KO, Pethambaram P, et al. Interleukin-10 levels are often elevated in serum of adults with Hodgkin’s disease and are associated with inferior failure-free survival. Ann Oncol. 1999;10(4):433–40. doi: 10.1023/a:1008301602785. [DOI] [PubMed] [Google Scholar]
- 30.Viviani S, Notti P, Bonfante V, Verderio P, Valagussa P, Bonadonna G. Elevated pretreatment serum levels of Il-10 are associated with a poor prognosis in Hodgkin’s disease, the milan cancer institute experience. Med Oncol. 2000;17(1):59–63. doi: 10.1007/BF02826218. [DOI] [PubMed] [Google Scholar]
- 31.Vassilakopoulos TP, Nadali G, Angelopoulou MK, et al. Serum interleukin-10 levels are an independent prognostic factor for patients with Hodgkin’s lymphoma. Haematologica. 2001;86(3):274–81. [PubMed] [Google Scholar]
- 32.Rautert R, Schinköthe T, Franklin J, et al. Elevated pretreatment interleukin-10 serum level is an International Prognostic Score (IPS)-independent risk factor for early treatment failure in advanced stage Hodgkin lymphoma. Leuk Lymphoma. 2008;49(11):2091–8. doi: 10.1080/10428190802441339. [DOI] [PubMed] [Google Scholar]
- 33.Peh SC, Kim LH, Poppema S. TARC, a CC chemokine, is frequently expressed in classic Hodgkin’s lymphoma but not in NLP Hodgkin’s lymphoma, T-cell-rich B-cell lymphoma, and most cases of anaplastic large cell lymphoma. Am J Surg Pathol. 2001;25(7):925–9. doi: 10.1097/00000478-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Niens M, Visser L, Nolte IM, et al. Serum chemokine levels in Hodgkin lymphoma patients: highly increased levels of CCL17 and CCL22. Br J Haematol. 2008;140(5):527–36. doi: 10.1111/j.1365-2141.2007.06964.x. [DOI] [PubMed] [Google Scholar]
- 35.Weihrauch MR, Manzke O, Beyer M, et al. Elevated serum levels of CC thymus and activation-related chemokine (TARC) in primary Hodgkin’s disease: potential for a prognostic factor. Cancer Res. 2005;65(13):5516–9. doi: 10.1158/0008-5472.CAN-05-0100. 1. [DOI] [PubMed] [Google Scholar]
- 36.Hnátková M, Mociková H, Trnený M, Zivný J. The biological environment of Hodgkin’s lymphoma and the role of the chemokine CCL17/TARC. Prague Med Rep. 2009;110(1):35–41. [PubMed] [Google Scholar]
- 37.Specht L, Gray RG, Clarke MJ, Peto R. Influence of more extensive radiotherapy and adjuvant chemotherapy on long-term outcome of early-stage Hodgkin’s disease: a meta-analysis of 23 randomized trials involving 3,888 patients. International Hodgkin’s Disease Collaborative Group. J Clin Oncol. 1998;16(3):830–43. doi: 10.1200/JCO.1998.16.3.830. [DOI] [PubMed] [Google Scholar]
- 38.Bonadonna G, Bonfante V, Viviani S, Di Russo A, Villani F, Valagussa P. ABVD plus subtotal nodal versus involved-field radiotherapy in early-stage Hodgkin’s disease: long-term results. J Clin Oncol. 2004;22(14):2835–41. doi: 10.1200/JCO.2004.12.170. [DOI] [PubMed] [Google Scholar]
- 39.Engert A, Franklin J, Eich HT, et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended-field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin’s lymphoma: final results of the GHSG HD7 trial. J Clin Oncol. 2007;25(23):3495–502. doi: 10.1200/JCO.2006.07.0482. [DOI] [PubMed] [Google Scholar]
- 40.Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21(19):3601–8. doi: 10.1200/JCO.2003.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Fermé C, Eghbali H, Meerwaldt JH, et al. EORTC-GELA H8 Trial. Chemotherapy plus involved-field radiation in early-stage Hodgkin’s disease. N Engl J Med. 2007;357(19):1916–27. doi: 10.1056/NEJMoa064601. [DOI] [PubMed] [Google Scholar]
- 42.Engert A, Diehl V, Pluettschow A, et al. Two cycles of ABVD followed by involved field radiotherapy with 20 gray (Gy) is the new standard of care in the treatment of patients with early-stage Hodgkin lymphoma: Final analysis of the randomized German Hodgkin Study Group (GHSG) HD10. Blood (ASH abstract) 2009;114 abst. 716. [Google Scholar]
- 43.Borchmann P, Diehl V, Goergen H, et al. Dacarbazine is an essential component of ABVD in the treatment of early favourable Hodgkin lymphoma: results of the second interim analysis of the GHSG HD13 trial. Haematologica. 2010;95(suppl.2):473. abs. 1146. [Google Scholar]
- 44.Borchmann P, Diehl V, Goergen H, et al. Combined modality treatment with intensified chemotherapy and dose-reduced involved field radiotherapy in patients with early unfavourable Hodgkin lymphoma (HL): Final analysis of the German Hodgkin Study Group (GHSG) HD11 trial. Blood (ASH Abstracts) 2009;114 doi: 10.1200/JCO.2010.29.8018. abs. 717. [DOI] [PubMed] [Google Scholar]
- 45.Advani RH, Hoppe RT, Baer DM, et al. Efficacy of abbreviated Stanford V chemotherapy and involved field radiotherapy in early stage Hodgkin’s disease: mature results of the G4 trial. Blood (ASH abstr) 2009;114:1670. doi: 10.1093/annonc/mds542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer RM, Gospodarowicz MK, Connors JM, et al. National Cancer Institute of Canada Clinical Trials Group; Eastern Cooperative Oncology Group Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited-stage Hodgkin’s lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23(21):4634–42. doi: 10.1200/JCO.2005.09.085. [DOI] [PubMed] [Google Scholar]
- 47.Specht L. 2-[18F]fluoro-2-deoxyglucose positron-emission tomography in staging, response evaluation, and treatment planning of lymphomas. Semin Radiat Oncol. 2007;17(3):190–7. doi: 10.1016/j.semradonc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Pommier P, Dussart S, Girinsky T, et al. Impact of 18F-Fluoro-2-deoxyglucose Positron Emission Tomography on Treatment Strategy and Radiotherapy Planning for Stage I-II Hodgkin Disease: A Prospective Multicenter Study. Int J Radiat Oncol Biol Phys. 2010 May 7; doi: 10.1016/j.ijrobp.2009.11.048. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Devita VT, Jr, Serpick AA, Carbone PP. Combination chemotherapy in the treatment of advanced Hodgkin’s disease. Ann Intern Med. 1970;73(6):881–95. doi: 10.7326/0003-4819-73-6-881. [DOI] [PubMed] [Google Scholar]
- 50.Longo DL, Young RC, Wesley M, et al. Twenty years of MOPP therapy for Hodgkin’s disease. J Clin Oncol. 1986;4(9):1295–306. doi: 10.1200/JCO.1986.4.9.1295. [DOI] [PubMed] [Google Scholar]
- 51.Viviani S, et al. Gonadal toxicity after combination chemotherapy for Hodgkin’s disease. Comparative results of MOPP vs ABVD. Eur J Cancer Clin Oncol. 1985;21(5):601–5. doi: 10.1016/0277-5379(85)90088-4. [DOI] [PubMed] [Google Scholar]
- 52.Valagussa P, et al. Secondary acute leukemia and other malignancies following treatment for Hodgkin’s disease. J Clin Oncol. 1986;4(6):830–7. doi: 10.1200/JCO.1986.4.6.830. [DOI] [PubMed] [Google Scholar]
- 53.Bonadonna G, et al. Combination chemotherapy of Hodgkin’s disease with adriamycin, bleomycin, vinblastine, and imidazole carboxamide versus MOPP. Cancer. 1975;36(1):252–9. doi: 10.1002/1097-0142(197507)36:1<252::aid-cncr2820360128>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 54.Santoro A, Bonadonna G, Valagussa P, et al. Long-term results of combined chemotherapy-radiotherapy approach in Hodgkin’s disease: superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. J Clin Oncol. 1987;5(1):27–37. doi: 10.1200/JCO.1987.5.1.27. [DOI] [PubMed] [Google Scholar]
- 55.Bonadonna G, Valagussa P, Santoro A. Alternating non-cross-resistant combination chemotherapy or MOPP in stage IV Hodgkin’s disease. A report of 8-year results. Ann Intern Med. 1986;104(6):739–46. doi: 10.7326/0003-4819-104-6-739. [DOI] [PubMed] [Google Scholar]
- 56.Klimo P, Connors JM. MOPP/ABV hybrid program: combination chemotherapy based on early introduction of seven effective drugs for advanced Hodgkin’s disease. J Clin Oncol. 1985;3(9):1174–82. doi: 10.1200/JCO.1985.3.9.1174. [DOI] [PubMed] [Google Scholar]
- 57.Glick JH, Young ML, Harrington D, et al. MOPP/ABV hybrid chemotherapy for advanced Hodgkin’s disease significantly improves failure-free and overall survival: the 8-year results of the intergroup trial. J Clin Oncol. 1998;16(1):19–26. doi: 10.1200/JCO.1998.16.1.19. [DOI] [PubMed] [Google Scholar]
- 58.Canellos GP, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327(21):1478–84. doi: 10.1056/NEJM199211193272102. [DOI] [PubMed] [Google Scholar]
- 59.Connors JM, et al. Treatment of advanced Hodgkin’s disease with chemotherapy – comparison of MOPP/ABV hybrid regimen with alternating courses of MOPP and ABVD: a report from the National Cancer Institute of Canada clinical trials group. J Clin Oncol. 1997;15(4):1638–45. doi: 10.1200/JCO.1997.15.4.1638. [DOI] [PubMed] [Google Scholar]
- 60.Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: report of an intergroup trial. J Clin Oncol. 2003;21(4):607–14. doi: 10.1200/JCO.2003.12.086. [DOI] [PubMed] [Google Scholar]
- 61.Hasenclever D, Loeffler M, Diehl V. Rationale for dose escalation of first line conventional chemotherapy in advanced Hodgkin’s disease. German Hodgkin’s Lymphoma Study Group. Ann Oncol. 1996;7(Suppl 4):95–8. doi: 10.1093/annonc/7.suppl_4.s95. [DOI] [PubMed] [Google Scholar]
- 62.Diehl V, Sieber M, Rüffer U, et al. BEACOPP: an intensified chemotherapy regimen in advanced Hodgkin’s disease. The German Hodgkin’s Lymphoma Study Group. Ann Oncol. 1997;8(2):143–8. doi: 10.1023/a:1008294312741. [DOI] [PubMed] [Google Scholar]
- 63.Tesch H, et al. Moderate dose escalation for advanced stage Hodgkin’s disease using the bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone scheme and adjuvant radiotherapy: a study of the German Hodgkin’s Lymphoma Study Group. Blood. 1998;92(12):4560–7. [PubMed] [Google Scholar]
- 64.Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003;348(24):2386–95. doi: 10.1056/NEJMoa022473. [DOI] [PubMed] [Google Scholar]
- 65.Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27(27):4548–54. doi: 10.1200/JCO.2008.19.8820. [DOI] [PubMed] [Google Scholar]
- 66.Federico M, Luminari S, Iannitto E, et al. HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin’s lymphoma: results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol. 2009;27(5):805–11. doi: 10.1200/JCO.2008.17.0910. [DOI] [PubMed] [Google Scholar]
- 67.Gianni AM, Rambaldi A, Zinzani P, et al. Comparable 3-year outcome following ABVD or BEACOPP first-line chemotherapy, plus pre-planned high-dose salvage, in advanced Hodgkin lymphoma: A randomized trial of the Michelangelo, GITIL and IIL cooperative groups. J Clin Oncol. 2008 May 20;26(suppl) abstr 8506. [Google Scholar]
- 68.Bartlett NL, Rosenberg SA, Hoppe RT, Hancock SL, Horning SJ. Brief chemotherapy, Stanford V, and adjuvant radiotherapy for bulky or advanced-stage Hodgkin’s disease: a preliminary report. J Clin Oncol. 1995;13(5):1080–8. doi: 10.1200/JCO.1995.13.5.1080. [DOI] [PubMed] [Google Scholar]
- 69.Horning SJ, Hoppe RT, Breslin S, et al. Stanford V and radiotherapy for locally extensive and advanced Hodgkin’s disease: Mature results of a prospective clinical trial. J Clin Oncol. 2002;20:630–637. doi: 10.1200/JCO.2002.20.3.630. [DOI] [PubMed] [Google Scholar]
- 70.Gobbi PG, Levis A, Chisesi T, Levis A, Chisesi T, et al. ABVD versus modified Stanford V versus MOPPEBVCAD with optional and limited radiotherapy in intermediate-and advanced-stage Hodgkin’s lymphoma: final results of a mulitcenter randomized trial by the Intergruppo Italiano Linfomi. J Clin Oncol. 2005;23(36):9198–207. doi: 10.1200/JCO.2005.02.907. [DOI] [PubMed] [Google Scholar]
- 71.Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the stanford V regimen and ABVD in the treatment of advanced Hodgkin’s Lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol. 2009;27(32):5390–6. doi: 10.1200/JCO.2009.23.3239. [DOI] [PubMed] [Google Scholar]
- 72.Federico M, Bellei M, Brice P, et al. High-dose therapy and autologous stem-cell transplantation versus conventional therapy for patients with advanced Hodgkin’s lymphoma responding to front-line therapy. J Clin Oncol. 2003;21(12):2320–5. doi: 10.1200/JCO.2003.11.103. [DOI] [PubMed] [Google Scholar]
- 73.Proctor SJ, Mackie M, Dawson A, et al. A population-based study of intensive multi-agent chemotherapy with or without autotransplant for the highest risk Hodgkin’s disease patients identified by the Scotland and Newcastle Lymphoma Group (SNLG) prognostic index. A Scotland and Newcastle Lymphoma Group study (SNLG HD III) Eur J Cancer. 2002;38(6):795–806. doi: 10.1016/s0959-8049(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 74.Nogová L, Reineke T, Brillant C, et al. Lymphocyte-predominant and classical Hodgkin’s lymphoma: A comprehensive analysis from the German Hodgkin Study Group. J Clin Oncol. 2008;26:434–39. doi: 10.1200/JCO.2007.11.8869. [DOI] [PubMed] [Google Scholar]
- 75.Diehl V, Sextro M, Franklin J, et al. Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin’s disease and lymphocyte-rich classical Hodgkin’s disease: Report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin’s Disease. J Clin Oncol. 1999;17:776–83. doi: 10.1200/JCO.1999.17.3.776. [DOI] [PubMed] [Google Scholar]
- 76.Quddus F, Armitage JO. Salvage therapy for Hodgkin’s lymphoma. Cancer J. 2009;15(2):161–3. doi: 10.1097/PPO.0b013e3181a1438a. [DOI] [PubMed] [Google Scholar]
- 77.Longo DL, Duffey PL, Young RC, et al. Conventional-dose salvage combination chemotherapy in patients relapsing with Hodgkin’s disease after combination chemotherapy: the low probability for cure. J Clin Oncol. 1992;10(2):210–8. doi: 10.1200/JCO.1992.10.2.210. [DOI] [PubMed] [Google Scholar]
- 78.Josting A, Franklin J, May M, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin’s lymphoma registered in the database of the German Hodgkin’s lymphoma study group. J Clin Oncol. 2002;20(1):221–30. doi: 10.1200/JCO.2002.20.1.221. [DOI] [PubMed] [Google Scholar]
- 79.Brice P, Bouabdallah R, Moreau P, et al. Prognostic factors for survival after high-dose therapy and autologous stem cell transplantation for patients with relapsing Hodgkin’s disease: analysis of 280 patients from the French registry. Société Française de Greffe de Moëlle. Bone Marrow Transplant. 1997;20(1):21–6. doi: 10.1038/sj.bmt.1700838. [DOI] [PubMed] [Google Scholar]
- 80.Josting A, Rudolph C, Mapara M, et al. Cologne high-dose sequential chemotherapy in relapsed and refractory Hodgkin lymphoma: results of a large multicenter study of the German Hodgkin Lymphoma Study Group (GHSG) Ann Oncol. 2005;16(1):116–23. doi: 10.1093/annonc/mdi003. [DOI] [PubMed] [Google Scholar]
- 81.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97(3):616–23. doi: 10.1182/blood.v97.3.616. [DOI] [PubMed] [Google Scholar]
- 82.Mendler JH, Friedberg JW. Salvage therapy in Hodgkin’s lymphoma. The Oncologist. 2009;14(4):425–32. doi: 10.1634/theoncologist.2009-0002. [DOI] [PubMed] [Google Scholar]
- 83.Sirohi B, Cunningham D, Powles R, et al. Long-term outcome of autologous stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma. Ann Oncol. 2008;19(7):1312–9. doi: 10.1093/annonc/mdn052. [DOI] [PubMed] [Google Scholar]
- 84.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341(8852):1051–4. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 85.Schmitz N, Pfistner B, Sextro M, et al. German Hodgkin’s Lymphoma Study Group; Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065–71. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 86.Fermé C, Mounier N, Diviné M, et al. Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin’s disease in relapse or failure after initial chemotherapy: results of the Groupe d’Etudes des Lymphomes de l’Adulte H89 Trial. J Clin Oncol. 2002;20(2):467–75. doi: 10.1200/JCO.2002.20.2.467. [DOI] [PubMed] [Google Scholar]
- 87.Horning SJ, Chao NJ, Negrin RS, et al. High-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin’s disease: analysis of the Stanford University results and prognostic indices. Blood. 1997;89(3):801–13. [PubMed] [Google Scholar]
- 88.Sweetenham JW, Carella AM, Taghipour G, et al. High-dose therapy and autologous stem-cell transplantation for adult patients with Hodgkin’s disease who do not enter remission after induction chemotherapy: results in 175 patients reported to the European Group for Blood and Marrow Transplantation. Lymphoma Working Party. J Clin Oncol. 1999;17(10):3101–9. doi: 10.1200/JCO.1999.17.10.3101. [DOI] [PubMed] [Google Scholar]
- 89.Constans M, Sureda A, Terol MJ, et al. Autologous stem cell transplantation for primary refractory Hodgkin’s disease: results and clinical variables affecting outcome. Ann Oncol. 2003;14(5):745–51. doi: 10.1093/annonc/mdg206. [DOI] [PubMed] [Google Scholar]
- 90.Chopra R, McMillan AK, Linch DC, et al. The place of high-dose BEAM therapy and autologous bone marrow transplantation in poor-risk Hodgkin’s disease. A single-center eight-year study of 155 patients. Blood. 1993;81(5):1137–45. [PubMed] [Google Scholar]
- 91.Stiff PJ, Unger JM, Forman SJ, et al. The value of augmented preparative regimens combined with an autologous bone marrow transplant for the management of relapsed or refractory Hodgkin disease: a Southwest Oncology Group phase II trial. Biol Blood Marrow Transplant. 2003;9(8):529–39. doi: 10.1016/s1083-8791(03)00205-2. [DOI] [PubMed] [Google Scholar]
- 92.Laport GG. Allogeneic hematopoietic cell transplantation for Hodgkin lymphoma: a concise review. Leuk Lymphoma. 2008;49(10):1854–9. doi: 10.1080/10428190802304974. [DOI] [PubMed] [Google Scholar]
- 93.Akpek G, Ambinder RF, Piantadosi S, et al. Long-term results of blood and marrow transplantation for Hodgkin’s lymphoma. J Clin Oncol. 2001;19(23):4314–21. doi: 10.1200/JCO.2001.19.23.4314. [DOI] [PubMed] [Google Scholar]
- 94.Milpied N, Fielding AK, Pearce RM, et al. Allogeneic bone marrow transplant is not better than autologous transplant for patients with relapsed Hodgkin’s disease. European Group for Blood and Bone Marrow Transplantation. J Clin Oncol. 1996;14:1291–6. doi: 10.1200/JCO.1996.14.4.1291. [DOI] [PubMed] [Google Scholar]
- 95.Sureda A, Robinson S, Canals C, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26(3):455–62. doi: 10.1200/JCO.2007.13.2415. [DOI] [PubMed] [Google Scholar]
- 96.Sarina B, Castagna L, Farina L, et al. Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: a retrospective study based on the time of HLA typing and donor availability. Blood. 2010;115(18):3671–7. doi: 10.1182/blood-2009-12-253856. [DOI] [PubMed] [Google Scholar]
- 97.Devetten MP, Hari PN, Carreras J, et al. Unrelated donor reduced-intensity allogeneic hematopoietic stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2009;15(7):804–11. doi: 10.1016/j.bbmt.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Horie R, Watanabe T, Morishita Y, et al. Ligand-independent signaling by overexpressed CD30 drives NF-kappaB activation in Hodgkin-Reed-Sternberg cells. Oncogene. 2002;21(16):2493–503. doi: 10.1038/sj.onc.1205337. [DOI] [PubMed] [Google Scholar]
- 99.Zheng B, Fiumara P, Li YV, Georgakis G, et al. MEK/ERK pathway is aberrantly active in Hodgkin disease: a signaling pathway shared by CD30, CD40, and RANK that regulates cell proliferation and survival. Blood. 2003;102(3):1019–27. doi: 10.1182/blood-2002-11-3507. [DOI] [PubMed] [Google Scholar]
- 100.Foyil KV, Bartlett NL. Anti-CD30 Antibodies for Hodgkin Lymphoma. Curr Hematol Malig Rep. 2010;5(3):140–7. doi: 10.1007/s11899-010-0053-y. [DOI] [PubMed] [Google Scholar]
- 101.Forero-Torres A, Leonard JP, Younes A, et al. A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol. 2009;146(2):171–9. doi: 10.1111/j.1365-2141.2009.07740.x. [DOI] [PubMed] [Google Scholar]
- 102.Ansell SM, Horwitz SM, Engert A, et al. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin’s lymphoma and anaplastic large-cell lymphoma. J Clin Oncol. 2007;25(19):2764–9. doi: 10.1200/JCO.2006.07.8972. [DOI] [PubMed] [Google Scholar]
- 103.Blum KA, Jung SH, Johnson JL, et al. for the Cancer and Leukemia Group B Serious pulmonary toxicity in patients with Hodgkin’s lymphoma with SGN-30, gemcitabine, vinorelbine, and liposomal doxorubicin is associated with an Fc{gamma}RIIIa- 158 V/F polymorphism. Ann Oncol. 2010 Apr 27; doi: 10.1093/annonc/mdq211. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dietlein M, Börner SM, Fischer T, et al. Development of anti-CD30 radioimmunoconstructs (RICs) for treatment of Hodgkin’s lymphoma. Studies with cell lines and animal studies. Nuklearmedizin. 2010;49(3):97–105. doi: 10.3413/nukmed-0258. [DOI] [PubMed] [Google Scholar]
- 105.Fanale M, Bartlett NL, Forero-Torres A, et al. The Antibody-Drug Conjugate Brentuximab Vedotin (SGN-35) Induced Multiple Objective Responses in Patients with Relapsed or Refractory CD30-Positive Lymphomas in a Phase 1 Weekly Dosing Study. Blood (ASH Annual Meeting Abstracts) 2009;114 Abstract 2731. [Google Scholar]
- 106.Younes A, Romaguera J, Hagemeister F, et al. A pilot study of rituximab in patients with recurrent, classic Hodgkin disease. Cancer. 2003;98(2):310–4. doi: 10.1002/cncr.11511. [DOI] [PubMed] [Google Scholar]
- 107.Oki Y, Pro B, Fayad LE, et al. Phase 2 study of gemcitabine in combination with rituximab in patients with recurrent or refractory Hodgkin lymphoma. Cancer. 2008;112(4):831–6. doi: 10.1002/cncr.23237. [DOI] [PubMed] [Google Scholar]
- 108.Schulz H, Rehwald U, Morschhauser F, et al. Rituximab in relapsed lymphocyte-predominant Hodgkin lymphoma: long-term results of a phase 2 trial by the German Hodgkin Lymphoma Study Group (GHSG) Blood. 2008;111(1):109–11. doi: 10.1182/blood-2007-03-078725. [DOI] [PubMed] [Google Scholar]
- 109.Horning SJ, Bartlett NL, Breslin S, et al. Results of a prospective phase II trial of limited and extended Rituximab treatment in Nodular Lymphocyte Predominant Hodgkin’s Disease (NLPHD) Blood (ASH Annual Meeting Abstracts) 2007;110 Abstract 644. [Google Scholar]
- 110.Bollard CM, Straathof KC, Huls MH, et al. The generation and characterization of LMP2-specific CTLs for use as adoptive transfer from patients with relapsed EBV-positive Hodgkin disease. J Immunother. 2004;27(4):317–27. doi: 10.1097/00002371-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 111.Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin’s disease treated at age 50 or younger. J Clin Oncol. 2002;20(8):2101–8. doi: 10.1200/JCO.2002.08.021. [DOI] [PubMed] [Google Scholar]
- 112.Hancock SL, Hoppe RT, Horning SJ, Rosenberg SA. Intercurrent death after Hodgkin disease therapy in radiotherapy and adjuvant MOPP trials. Ann Intern Med. 1988;109(3):183–9. doi: 10.7326/0003-4819-109-3-183. [DOI] [PubMed] [Google Scholar]
- 113.Moskowitz CH, Zelenetz A, Schoder H. An update on the role of interim restaging FDG-PET in patients with diffuse large B-cell lymphoma and Hodgkin lymphoma. J Natl Compr Canc Netw. 2010;8(3):347–52. doi: 10.6004/jnccn.2010.0023. [DOI] [PubMed] [Google Scholar]
- 114.Pazianas M. Macrophages in Hodgkin’s lymphoma. N Engl J Med. 2010;362(22):2135. [PubMed] [Google Scholar]
- 115.Pfreundschuh MG, Rueffer U, Lathan B, et al. Dexa-BEAM in patients with Hodgkin’s disease refractory to multidrug chemotherapy regimens: a trial of the German Hodgkin’s Disease Study Group. J Clin Oncol. 1994;12(3):580–6. doi: 10.1200/JCO.1994.12.3.580. [DOI] [PubMed] [Google Scholar]
- 116.Martín A, Fernández-Jiménez MC, et al. Long-term follow-up in patients treated with Mini-BEAM as salvage therapy for relapsed or refractory Hodgkin’s disease. Br J Haematol. 2001;113(1):161–71. doi: 10.1046/j.1365-2141.2001.02714.x. [DOI] [PubMed] [Google Scholar]
- 117.Rodriguez J, Rodriguez MA, Fayad L, et al. ASHAP: a regimen for cytoreduction of refractory or recurrent Hodgkin’s disease. Blood. 1999;93(11):3632–6. [PubMed] [Google Scholar]
- 118.Santoro A, Magagnoli M, Spina M, et al. Ifosfamide, gemcitabine, and vinorelbine: a new induction regimen for refractory and relapsed Hodgkin’s lymphoma. Haematologica. 2007;92(1):35–41. doi: 10.3324/haematol.10661. [DOI] [PubMed] [Google Scholar]
- 119.Bartlett NL, Niedzwiecki D, Johnson JL, et al. Cancer Leukemia Group B. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin’s lymphoma: CALGB 59804. Ann Oncol. 2007;18(6):1071–9. doi: 10.1093/annonc/mdm090. [DOI] [PubMed] [Google Scholar]
- 120.Kuruvilla J, Nagy T, Pintilie M, Tsang R, Keating A, Crump M. Similar response rates and superior early progression-free survival with gemcitabine, dexamethasone, and cisplatin salvage therapy compared with carmustine, etoposide, cytarabine, and melphalan salvage therapy prior to autologous stem cell transplantation for recurrent or refractory Hodgkin lymphoma. Cancer. 2006;106(2):353–60. doi: 10.1002/cncr.21587. [DOI] [PubMed] [Google Scholar]