Abstract
Mutations of LAMB2 typically cause autosomal recessive Pierson syndrome, a disorder characterized by congenital nephrotic syndrome, ocular and neurologic abnormalities, but may occasionally be associated with milder or oligosymptomatic disease variants. LAMB2 encodes the basement membrane protein laminin β2 which is incorporated in specific heterotrimeric laminin isoforms and has an expression pattern corresponding to the pattern of organ manifestations in Pierson syndrome. Herein we review all previously reported and several novel LAMB2 mutations in relation to the associated phenotype in patients from 39 unrelated families. The majority of disease-causing LAMB2 mutations are truncating, consistent with the hypothesis that loss of laminin β2 function is the molecular basis of Pierson syndrome. While truncating mutations are distributed across the entire gene, missense mutations are clearly clustered in the N-terminal LN domain, which is important for intermolecular interactions. There is an association of missense mutations and small in frame deletions with a higher mean age at onset of renal disease and with absence of neurologic abnormalities, thus suggesting that at least some of these may represent hypomorphic alleles. Nevertheless, genotype alone does not appear to explain the full range of clinical variability, and therefore hitherto unidentified modifiers are likely to exist.
Keywords: LAMB2, Pierson syndrome, nephrotic syndrome, autosomal recessive, podocyte, laminin, ocular malformation
BACKGROUND
Mutations of LAMB2 (MIM# 150325), the gene encoding laminin β2, were first detected in patients who suffered from congenital nephrotic syndrome (NS) histologically presenting as diffuse mesangial sclerosis, in combination with a complex ocular maldevelopment the most impressing clinical sign of which is extreme and fixed narrowing of the pupils (microcoria) (Zenker et al., 2004a). This unusual association was first described by Pierson et al. in 1963 (Pierson et al., 1963), and therefore the term Pierson syndrome was coined for this disorder (MIM# 609049) (Zenker et al., 2004b). Microcoria-congenital nephrosis syndrome is a synonym. Patients with Pierson syndrome are also at risk for severe neurodevelopmental deficits including congenital muscular weakness/myasthenia and developmental retardation (Maselli et al., 2009; Wuhl et al., 2007). The clinical manifestations in Pierson syndrome correspond well to the defects observed in mice deficient of laminin β2, who display severe glomerular kidney disease, ocular and neurologic abnormalities (Miner et al., 2006; Noakes et al., 1995a; Noakes et al., 1995b).
Laminins represent a group of cross-shaped heterotrimeric proteins each consisting of α, β and γ subunits joined together through a coiled coil. Laminins are indispensable basement membranes constituents with important roles in cell adhesion, proliferation, differentiation and migration (Miner and Yurchenco, 2004; Ryan et al., 1996; Tunggal et al., 2000). Laminin-521 (consisting of α5, β2, and γ1 subunits; formerly called laminin-11) is the most common laminin isoform that contains a β2-chain (Miner and Patton, 1999). This isoform is specifically expressed at distinct sites such as the glomerular basement membrane, various ocular structures, and the neuromuscular system, consistent with the pattern of organ involvement in Pierson syndrome (Zenker et al., 2004a).
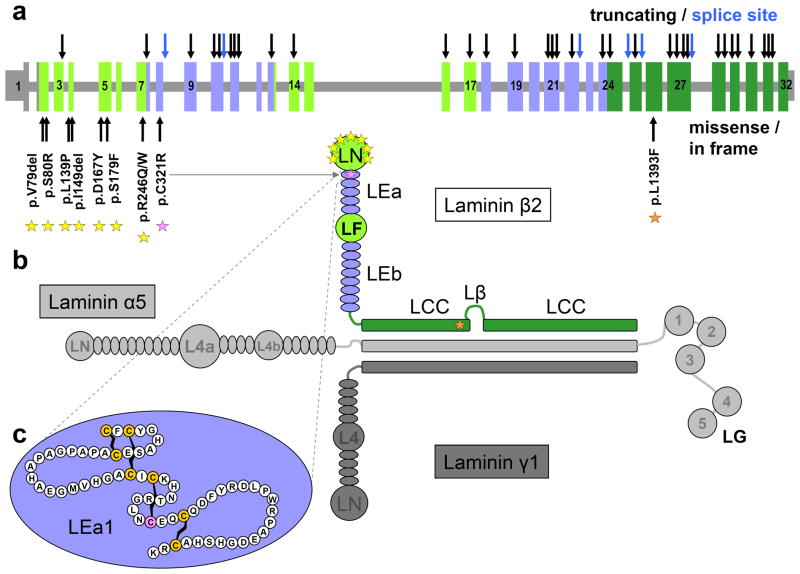
The human LAMB2 gene maps to chromosome band 3p21 and is composed of 32 densely packed exons spanning about 12 kb of genomic DNA (Fig. 1a). The gene encodes a protein of 1,798 amino acids, containing the typical laminin domains: the N-terminal globular laminin domain (LN) making interactions with neighboring laminins, multiple EGF-like repeats (LE) with an interjacent second globular domain (LF) whose function is currently unknown, and a coiled coil domain (LCC) (Fig. 1b). The most N-terminal 32 amino acids represent a cleavable signal peptide.
Figure 1.
LAMB2 gene, laminin β2 protein domains, and distribution of known mutations. a, Genomic representation of the human LAMB2 gene. Vertical bars represent exons. Untranslated regions are shown in reduced height. Coloring corresponds to functional protein domains with grey color in parts of exon 1 and 2 encoding the signal peptide. Numerals within the boxes indicate exon numbers. Location of truncating mutations (nonsense, frameshift) and splice site mutations are indicated by arrows on top of the cartoon in black and blue, respectively. Positions of missense mutations and small in frame deletions are indicated by arrows at the bottom of the cartoon and show obvious clustering in exons 2 to 7. b, In its biologically active form laminin β2 is part of a heterotrimeric complex shown here exemplarily as laminin-521. Laminin α5 and γ1 chains are depicted in light and dark grey, respectively. Coloring of the human laminin β2 chain corresponds to functional protein domains: LN, laminin N-terminal globular domain; LEa/b, laminin EGF-like modules; LF, domain IV, globular domain; LCC, laminin coiled coil domain; Lβ, Laminin β loop; LG, C-terminal globular modules (belonging to α chain). Positions of non-truncating mutations are indicated by asterisks. Most of them affect the LN domain (yellow asterisks), while one is located in the first EGF-like module (pink) and another one in the LCC domain (orange). c, The first EGF-like module (LEa1) is shown in detail with letters corresponding to the one letter amino acid code. The highly conserved cysteine residues are highlighted and connecting lines indicate disulfide bonds. The mutated Cys-321 is shown in pink.
Although Pierson syndrome was not recognized as a separate entity before 2004, several reports on single cases and a few patient series have appeared in the years since then (Bredrup et al., 2008; Choi et al., 2008; Hasselbacher et al., 2006; Kagan et al., 2008; Maselli et al., 2009; Matejas et al., 2006; VanDeVoorde et al., 2006; Wuhl et al., 2007), indicating that this disease had likely been overlooked before. The current literature also includes some descriptions of milder variants of the disease as well as two observations of apparent isolated infantile NS caused by homozygous or compound heterozygous mutations of LAMB2 (Choi et al., 2008; Hasselbacher et al., 2006; Kagan et al., 2008; Matejas et al., 2006). Based on the finding of missense mutations or small in frame deletions at least on one allele in these cases in contrast to the predominance of biallelic truncating mutations in the classic Pierson syndrome, it has been proposed that the level of residual laminin β2 function/expression is the main modifier of the phenotype (Hasselbacher et al., 2006; Kagan et al., 2008). Herein we review a total of 49 mutations of LAMB2 including 12 novel ones, we summarize clinical findings and discuss genotype phenotype correlations.
VARIANTS IN THE LAMB2 GENE
All LAMB2 sequence changes published thus far, together with the novel variants characterized in our laboratories since 2004, are divided into three subgroups and listed in Tables 1, 3, and 4. The genotype and phenotype features of patients with LAMB2 mutations are listed in Table 2.
Table 1.
Mutations in the LAMB2 gene causing Pierson syndrome and milder disease variants*
| Exon/Intron | DNA Varianta | Predicted and/or | Family-ID | Previous Publications |
|---|---|---|---|---|
| Exon 2 | c.235_237delGTC | p.V79del Demonstrated Effect | 1 | Matejas et al. [2006] |
| Exon 2 | c.240T>G | p.S80R | 2 | This report |
| Exon 3 | c.373C>T | p.Q125X | 3 | Bredrup et al. [2008] |
| Exon 4 | c.416T>C | p.L139P | 4 | This report |
| Exon 4 | c.447_449delTAT | p.I149del | 5 | Bredrup et al. [2008] |
| Exon 5 | c.499G>T | p.D167Y | 6 | Kagan et al. [2008] |
| Exon 5 | c.536C>T | p.S179F | 7 | Choi et al. [2008] |
| Exon 7 | c.736C>T | p.R246W | 8, 9, 10, 11,12 | Zenker et al. [2004] |
| Exon 7 | c.737G>A | p.R246Q | 13 | Hasselbacher et al. [2006] |
| Exon 7 | c.825T>A | p.Y275X | 14 | This report |
| Exon 8 | c.961T>C | p.C321R | 15 | Hasselbacher et al. [2006] |
| Intron 8 | c.1036+6_9delTGAG | No mRNA studies | 16 | This report |
| Exon 9 | c.1122T>A | p.C374X | 17 | Zenker et al. [2005] |
| Exon 10 | c.1241_1242dupCC | p.M415PfsX83 | 18 | Wühl et al. [2007] |
| Exon 10 | c.1252C>T | p.Q418X | 18 | Wühl et al. [2007] |
| Intron 10 | c.1405+1G>A | p.S409X | 19, 20 | Bredrup et al. [2008] |
| Exon 11 | c.1477delT | p.C493AfsX4 | 21, 22 | Wühl et al. [2007] |
| Exon 11 | c.1478delG | p.C493SfsX4 | 23 | Maselli et al. [2009] |
| Exon 11 | c.1503_1504delAT | p.C502X | 24 | Choi et al. [2008] |
| Exon 13 | c.1723C>T | p.R575X | 9 | Bredrup et al. [2008] |
| Exon 14 | c.1875_1879delGCGCT | p.L627AfsX5 | 25 | Bredrup et al. [2008] |
| Exon 16 | c.2067C>G | p.Y689X | 17 | Zenker et al. [2005] |
| Exon 17 | c.2283_2286delCTCT | p.S762RfsX29 | 7 | Choi et al. [2008] |
| Exon 18 | c.2422delG | p.V808WfsX343 | 26 | Bredrup et al. [2008] |
| Exon 19 | c.2602C>T | p.Q868X | 27 | Bredrup et al. [2008] |
| Exon 21 | c.3015delG | p.Q1006NfsX145 | 28 | Zenker et al. [2004] |
| Exon 21 | c.3015dupG | p.Q1006AfsX49 | 29 | This report |
| Exon 21 | c.3094C>T | p.R1032X | 30 | Bredrup et al. [2008] |
| Exon 22 | c.3174_3175delTG | p.C1058X | 27, 31 | Bredrup et al. [2008] |
| Intron 22 | c.3327+2T>C | p.R1037LfsX18 | 21 | Wühl et al. [2007] |
| Exon 24 | c.3440dupC | p.R1148SfsX27 | 29 | This report |
| Exon 24 | c.3780_3781delGG | p.E1260DfsX8 | 19 | Bredrup et al. [2008] |
| Intron 24 | c.3798-2A>C | No mRNA studies | 31 | Bredrup et al. [2008] |
| Exon 25 | c.3902delA | p.E1301GfsX58 | 22 | Wühl et al. [2007] |
| Intron 25 | c.3982+1G>T | No mRNA studies | 32 | This report |
| Exon 26 | c.4177C>T | p.L1393F | 15 | Hasselbacher et al. [2006] |
| Exon 27 | c.4267delT | p.C1423VfsX29 | 24 | Choi et al. [2008] |
| Exon 27 | c.4504delA | p.R1502GfsX18 | 33, 25 | Zenker et al. [2004] |
| Exon 27 | c.4519C>T | p.Q1507X | 33 | Zenker et al. [2004] |
| Exon 27 | c.4534delC | p.L1512FfsX8 | 34 | This report |
| Intron 27 | c.4573+1G>A | No mRNA studies | 35 | This report |
| Exon 28 | c.4684C>T | p.R1562X | 36 | Zenker et al. [2004] |
| Exon 28 | c.4780dupA | p.R1594KfsX5 | 37 | This report |
| Exon 29 | c.4804delC | p.Q1602RfsX52 | 23 | Maselli et al. [2009] |
| Exon 29 | c.4907_4908delAG | p.E1636AfsX22 | 38 | This report |
| Exon 30 | c.5078delG | p.G1693VfsX21 | 5 | Bredrup et al. [2008] |
| Exon 31 | c.5182C>T | p.Q1728X | 1 | Matejas et al. [2006] |
| Exon 31 | c.5197C>T | p.Q1733X | 10 | This report |
| Exon 31 | c.5258dupA | p.E1754GfsX7 | 39 | Zenker et al. [2004] |
The numbering for the nucleotide changes are based on cDNA sequence in accordance with the GenBank entries NM_002292.3, NP_002283.3, and NT_022517.18 (Genome Reference Consortium Human Build 37). Novel mutations are printed in bold.
For cDNA numbering, +1 corresponds to the A of the ATG translation initiation codon in the reference sequence
Table 3.
Single nucleotide polymorphisms in the LAMB2 gene
| Exon/Intron | DNA varianta | Predicted and/or demonstrated effect on protein | Status | Protein domain |
|---|---|---|---|---|
| Exon 3 | c.306C>T | p.= | novel | LN |
| Intron 3 | c.250-97A>G | - | novel | - |
| Exon 8 | c.1014C>T | p.= | novel | LEa1 |
| Exon 14 | c.1764C>T | p.= | rs33942096 | LF |
| Intron 14 | c.1890+25G>A | - | rs9865051 | - |
| Exon 16 | c.2034T>C | p.= | novel | LF |
| Exon 17 | c.2307C>T | p.= | novel | LF |
| Intron 18 | c.2489-62C>T | - | novel | - |
| Exon 19 | c.2673C>T | p.= | novel | LEb3 |
| Exon 20 | c.2740G>A | p.G914R | rs35713889 | LEb3 |
| Exon 20 | c.2754G>T | p.= | novel | LEb3 |
| Exon 21 | c.2959G>A | p.E987K | rs34759087 | LEb5 |
| Intron 21 | c.3110-15T>C | - | novel | - |
| Intron 22 | c.3327+28T>C | - | novel | - |
| Intron 22 | c.3328-36T>G | - | novel | - |
| Exon 23 | c.3387A>G | p.= | rs34290943 | LEb7 |
| Exon 24 | c.3645G>A | p.= | rs13082063 | LCC |
| Exon 24 | c.3727G>C | p.G1243R | novel | LCC |
| Exon 25 | c.3858G>T | p.= | rs34967349 | LCC |
| Exon 26 | c.4140C>A | p.N1380K | novel | LCC |
| Intron 26 | c.4224+19G>A | - | novel | - |
| Intron 27 | c.4573+26A>G | - | novel | - |
| Intron 29 | c.4923+17A>G | - | novel | - |
| Intron 29 | c.4923+49G>A | - | novel | - |
| Intron 29 | c.4924-35G>A | - | rs72936885 | - |
| Exon 32 | c.5293G>A | p.A1765T | novel | LCC |
The numbering for the nucleotide changes are based on cDNA sequence in accordance with the GenBank entries NM_002292.3, NP_002283.3, and NT_022517.18 (GRCh37).
For cDNA numbering, +1 corresponds to the A of the ATG translation initiation codon in the reference sequence
Table 4.
LAMB2 sequence variants with unknown phenotypic effects
| Exon/Intron | DNA varianta | Predicted and/or demonstrated effect on protein | Status | Protein Domain |
|---|---|---|---|---|
| 5′UTR | c.-1925G>C | - | novel | - |
| 5′UTR | c.-404_408delTAGTT | - | novel | - |
| 5′UTR | c.-165C>A | - | novel | - |
| Exon 2 | c.109C>G | p.P37A | novel | Signal peptide cleavage site |
| Exon 9 | c.1193C>T | p.T398I | novel | LEa2 |
| Exon 10 | c.1403G>T | p.R468L | novel | LEa3 |
| Exon 13 | c.1724G>A | p.R575Q | novel | LF |
| Exon 16 | c.2099G>A | p.G700E | novel | LF |
| Exon 19 | c.2644C>T | p.H882Y | novel | LEb3 |
| Exon 22 | c.3155_3157delCTC | p.P1053del | novel | LEb6 |
| Exon 26 | c.4118A>G | p.D1373G | novel | LCC |
| Exon 27 | c.4370G>A | p.R1457Q | novel | LCC |
The numbering for the nucleotide changes are based on cDNA sequence in accordance with the GenBank entries NM_002292.3, NP_002283.3, and NT_022517.18 (GRCh37).
For cDNA numbering, +1 corresponds to the A of the ATG translation initiation codon in the reference sequence
Table 2.
Patients with LAMB2 mutations*: genotype and phenotypic features
| Family # | Patient | Genotypea | Total number of affected individuals |
Age of death (†)/ last follow-up |
Age at diagnosis of nephrotic proteinuria |
Onset of ESRD/Age at initiation of RRT |
Kidney biopsy result |
Microcoria | Other major ocular abnormalities |
Microcephaly | Neurodevelopmental deficits |
Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.1 | [p.V79del]+[p.Q1728X] | 7 | 20 y | NA | 4 y | NA | − | NA | − | - | Matejas et al. |
| 1.2 | 8y (†) | NA | 5 y | DMS | − | C, R, VI | − | - | ||||
| 1.3 | NA | 4.5 y | 16 y | FSGS | − | Mi, C, R, VI | − | - | ||||
| 2 | 2.1 | [p.S80R]+ [p.S80R] | 1 | 12 y | 6.5 | - | atypical DMS | − | My, R | − | - | This report |
| 3 | 3.1 | [p.Q125X] + ? | 1 | 2m (†) | 1 w | 2 w | DMS, MGN | + | L | + | H | Bredrup et al.[2008] |
| 4 | 4.1 | [p.L139P] + [p.L139P] | 3 | 12m (†) | 3 m | - | DMS? | + | L, VI | − | H, M, C | This report |
| 4.2 | 1.5w (†) | < 1 w | - | NA | − | VI | − | NA | ||||
| 4.3 | 5m (†) | < 1 w | - | NA | − | L, VI | − | H, M | ||||
| 5 | 5.1 | [p.I149del]+[p.G1693VfsX21] | 1 | 2m (†) | 1 w | < 1m | NA | + | VI | − | H | Bredrup et al.[2008] |
| 6 | 6.1 | [p.D167Y]+ [p.D167Y] | 2 | 3.7 y | 1 m | 19 m | MCD | − | My, R, VI | − | - | Kagan et al. [2008] |
| 7 | 7.1 | [p.S179F]+[p.S762RfsX29] | 1 | 1.5 y | 2 w | 1.5 y | FSGS | − | R, VI | − | - | Choi et al. [2008] |
| 8 | 8.1 | [p.R246W]+[p.R246W] | 2 | 8m (†) | 5 m | - | DMS | + | L | − | H, M | Zenker et al. [2004]2 |
| 8.2 | 8m (†) | 1 w | - | DMS | + | L | − | H. M | ||||
| 9 | 9.1 | [p.R246W]+[p.R575X] | 2 | 16 m | 1 w | 5 m | NA | + | L, VI | + | H, M, C | Bredrup et al.[2008] |
| 9.2 | 1y (†) | < 1 m | - | DMS | + | N, My | − | H, M, C | ||||
| 10 | 10.1 | [p.R246W]+[p.Q1733X] | 1 | 2 m | 1 m | - | NA | + | NA | − | NA | This report |
| 11 | 11.1 | [p.R246W]+[p.R246W ] | 1 | 19m (†) | 2 w | 13 m | FSGS | + | L | − | M | Bredrup et al.[2008] |
| 12 | 12.1 | [p.R246W]+[p.R246W] | 5 | 3m (†) | < 1 m | NA | NA | NA | N | − | NA | This report |
| 13 | 13.1 | [p.R246Q]+[p.R246Q] | 2 | 5y (†) | 1 m | < 12m | FSGS | − | - | − | - | Hasselbacher et al. [2006] |
| 13.2 | 3 y | 1 w | < 12 m | NA | − | - | − | - | ||||
| 14 | 14.1 | [p.Y275X]+[p.Y275X] | 2 | 1 m | < 1 w | < 1 w | DMS | + | VI | − | NA | This report |
| 15 | 15.1 | [p.C321R]+[p.L1393F] | 2 | 7 y | 3 m | 5.7 y | MCD | − | N, My, R, VI | − | - | Hasselbacher et al. [2006] |
| 15.2 | 3.5 y | 1 w | 8 m | O | (+) | R, VI | − | - | ||||
| 16 | 16.1 | [c.1036+6_9del]+[c.1036+6_9del] | 1 | 2 m | 7 w | 7 w | NA | + | Mi, R | − | M, S | This report |
| 17 | 17.1 | [p.C374X]+[p.Y689X] | 2 | 2w (†) | 1 w | 1 w | DMS | + | L | − | H | Zenker et al. [2005] |
| 17.2 | 1.5w (†) | 1 w | 1 w | DMS | + | L | − | NA | ||||
| 18 | 18.1 | [p.M415PfsX83]+[p.Q418X] | 1 | 15m (†) | < 1mo | 3 m | NA | + | Mi, L, VI | + | H, M, C | Wühl et al. [2007] |
| 19 | 19.1 | [c.1405+1G>A]+[p.E1260DfsX8] | 2 | 6w (†) | < 1w | 1 m | NA | + | L, VI | NA | NA | Bredrup et al.[2008] |
| 20 | 20.1 | [c.1405+1G>A]+[c.1405+1G>A] | 1 | 16m (†) | 1 w | 3 w | DMS | + | N, R, VI | − | H, M, C | Bredrup et al.[2008] |
| 21 | 21.1 | [p.C493AfsX4]+[c.3327+2T>C] | 1 | 8.3 y | 1.5 m | 2.9 y | DMS | + | My, L, VI | − | - | Wühl et al. [2007] |
| 22 | 22.1 | [p.C493AfsX4]+[p.E1301GfsX58] | 1 | 5 y | < 1m | < 1m | DMS | + | VI | − | H, M, C | Wühl et al. [2007] |
| 23 | 23.1 | [p.C493SfsX4]+[p.Q1602RfsX52] | 1 | 20 y | 1 w | 12 m | DMS | + | My | − | H, M | Maselli et al. [2009] |
| 24 | 24.1 | [p.C502X]+[p.C1423VfsX29] | 1 | 7.5 y | 8 m | - | MCD | + | N, My, VI | − | - | Choi et al. [2008] |
| 25 | 25.1 | [p.L627AfsX5]+[p.R1502GfsX18] | 2 | 4w (†) | 1 w | 2 w | DMS | + | L, Mi, VI | − | H | Bredrup et al.[2008] |
| 26 | 26.1 | [p.V808WfsX343]+[p.V808WfsX343] | 2 | 8m (†) | 1 w | 2 m | DMS | + | N | + | H, M, S | Bredrup et al.[2008] |
| 27 | 27.1 | [p.Q868X]+[p.C1058X] | 1 | 4.5m (†) | 2 w | 4 m | atypical DMS | + | N, VI | − | H | Bredrup et al.[2008] |
| 28 | 28.1 | [p.Q1006NfsX145]+[p.Q1006NfsX145] | 8 | 2–8 w | < 1m | < 1m | DMS | + | NA | NA | Zenker et al. [2004]1 | |
| 29 | 29.1 | [p.Q1006AfsX49]+[p.R1148SfsX27] | 1 | 18 y | 3 m | 3 y | DMS | − | VI (glaucoma) | − | - | This report |
| 30 | 30.1 | [p.R1032X]+[p.R1032X] | 1 | 4m (†) | 1 w | 1 m | DMS | + | L | + | NA | Bredrup et al.[2008] |
| 31 | 31.1 | [p.C1058X]+[c.3798-2A>C] | 1 | 10 y | 2 m | 3 m | NA | + | N, My, VI | − | - | Bredrup et al.[2008] |
| 32 | 32.1 | [c.3982+1G>T]+[c.3982+1G>T] | 2 | 5 y | < 3 y | - | DMS | + | L, N, Mi, VI | − | - | This report |
| 32.2 | 6m (†) | < 6 m | 6 m | NA | NA | NA | NA | NA | ||||
| 33 | 33.1 | [p.R1502GfsX18]+[p.Q1507X] | 1 | 2m (†) | 1w | 2 m | DMS | + | N, VI, L | − | NA | Zenker et al. [2004]2 |
| 34 | 34.1 | [p.L1512FfsX8]+[ p.L1512FfsX8] | 1 | 1m | < 1w | 2 w | DMS | + | - | − | - | This report |
| 35 | 35.1 | [c.4573+1G>A]+[c.4573+1G>A] | 3 | 5 y | < 1 w | - | NA | − | N, My, R | − | - | This report |
| 35.2 | 21 y | 3 y | 21 y | MGN | − | N, My, R | − | - | ||||
| 35.3 | 15 y | 5 y | 9 y | FSGS | − | N, My, R | − | - | ||||
| 36 | 36.1 | [p.R1562X]+[p.R1562X] | 3 | 1m (†) | < 1m | < 1m | DMS | + | L | H | Zenker et al. [2004]1 | |
| 37 | 37.1 | [p.R1594KfsX5]+[p.R1594KfsX5] | 1 | 1m (†) | < 1w | < 1m | NA | + | L | − | C | This report |
| 38 | 38.1 | [p.E1636AfsX22]+[p.E1636AfsX22] | 1 | 6w (†) | 1 w | < 1 m | DMS | + | L | − | H, M | This report |
| 39 | 39.1 | [p.E1754GfsX7]+[p.E1754GfsX7] | 1 | 19m (†) | < 1m | < 1m | DMS | + | L, N, VI | + | H, M | Zenker et al. [2004]2 |
The numbering for the nucleotide changes are based on cDNA sequence in accordance with the GenBank entries NM_002292.3, NP_002283.3, and NT_022517.18 (GRCh37).
For cDNA numbering, +1 corresponds to the A of the ATG translation initiation codon in the reference sequence y, year(s); m, month(s); w, week(s); NA, not done/not available; MCD, minimal changes; DMS, diffuse mesangial sclerosis; FSGS, focal and segmental glomerulosclerosis; MGN, membranous glomerulonephritis; O, other; +, present; −, not present/none; Mi, microphthalmia; My, high myopia (> 5 diopters); N, nystagmus; L, abnormal lens (either lenticonus or cataract); R, retinal detachment; VI, severe visual impairment of any cause and despite correcting glasses; H, significant hypotonia/muscular weakness/myasthenia; M, significant motor delay; C, suspected or proven cognitive deficits, speech delay; S, seizures; MR, mental retardaion
Mutations
Sequence changes regarded as disease-causing mutations are presented in Table 1. They comprise missense, nonsense, and splice site mutations, as well as small deletions and insertions, found either as homozygous or compound heterozygous sequence changes in patients affected by typical Pierson syndrome or its milder variants (Choi et al., 2008; Hinkes et al., 2007; Kagan et al., 2008; Maselli et al., 2009; Matejas et al., 2006; VanDeVoorde et al., 2006; Wuhl et al., 2007; Zenker et al., 2004a, 2005), as well as in two previously published siblings with isolated NS (Hasselbacher et al., 2006). The 12 novel mutations were identified by automated sequencing of PCR products of genomic DNA as described previously (Zenker et al., 2004a). All coding exons and flanking intronic regions were analyzed in each patient. Sequence changes were classified as causative mutations, if they produce a premature translational stop codon, if they affect the conserved nucleotides at the splice acceptor and donor sites, respectively, or if they delete or substitute a conserved amino acid and were observed together with a mutation on the second allele.
The majority of mutations (35 out of 49) are predicted to lead to a premature translational stop codon. These mutations include 14 nonsense, 19 frameshift, and two splice site mutations whose consequences on splicing could be confirmed on the mRNA level. The precise effect of four additional splice site mutations (c.1036+6_9delTGAG; c.3798-2A>C; c.3982+1G>T; c.4573+1G>A) has not been determined experimentally, because no appropriate material from the respective patients was available. However, in all of them the most likely consequence predicted by in silico analysis is either exon skipping or intron inclusion, shifting the reading frame. This is also true for the mutation c.1036+6_9delTGAG, the only identified splice site mutation that does not affect the invariant nucleotides +1 or +2 of the splice donor, but which is nevertheless predicted to lead to loss of splice donor function. Only eight missense changes and two in frame deletions leading to the loss of a single amino acid have been identified as likely causative mutations, to date. All of them affect highly conserved amino acid residues of the laminin β2 protein (Supp. Figure S1) and were found either in the homozygous state or in compound heterozygosity with another bona fide mutation on the second allele (Table 2). All missense mutations except for two (p.L139P, p.S80R) have been reported previously (Choi et al., 2008; Hasselbacher et al., 2006; Kagan et al., 2008; Matejas et al., 2006; Zenker et al., 2004a). None of these changes was found in over 200 controls. Five mutations were recurrent (Table 2). Four of them (c.1405+1G>A, c.1477delT, c.3174_3175delTG, and c.4504delA) were found in two unrelated families each, while the mutation p.R246W was independently observed in 5 unrelated families. The remaining changes are “private mutations” observed only in single families.
Mutations creating premature stop codons are almost evenly distributed along the LAMB2 gene (Fig. 1a). They may either lead to nonsense mediated mRNA decay or result in truncated proteins. Notably, the mutation c.5258dupA, which is predicted to produce the premature translational stop located most closely to the 3′ end of the mRNA and to delete only 39 amino acids from the protein, has been demonstrated to result in complete lack of protein expression (Zenker et al., 2004a). This may be due to the fact that the C-terminus of laminin β2 is important for the proper assembly of the laminin chains. Mutant laminin β2 chains that cannot be stably assembled into a trimeric laminin complex are probably degraded (Utani et al., 1994). Consequently, all truncating mutations known to date likely represent functional null alleles.
In contrast, missense mutations and small in frame deletions obviously cluster in the LN domain of laminin β2 (Fig. 1b). This protein domain is critical for interacting with the LN domains of α and γ chains of neighboring laminins to form the monolayer network which represents a scaffold for basement membrane assembly (Colognato et al., 1999; Yurchenco and Cheng, 1993). This suggests that changes of highly conserved amino acid residues in that domain might perturb laminin polymerization. However, it has also been shown that the mutation p.R246W leads to significant reduction in protein expression (Zenker et al., 2004a). This may be due to disturbances at various stages of protein processing. Consistently, studies on a mouse model expressing the laminin β2 mutant R246Q suggested that the impact of this mutant on glomerular function stems in part from impaired laminin secretion (Cheng et al., 2008). The missense mutation p.C321R affects one of the invariant cysteines in the first EGF-like domain, LEa1 (Fig. 1c). As these cysteine residues form disulfide bonds that stabilize the structure of the EGF-like domains, substitution by other amino acids probably result in alteration or destabilization of protein structure. The consequences of the missense change p.L1393F affecting the LCC domain remain elusive (Hasselbacher et al., 2006).
In our own study population, the causative sequence changes were identified on both alleles in 29 out of 30 unrelated patients with typical Pierson syndrome (as defined by the presence of congenital microcoria plus NS). In a single patient (case 3.1, Table 2) only one allele, a nonsense mutation (p.Q125X) was identified, while the second allele has remained undetected despite sequencing of all introns and the presumed promoter region as well as screening for larger genomic deletions (using long range PCR covering the entire gene). RT-PCR on mRNA from kidney tissue of this patient, however, showed severely reduced LAMB2 mRNA expression from the allele that was not affected by the nonsense mutation (data not shown). These findings strongly support the existence of a mutation on the second allele, which escaped the employed screening methods (maybe an inversion or translocation affecting the LAMB2 locus). Together, these results provide clear evidence that Pierson syndrome is not heterogeneous and that LAMB2 mutation detection rate reaches 98–100% in typical cases.
Polymorphisms
Table 3 lists sequence variants that likely do not lead to development of Pierson syndrome or NS. These changes were quoted as probable neutral polymorphisms because they have been found either in homozygous state in healthy controls, in compound heterozygosity together with a clearly disease causing mutation in healthy Pierson syndrome carriers, or together with two bona fide mutations in patients. Altogether, 26 polymorphisms have been detected by sequencing of more than 200 individuals of various ethnic backgrounds (Table 3). Eight of them are known polymorphisms listed in the dbSNP database, while 18 variants are novel. 15 of the latter are either intronic with no obvious effect on splicing or do not cause changes on the protein level and, thus, are not supposed to affect the protein structure or function. A heterozygous variant in exon 24, predicting a substitution of glycine by arginine (p.G1243R) was identified in a patient with Pierson syndrome together with the mutation p.G1693VfsX20 on the same allele. Moreover, interspecies alignment revealed only poor conservation of the glycine at position 1243, together suggesting that this change likely represents a neutral polymorphism. Similarily, the sequence variant c.4140C>A in exon 26 leading to an exchange of asparagine by lysine (p.N1380K) was detected in a patient together with the mutation p.L1393F on the same allele. Based on evolutionary conservation and on the fact that p.N1380K but not p.L1393F was also found in one of 96 controls, the former was regarded as a probable polymorphism and the latter as the disease-causing mutation (Hasselbacher et al., 2006). The c.5293G>A variant (predicting the change p.A1765T) was found repeatedly in both patients and healthy controls, and it was found in one family to be located on the same allele as a truncating mutation (p.Q1006NfsX144) (Matejas et al., 2006). For evolutionary conservation of these missense variants see Supp. Figure S2.
Sequence Variants of LAMB2 with Unknown Phenotype Effect
For nine missense variations in the LAMB2 gene and three variations located 5′ to the start codon in the potential promoter region, the pathogenetic significance could not definitively be determined. These changes are listed in Table 4. All but two of them have been found as heterozygous changes in patients with NS who lacked other typical features of Pierson syndrome. None of these patients was found to have a disease-causing change on the second allele. Nevertheless, we cannot exclude the possibility that the LAMB2 alteration might have contributed to the renal disorder in these patients. The amino acids affected by those changes show various levels of evolutionary conservation (Supp. Figure S2). Three variations are located in the putative LAMB2 promoter region. Two of them were heterozygous (c.-1925G>C and c.-408_404delTAGTT) while the c.-165C>A substitution was found in a homozygous state in a patient with isolated NS. Their significance cannot be determined clearly, since the LAMB2 promoter is poorly characterized, so far. The heterozygous variant p.P37A affects the relatively conserved signal peptide cleavage site and is predicted to possibly favour aberrant cleavage 4 amino acids more downstream (SignalP 3.0 Server at http://www.cbs.dtu.dk/services/SignalP/), thus leading to a protein that is slightly shortened at its N-terminus. A heterozygous substitution p.H882Y was found in a healthy carrier for Pierson syndrome, who was heterozygous for the mutation p.C374X, but whether the variation p.H882Y was on the same allele could not be determined, because the affected children were not available for genetic testing (Zenker et al., 2005). Since His-882 is relatively conserved (Supp. Figure S2) and we cannot absolutely exclude the possibility of two clinically significant sequence changes on the same allele, we conservatively classified p.H882Y in this category.
Haplotype Analysis of Recurrent LAMB2 Mutations
In patients harbouring the five recurrent LAMB2 mutations we determined haplotypes by genotyping 13 microsatellite markers within a range of 15 Mb flanking the LAMB2 gene as well as 5 to 6 common intragenic single nucleotide polymorphisms in the index patient and his/her parents, as far as parental DNAs were available (Supp. Figure S3). The mutation p.R246W, which has been observed in 5 unrelated families, was found on the same haplotype in three families of Portuguese origin (two from Portugal and one from Brazil), thus suggesting a founder effect in this population. However, the same mutation was also found in one family of Asian origin on a different haplotype and in a patient of African origin. Shared haplotypes were also found in two families with Slavic background (originating from Poland and the Czech Republic), who carried the mutation c.4504delA, two families of German/French ancestry (mutation: c.1477delT), and two families of Middle European origin (mutation: p.C1058X), respectively. In all cases the shared haplotypes encompassed about 5 Mb. However, the precise extent could not be determined because it is impossible to distinguish identity by descent from identity by state for individual markers.
GENOTYPE-PHENOTYPE CORRELATIONS
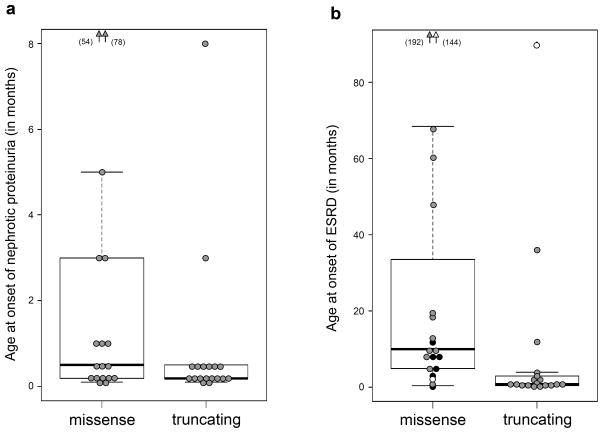
All individuals carrying homozygous or compound heterozygous LAMB2 mutations were affected by NS in the first decade, the vast majority in the first year of life. All but two patients (affected siblings from family 13) had ocular anomalies (Table 2). Although an ascertainment bias cannot be excluded, the current data suggest that kidney involvement is an invariant manifestation of genetic defects of the LAMB2 gene. However, some patients may be recognized because of their eye findings, and the renal symptoms only arrive thereafter (Bredrup et al., 2008). As mentioned above, there is strong evidence that truncating mutations, even those creating truncations of less than 50 amino acids from the C-terminal part of the protein, represent functional null alleles. Comparison of the group of patients with biallelic truncating mutations versus those with non-truncating mutations on at least one allele reveals a somewhat earlier onset of nephrotic symptoms and delayed occurrence of end stage renal disease (ESRD) at least in some of them (Fig. 2). Patients with two putative functional null alleles typically develop ESRD within the first year of life. ESRD may already be present at birth and mask nephrotic symptoms (hypoproteinemia, edema) through the limitation of renal protein waste in the presence of oliguria. However, in two patients with biallelic truncating mutations and presumed complete loss of laminin β2 production, ESRD was delayed until childhood age (patients 24.1 and 29.1) (Choi et al., 2008 and unpublished observation). Regarding ocular manifestations, all patients harboring biallelic nonsense or frameshift mutations exhibited congenital microcoria in association with variable other eye abnormalities as reviewed previously (Bredrup et al., 2008), except for one unpublished case (patient 29.1). With this single exception, patients who lacked this typical ocular sign of Pierson syndrome, including the two reported families with initial presentation of isolated NS (Choi et al., 2008; Hasselbacher et al., 2006), had at least one non-truncating allele or a splice site mutation whose effect on protein expression could not be definitely determined. This may suggest that only little residual function of laminin β2 is required for an apparently normal development and maintenance of the iris muscles. Notably, those patients who presented initially with only minor or without ocular changes appear to remain at high risk of developing serious ocular complications, e.g. retinal detachment, in later infancy or childhood (Bredrup et al., 2008; Choi et al., 2008; Matejas et al., 2006). Generally, isolated NS is rarely caused by LAMB2 mutations (Hinkes et al., 2007). In addition to what was published before (Choi et al., 2008; Hasselbacher et al., 2006; Hinkes et al., 2007), we have meanwhile studied more than 70 further cases with isolated NS without detecting another LAMB2 mutation-positive individual in this cohort. The same is true for nonspecific syndromic NS cases lacking ocular abnormalities of the Pierson syndrome spectrum. Significant neurodevelopmental abnormalities (muscular weakness/myasthenia, global delay, presumed retinal blindness) have been described repeatedly in patients with presumed complete deficiency of laminin β2 (Maselli et al., 2009; Wuhl et al., 2007). However, the nature of these deficits is not well characterized, and only few patients with the classic Pierson syndrome phenotype survived beyond the age of 2. One long-term survivor has recently been described with a neurologic picture resembling congenital myasthenia (Maselli et al., 2009). In contrast, we are aware of several patients (case 21.1, 24.1, 29.1, 31.1, 32.1, and 35.1; Table 2) with either truncating or splice site mutations on each allele, who had a normal neurologic and cognitive development up to the age of 4–21 years (Bredrup et al., 2008; Choi et al., 2008; Wuhl et al., 2007). In one of them, possible residual function of one allele carrying a de novo splice site mutation (c.3798-2A>C) was discussed but could not be demonstrated (Wuhl et al., 2007). Most patients who showed a favourable neurodevelopmental outcome despite the presence of biallelic mutations predicting probable complete loss of function also had delayed onset of ESRD. This observation would be compatible with the existence of genetic modifiers that may to some extent compensate for the laminin β2 defect, thereby rescuing the neurologic deficits and ameliorating the renal phenotype. Admittedly, considering truncating mutations as functional null alleles may be an oversimplification and does not account for possible rescue mechanisms on the transcriptional level (Kellermayer, 2006).
Figure 2.
Age of onset of gross proteinuria/nephrosis (a) and age of onset of end stage renal disease (ESRD) (b) are plotted on the Y axis in two genotypic classes: patients with either nonsense or frameshift mutations on both alleles (“truncating”) and patients with a missense mutation or small in frame deletion on at least one allele (“missense”). Each bullet represents one individual patient. Grey filled circles indicate the age when the respective patient developed NS and ESRD, respectively, while open circles represent patients who have not developed this feature (nephrosis or ESRD) at the given age of their last follow-up examination. Black bullets in (b) represent the age of death of those patients who died without having developed ESRD. Boxes indicate the range between upper and lower quartiles. Black horizontal line represents median. Whiskers reach from maximum to minimum excluding outliers. Arrowheads represent observations out of the range of the diagram (age in months given in parentheses).
Taken together, the current data provide evidence of significant genotype phenotype correlations. On the other hand, it is has become obvious that the genotype alone does not explain all the clinical variability among LAMB2-associated disorders. The neurological manifestations of Pierson syndrome remain the most enigmatic part of the disease spectrum and require further elucidation.
CLINICAL AND DIAGNOSTIC RELEVANCE
The diagnosis of Pierson syndrome is based on the recognition of the typical association of glomerular kidney disease and ocular abnormalities. In the typical cases with microcoria and early onset NS the diagnosis is obvious. Molecular testing of LAMB2 will very likely confirm the diagnosis in such patients. Mutational screening by sequencing of all coding exons and flanking intronic regions should particularly be attempted, if the parents wish to have prenatal testing in a further pregnancy. Although affected fetuses may also present with kidney abnormalities on prenatal ultrasound (Mark et al., 2006), only molecular genetic testing allows an early and reliable prenatal diagnosis. Molecular analysis of the LAMB2 gene may also be indicated to identify a familial mutation in order to offer subsequent carrier testing in healthy family members, although the risk of having a child with Pierson syndrome in relatives of a patient is very small (excluding consanguinity of the partners), given a presumably low carrier frequency in the general population. In cases with congenital or infantile NS and less specific ocular symptoms, molecular analysis of the LAMB2 gene provides a clue in the differential diagnosis. Among patients with isolated NS LAMB2 mutations are apparently rare (Hinkes et al., 2007 and unpublished results). Analysis of this gene may however still be justified in patients with congenital or infantile nephrosis, mesangial sclerosis on biopsy, and negative results upon testing for mutations in the genes NPHS1, NPHS2, WT1, and PLCE1. Early development of ESRD (from birth or in the first 3 months of life), which is unusual in other types of congenital NS, may be a further indication for testing for LAMB2 mutations. Considering the fact that microcoria may be the first presenting symptom of Pierson syndrome, LAMB2 testing may be of important diagnostic and prognostic value in any child with congenital microcoria.
Considering the clinical variability of LAMB2-associated disorders, predictions on the phenotypic expression on the basis of the genotype should be made with caution. Unfortunately, this is particularly true with respect to the possibility of relevant neurologic involvement which is an important determinant of long-term prognosis. Within the same family, however, our current experience suggests a rather high consistency of the phenotype.
Patients with Pierson syndrome are mainly taken care of by pediatric nephrologists and ophthalmologists. There is so far no specific treatment available. Nephrectomy for the treatment of severe renal protein waste may be considered similar to the management of patients with Finnish type nephrosis (Holmberg et al., 1995), but it should be taken into account that ESRD, which usually occurs much earlier in patients with Pierson syndrome compared to Finnish type nephrosis, will spontaneously limit protein loss. Kidney transplantation is currently the only long-term renal treatment option. There is so far no evidence of recurrence of mesangial sclerosis in the transplant, but the number of successfully transplanted patients who could be monitored for a longer time is very small. Of note, patients with a proven laminin β2 defect should receive careful ophthalmological follow-up, as they are obviously at high risk of retinal detachment even in the absence of significant congenital ocular anomalies. High grade myopia seems to be another common feature developing in infancy and early childhood (Bredrup et al., 2008).
An important differential diagnosis to Pierson syndrome is the nephrosis-microcephaly syndrome (Galloway-Mowat syndrome; GMS; MIM# 251300). Microcephaly which is a key finding in GMS has also been described in some patients with Pierson syndrome (Wuhl et al., 2007). However, in the latter microcephaly is usually not congenital, but may develop during the first year of life. Moreover, other features, such as structural brain anomalies, epilepsy, and hiatus hernia usually allow distinguishing GMS from Pierson syndrome clinically. Notably, however, there have been a few reports on patients with GMS and ocular changes resembling the manifestations in Pierson syndrome (Mildenberger et al., 1998; Shapiro et al., 1976). Despite the obvious clinical overlap, we have found no evidence that GMS and Pierson syndrome are allelic disorders (Dietrich et al., 2008).
There is so far no evidence for isolated ocular anomalies or ocular plus neurologic abnormalities without kidney involvement being caused by mutations in LAMB2. Isolated microcoria was been reported as an autosomal dominant trait (MIM# 156600). A provisional locus has been assigned to 13q31-q32 (Rouillac et al., 1998), thus ruling out LAMB2 as the causative gene for families linked to this locus. However, genetic heterogeneity is not excluded and studies of a possible significance of LAMB2 mutations in sporadic cases with isolated microcoria or related iris symptoms as well as in families unlinked to 13q31-q32 are warranted.
FUTURE PROSPECTS
Future research will be directed towards the identification of possible modifiers of the phenotype caused by laminin β2 defects. Considering that a lack of expression on theβ2-chain probably leads to the expression of aberrant laminin isoforms in the basement membrane rather than the complete absence of laminin (Noakes et al., 1995b), it is tempting to speculate that laminin β1 (or other laminin β isoforms) might to some extent be able to compensate for the lack of β2. Treatment prospects might arise from the knowledge on modifying factors. As a significant proportion of LAMB2 mutations are nonsense mutations, aminoglycosides which are known to be able to induce translational stop codon readthrough (Allamand et al., 2008; Linde et al., 2007), and which reach high concentrations particularly in the kidney, may be evaluated as a therapeutic target in the future.
A locus-specific mutation database is available at: http://www.med.uni-magdeburg.de/LAMB2mutdb.
Supplementary Material
Acknowledgments
We are indebted to the families of our patients for participation in our study. We are grateful to the members of the GPN (Gesellschaft für Pädiatrische Nephrologie) and Dr. Mike Urban for providing us with DNA samples from patients with syndromic forms of NS, and to Jeffrey H. Miner for helpful discussion. This work was supported by a grant from the German Research Foundation (DFG) to M.Z. (SFB 423, TP A19)
References
- Allamand V, Bidou L, Arakawa M, Floquet C, Shiozuka M, Paturneau-Jouas M, Gartioux C, Butler-Browne GS, Mouly V, Rousset JP, Matsuda R, Ikeda D, Guicheney P. Drug-induced readthrough of premature stop codons leads to the stabilization of laminin alpha2 chain mRNA in CMD myotubes. J Gene Med. 2008;10:217–224. doi: 10.1002/jgm.1140. [DOI] [PubMed] [Google Scholar]
- Bredrup C, Matejas V, Barrow M, Blahova K, Bockenhauer D, Fowler DJ, Gregson RM, Maruniak-Chudek I, Medeira A, Mendonca EL, Kagan M, Koenig J, Krastel H, Kroes HY, Saggar A, Sawyer T, Schittkowski M, Swietliński J, Thompson D, VanDeVoorde RG, Wittebol-Post D, Woodruff G, Zurowska A, Hennekam RC, Zenker M, Russell-Eggitt I. Ophthalmological aspects of Pierson syndrome. Am J Ophthalmol. 2008;146:602–611. doi: 10.1016/j.ajo.2008.05.039. [DOI] [PubMed] [Google Scholar]
- Chen YM, Kikkawa Y, Miner JH. Proteinuria in mice expressing a mutant laminin 2 chain (R246Q) in podocytes stems in part from impaired laminin secretion. J Am Soc Nephrol. 2008;19:102A. [Google Scholar]
- Choi HJ, Lee BH, Kang JH, Jeong HJ, Moon KC, Ha IS, Yu YS, Matejas V, Zenker M, Choi Y, Cheong HI. Variable phenotype of Pierson syndrome. Pediatr Nephrol. 2008;23:995–1000. doi: 10.1007/s00467-008-0748-7. [DOI] [PubMed] [Google Scholar]
- Colognato H, Winkelmann DA, Yurchenco PD. Laminin polymerization induces a receptor-cytoskeleton network. J Cell Biol. 1999;145:619–631. doi: 10.1083/jcb.145.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Matejas V, Bitzan M, Hashmi S, Kiraly-Borri C, Lin SP, Mildenberger E, Hoppe B, Palm L, Shiihara T, Steiss JO, Tsai JD, Vester U, Weber S, Wühl E, Zepf K, Zenker M. Analysis of genes encoding laminin beta2 and related proteins in patients with Galloway-Mowat syndrome. Pediatr Nephrol. 2008;23:1779–1786. doi: 10.1007/s00467-008-0880-4. [DOI] [PubMed] [Google Scholar]
- Hasselbacher K, Wiggins RC, Matejas V, Hinkes BG, Mucha B, Hoskins BE, Ozaltin F, Nurnberg G, Becker C, Hangan D, Pohl M, Kuwertz-Bröking E, Griebel M, Schumacher V, Royer-Pokora B, Bakkaloglu A, Nürnberg P, Zenker M, Hildebrandt F. Recessive missense mutations in LAMB2 expand the clinical spectrum of LAMB2-associated disorders. Kidney Int. 2006;70:1008–1012. doi: 10.1038/sj.ki.5001679. [DOI] [PubMed] [Google Scholar]
- Hinkes BG, Mucha B, Vlangos CN, Gbadegesin R, Liu J, Hasselbacher K, Hangan D, Ozaltin F, Zenker M, Hildebrandt F. Nephrotic syndrome in the first year of life: two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2) Pediatrics. 2007;119:e907–919. doi: 10.1542/peds.2006-2164. [DOI] [PubMed] [Google Scholar]
- Holmberg C, Antikainen M, Ronnholm K, Ala Houhala M, Jalanko H. Management of congenital nephrotic syndrome of the Finnish type. Pediatr Nephrol. 1995;9:87–93. doi: 10.1007/BF00858984. [DOI] [PubMed] [Google Scholar]
- Kagan M, Cohen AH, Matejas V, Vlangos C, Zenker M. A milder variant of Pierson syndrome. Pediatr Nephrol. 2008;23:323–327. doi: 10.1007/s00467-007-0624-x. [DOI] [PubMed] [Google Scholar]
- Kellermayer R. Translational readthrough induction of pathogenic nonsense mutations. Eur J Med Genet. 2006;49:445–450. doi: 10.1016/j.ejmg.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Linde L, Boelz S, Nissim-Rafinia M, Oren YS, Wilschanski M, Yaacov Y, Virgilis D, Neu-Yilik G, Kulozik AE, Kerem E, Kerem B. Nonsense-mediated mRNA decay affects nonsense transcript levels and governs response of cystic fibrosis patients to gentamicin. J Clin Invest. 2007;117:683–692. doi: 10.1172/JCI28523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark K, Reis A, Zenker M. Prenatal findings in four consecutive pregnancies with fetal Pierson syndrome, a newly defined congenital nephrosis syndrome. Prenat Diagn. 2006;26:262–266. doi: 10.1002/pd.1393. [DOI] [PubMed] [Google Scholar]
- Maselli RA, Ng JJ, Anderson JA, Cagney O, Arredondo J, Williams C, Wessel HB, Abdel-Hamid H, Wollmann RL. Mutations in LAMB2 causing a severe form of synaptic congenital myasthenic syndrome. J Med Genet. 2009;46:203–208. doi: 10.1136/jmg.2008.063693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matejas V, Al-Gazali L, Amirlak I, Zenker M. A syndrome comprising childhood-onset glomerular kidney disease and ocular abnormalities with progressive loss of vision is caused by mutated LAMB2. Nephrol Dial Transplant. 2006;21:3283–3286. doi: 10.1093/ndt/gfl463. [DOI] [PubMed] [Google Scholar]
- Mildenberger E, Lennert T, Kunze J, Jandeck C, Waldherr R, Versmold H. Diffuse mesangial sclerosis: association with unreported congenital anomalies and placental enlargement. Acta Paediatr. 1998;87:1301–1303. doi: 10.1080/080352598750031022. [DOI] [PubMed] [Google Scholar]
- Miner JH, Go G, Cunningham J, Patton BL, Jarad G. Transgenic isolation of skeletal muscle and kidney defects in laminin beta2 mutant mice: implications for Pierson syndrome. Development. 2006;133:967–975. doi: 10.1242/dev.02270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Patton BL. Laminin-11. Int J Biochem Cell Biol. 1999;31:811–816. doi: 10.1016/s1357-2725(99)00030-8. [DOI] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature. 1995a;374:258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP. The renal glomerulus of mice lacking s-laminin/laminin beta 2: nephrosis despite molecular compensation by laminin beta 1. Nat Genet. 1995b;10:400–406. doi: 10.1038/ng0895-400. [DOI] [PubMed] [Google Scholar]
- Pierson M, Cordier J, Hervouuet F, Rauber G. An Unusual Congenital and Familial Congenital Malformative Combination Involving the Eye and Kidney. J Genet Hum. 1963;12:184–213. [PubMed] [Google Scholar]
- Rouillac C, Roche O, Marchant D, Bachner L, Kobetz A, Toulemont PJ, Orssaud C, Urvoy M, Odent S, Le Marec B, Abitbol M, Dufier JL. Mapping of a congenital microcoria locus to 13q31-q32. Am J Hum Genet. 1998;62:1117–1122. doi: 10.1086/301841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MC, Christiano AM, Engvall E, Wewer UM, Miner JH, Sanes JR, Burgeson RE. The functions of laminins: lessons from in vivo studies. Matrix Biol. 1996;15:369–381. doi: 10.1016/s0945-053x(96)90157-2. [DOI] [PubMed] [Google Scholar]
- Shapiro LR, Duncan PA, Farnsworth PB, Lefkowitz M. Congenital microcephaly, hiatus hernia and nephrotic syndrome: an autosomal recessive syndrome. Birth Defects Orig Artic Ser. 1976;12:275–278. [PubMed] [Google Scholar]
- Tunggal P, Smyth N, Paulsson M, Ott MC. Laminins: structure and genetic regulation. Microsc Res Tech. 2000;51:214–227. doi: 10.1002/1097-0029(20001101)51:3<214::AID-JEMT2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Utani A, Nomizu M, Timpl R, Roller PP, Yamada Y. Laminin chain assembly. Specific sequences at the C terminus of the long arm are required for the formation of specific double- and triple-stranded coiled-coil structures. J Biol Chem. 1994;269:19167–19175. [PubMed] [Google Scholar]
- VanDeVoorde R, Witte D, Kogan J, Goebel J. Pierson syndrome: a novel cause of congenital nephrotic syndrome. Pediatrics. 2006;118:e501–505. doi: 10.1542/peds.2005-3154. [DOI] [PubMed] [Google Scholar]
- Wuhl E, Kogan J, Zurowska A, Matejas V, Vandevoorde RG, Aigner T, Wendler O, Lesniewska I, Bouvier R, Reis A, Weis J, Cochat P, Zenker M. Neurodevelopmental deficits in Pierson (microcoria-congenital nephrosis) syndrome. Am J Med Genet A. 2007;143:311–319. doi: 10.1002/ajmg.a.31564. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Cheng YS. Self-assembly and calcium-binding sites in laminin. A three-arm interaction model. J Biol Chem. 1993;268:17286–17299. [PubMed] [Google Scholar]
- Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wuhl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dötsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A. Human laminin β2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004a;13:2625–2632. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- Zenker M, Pierson M, Jonveaux P, Reis A. Demonstration of two novel LAMB2 mutations in the original Pierson syndrome family reported 42 years ago. Am J Med Genet A. 2005;138:73–74. doi: 10.1002/ajmg.a.30894. [DOI] [PubMed] [Google Scholar]
- Zenker M, Tralau T, Lennert T, Pitz S, Mark K, Madlon H, Dotsch J, Reis A, Muntefering H, Neumann LM. Congenital nephrosis, mesangial sclerosis, and distinct eye abnormalities with microcoria: an autosomal recessive syndrome. Am J Med Genet. 2004b;130A:138–145. doi: 10.1002/ajmg.a.30310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.