Abstract
Fasting has been used to control epilepsy since antiquity, but the mechanism of coupling between metabolic state and excitatory neurotransmission remains unknown. Previous work has shown that the vesicular glutamate transporters (VGLUTs) required for exocytotic release of glutamate undergo an unusual form of regulation by Cl−. Using functional reconstitution of the purified VGLUTs into proteoliposomes, we now show that Cl− acts as an allosteric activator, and the ketone bodies that increase with fasting inhibit glutamate release by competing with Cl− at the site of allosteric regulation. Consistent with these observations, acetoacetate reduced quantal size at hippocampal synapses, and suppresses glutamate release and seizures evoked with 4-aminopyridine in the brain. The results indicate an unsuspected link between metabolic state and excitatory neurotransmission through anion-dependent regulation of VGLUT activity.
Keywords: vesicular glutamate transporter, chloride, acetoacetate, epilepsy, ketone body, glutamatergic neurotransmission
INTRODUCTION
Epilepsy is a common neurological disorder affecting ~1% of the population world-wide. Although a large number of drugs have been developed to treat seizures (Meldrum and Rogawski, 2007), approximately one third of epileptic patients remain poorly controlled, and many continue to rely on fasting or a high-fat, low-protein and–carbohydrate ( ketogenic ) diet originally described in biblical times (Laffel, 1999; Vining, 1999; Bailey et al., 2005; Zupec-Kania and Spellman, 2008). Through β-oxidation in the liver, fasting and the ketogenic diet produce ketone bodies, in particular acetoacetate and β-hydroxybutyrate, and these circulating metabolites enter the brain where they serve as substrates for energy production in neurons (Laffel, 1999; Freeman et al., 2006; Hartman et al., 2007). Under normal circumstances, the blood level of ketone bodies is maintained at ~0.3 mM, but increases markedly up to ~10 mM with a ketogenic diet (Laffel, 1999; Freeman et al., 2006; Bough and Rho, 2007). Ketone bodies are thus considered to be responsible for the therapeutic effect of fasting and the ketogenic diet (Freeman et al., 2006; Bough and Rho, 2007; Hartman et al., 2007).
How do ketone bodies control epilepsy? They might suppress excitatory neurotransmission and/or stimulate inhibitory neurotransmission (Nordli and De Vivo, D.C 1997; Freeman et al., 2006; Bough and Rho, 2007; Hartman et al., 2007; Yudkoff et al., 2008). However, the effects on brain glutamate and GABA content remain controversial, and systemic levels of glutamate and GABA do not significantly change with a ketogenic diet (Appleton and De Vivo, 1974; Bough and Rho, 2007; Hartman et al., 2007; Yudkoff et al., 2008). The expression and activity of receptors and plasma membrane transporters for glutamate and GABA also do not change (Thio et al., 2000; Noh et al., 2004; Bough et al., 2007; Hartman et al., 2007). Recent work has suggested that acetoacetate reduces firing by lowering cytoplasmic ATP, thereby activating the ATP-sensitive K+ channel (KATP) (Ma et al., 2007). However, the effect on KATP has not been tested in vivo, and the effect of ketone bodies on cytoplamic ATP remains controversial (Nakazawa et al., 1983; Zhao et al., 2006).
The filling of synaptic vesicles with glutamate has a crucial role in glutamatergic neurotransmission, and depends on the activity of the vesicular glutamate transporters (VGLUT1-3) (Reimer, and Edwards, 2004, Fremeau et al., 2004a; Edwards 2007). The VGLUTs use a membrane potential (Δψ) established by the vacuolar proton pump (V-ATPase) as a driving force (Maycox et al., 1988; Moriyama and Yamamoto, 1995; Juge et al., 2006). The loss of VGLUT activity eliminates vesicular release and glutamatergic neurotransmission, with profound consequences for behavior and indeed survival, indicating the essential role(s) of VGLUTs in brain function (Fremeau et al., 2004b; Wojcik et al., 2004; Moechars et al., 2006; Wallén-Mackenzie et al., 2006; Smear et al., 2007; Gras et al., 2008; Seal et al., 2008; Seal et al., 2009).
One of the most unusual but unexplained features of VGLUT function is its Cl− dependence. ATP-dependent glutamate uptake in synaptic vesicles exhibits a biphasic dependence on Cl− (Naito and Ueda, 1985; Maycox et al., 1988; Moriyama and Yamamoto, 1995). In the absence of Cl−, glutamate uptake into synaptic vesicles is very low, but low [Cl−] (4 mM) strongly activates transport, with inhibition at higher concentrations. Cl− dependence is even more extreme in proteoliposomes containing purified VGLUT2 and bacterial F-ATPase; no transport activity is observed in the absence of Cl− but full activity appears ~4 mM Cl− (Juge et al., 2006). It has been proposed that the Cl− dependence on the glutamate uptake reflects an allosteric effect, and that the decrease at high [Cl−] reflects dissipation of Δψ (Hartinger and Jahn, 1993; Moriyama and Yamamoto, 1995; Juge et al, 2006). However, previous work using heterologous expression has suggested that VGLUT1 exhibits a Cl− conductance (Bellocchio et al., 2000), and more recently, functional reconstitution of purified VGLUT1 and bacterial F-ATPase has supported this possibility, although Cl− flux itself was not actually demonstrated (Schenck et al., 2009). These studies nonetheless indicate that Cl− dependence is an intrinsic property of VGLUTs, although the mechanism responsible and its physiological role in the regulation of quantal size remain poorly understood.
We have investigated the molecular mechanism for regulation of the VGLUTs by Cl−. In the course of these studies, we have found a surprising but strong link between the Cl− dependence of VGLUTs and the metabolic control of seizures by ketone bodies. We show that Cl− acts as an allosteric activator for VGLUTs, that ketone bodies and acetoacetate in particular compete with Cl− for VGLUT activation, and that this regulatory mechanism is conserved in the entire SLC17 transporter family. Finally, we show that acetoacetate reversibly inhibits glutamate release in vivo.
RESULTS
Cl− directly activates VGLUT2
In both native membranes and proteoliposomes containing an active proton pump as well as VGLUT, Cl− has a dual role in regulation of the transporter, and in expression of the Δψ that drives transport (Juge et al., 2006; Schenck et al., 2009). To characterize the effect of Cl− on the transporter independent of the driving force, we have therefore established an in vitro assay with proteoliposomes containing only the purified VGLUTs, where Cl− can be manipulated without affecting Δψ.
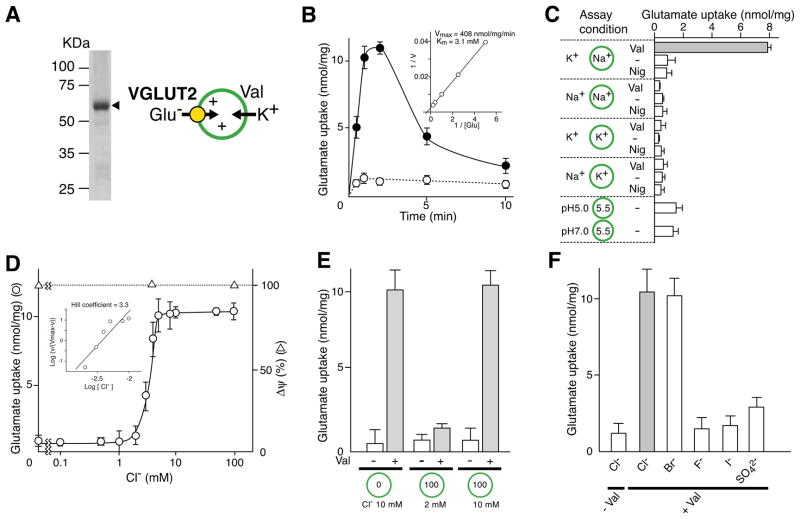
As a first step to characterizing the interaction of VGLUTs with Cl−, purified VGLUT2 was reconstituted into liposomes in the presence of Na-acetate (Figure 1A). The resultant proteoliposomes were suspended in buffer containing K-acetate. Upon addition of valinomycin (Val), a stable, inside positive Δψ of 87.2 ± 0.07 mV; n = 3 was established through the electrogenic entry of K+ into proteoliposomes and thus, triggered glutamate uptake (Figure 1B). The characteristics of Val-evoked glutamate uptake, including the kinetic parameters, sensitivity to Evans blue and diisothiocyanostilbene disulfonic acid (DIDS), and insensitivity to aspartate, were essentially identical to those reported previously (Figure 1B and Figure S1) (Juge et al., 2006). Bioenergetic analysis under defined Δψ and/or ΔpH conditions indicated that Δψ but not ΔpH primarily triggered glutamate uptake (Figure 1C). As observed in proteoliposomes containing VGLUT2 and F-ATPase (Juge et al., 2006; Schenck et. al., 2009), Δψ-mediated glutamate uptake absolutely required Cl−: glutamate transport was not detected in the absence of Cl−. Glutamate uptake appeared at 2 mM Cl−, increased drastically with a slight increase of [Cl−] and reached a plateau at 5 mM (Figure 1D). The steady glutamate uptake and magnitude of Δψ at Cl− concentrations above 5 mM contrasted with the results of proteoliposomes containing VGLUT and F-ATPase (Juge et al., 2006; Schenck et al., 2009). It is noteworthy that Cl−-dependent activation of VGLUT2 exhibited a strong and extraordinarly positive cooperativity for glutamate transport with a Hill co-efficient for Cl− of ~3 (Figure 1D). We further found that, in contrast to proteoliposomes containing VGLUT1 and F-ATPase (Schenck et al., 2009), intravesicular Cl− did not affect Cl− and Δψ-dependent glutamate uptake (Figure 1E). Br compensated for Cl−, while sulfate showed a slight stimulatory effect and neither iodide nor fluoride showed much stimulation (Figure 1F).
Figure 1. Cl− activates VGLUT2 externally.
A. Simple assay system for VGLUT. Proteoliposomes containing purified VGLUT2 (10 μg protein) were analyzed in an 11% polyacrylamide gel in the presence of SDS and visualized by Coomassie brilliant blue staining. The positions of molecular markers are indicated. The principle of Val-evoked glutamate uptake is also shown. B. Time course of glutamate uptake by reconstituted VGLUT2 was measured as described in Materials and Methods in the presence (closed circle) or absence (open circle) of Val. Kinetics of Val-evoked glutamate uptake at 1 min is shown in the inset. See also Figure S1. C. Energetics of glutamate uptake. Glutamate uptake was measured under the indicated ionic conditions. Nig, nigericin. D. Concentration dependence of Cl− on glutamate uptake. A sample was taken after 1 min. Δψ, measured as oxonol-V fluorescence quenching, is shown. The inset shows the Hill plot of glutamate uptake as a function of [Cl−]. E. Effect of internal and external Cl− on glutamate uptake. Proteoliposomes were prepared at the indicated concentrations of Cl− and suspended in buffer containing the indicated Cl− concentration. Val-evoked glutamate uptake was measured at 1 min. F. Cl− was replaced with the indicated anion and Val-evoked uptake of glutamate was measured at 1 min. Error bars represent mean ± SEM; n = 3.
VGLUT2 does not transport Cl−
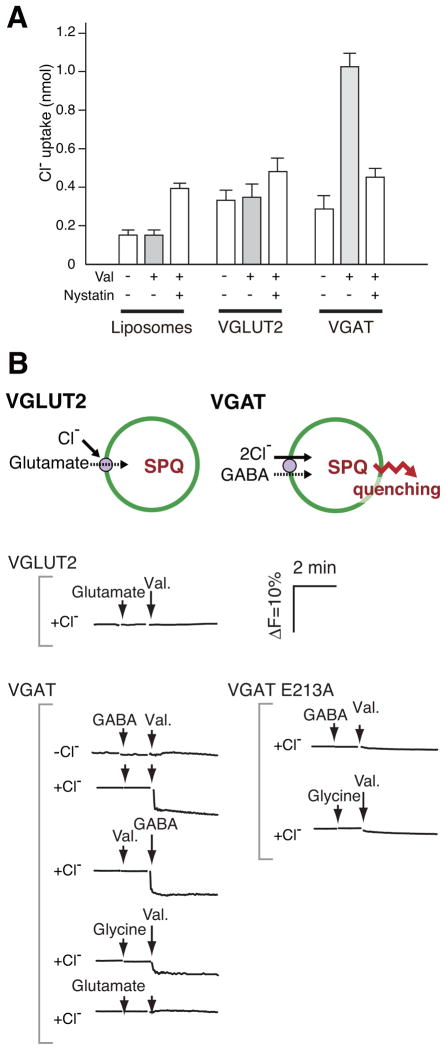
An important question was whether Cl− was actually transported through VGLUT2 during Cl− and Δψ-dependent glutamate uptake. We investigated this issue using two independent procedures: direct isotope tracing and a fluorometric procedure employing 6-methoxy-N-(3-sulfopropyl)-quinolinum monohydrate (SPQ), a fluorescent Cl− indicator (Biwersi et al., 1994).
The background level of 36Cl− uptake was shown in liposomes in the presence or absence of Δψ by radioisotope tracing (Figure 2A, left). 36Cl− uptake at equilibrium was determined by the addition of nystatin, an anion ionophore (Russell et al., 1977), to demonstrate the impermeability of the liposomal membrane to Cl− (Figure 2A). Δψ alone did not facilitate 36Cl− uptake by VGLUT2-containing proteoliposomes. The inward flux of Cl− during glutamate uptake was significantly lower than the influx at equilibrium in the presence of nystatin (Figure 2A). Vesicular GABA transporter (VGAT) is a Δψ-driven Cl−/GABA co-transporter, and was used as a positive control for vesicular Cl− uptake (Juge et al., 2009). As shown in Figure 2A, VGAT facilitates active transport of GABA-dependent Cl− uptake against a Cl− concentration gradient.
Figure 2. VGLUT2 does not transport Cl− during glutamate transport.
A. Uptake of radiolabeled Cl−. In the presence of 5 mM glutamate, 36Cl− uptake by liposomes or VGLUT2-containing proteoliposomes was measured in the presence (+) or absence (−) of Val. In some experiments, nystatin was included to estimate [36Cl−] at equilibrium. 36Cl− uptake by VGAT at 1 min is shown as a positive control for active Cl− transport. Essentially the same results were obtained when 10 % (w/w) cholesterol was included in the proteoliposomes. Error bars represent mean ± SEM; n = 3. B. Comparison of glutamate or GABA-evoked Cl− uptake by VGLUT or VGAT, respectively, as revealed by SPQ fluorescence. SPQ-trapped VGLUT2-containing proteoliposomes were prepared and SPQ fluorescence intensity was monitored. Proteoliposomes containing either VGAT or the E213A mutant VGAT were also measured. Additions: KCl, 10 mM; GABA, 5 mM; glutamate, 5 mM; glycine, 5 mM; Val, 2 μM; nystatin, 250 μM. A principle of assay system was also illustrated. See also Figure S2.
Entry of Cl− into proteoliposomes loaded with SPQ should result in decreased fluorescence intensity due to collisions between SPQ and Cl− (Accardi and Miller, 2004). Therefore, we prepared SPQ-trapped proteoliposomes and monitored changes of SPQ fluorescent intensity. SPQ fluorescence quenching was not observed in the absence or presence of external Cl− (10 mM) in VGLUT2-containing proteoliposomes (Figure 2B). On the other hand, in VGAT-containing proteoliposomes, the addition of GABA and Val quenched SPQ fluorescence (Figure 2B). Glycine, another substrate for VGAT (Juge et al., 2009), caused similar fluorescence quenching, while glutamate, which is not a substrate for VGAT, did not (Figure 2B). The addition of Val and GABA to liposomes containing the inactive mutant of VGAT, E213A (Juge et al., 2009), did not quench SPQ fluorescence (Figure 2B). The quenching induced by Cl−, GABA and Val in VGAT-containing liposomes exhibited dose dependence with apparent Km values for GABA and Cl− of 0.79 and 2.7 mM, respectively, while no such dependence was observed in VGLUT2-containing proteoliposomes (Figure S2).
Acetoacetate is an allosteric modulator of VGLUT2 Cl− dependence
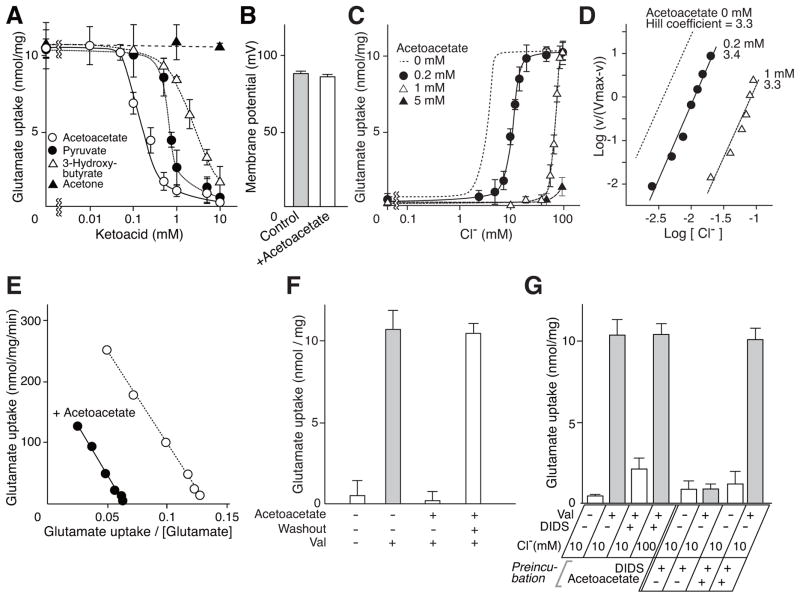
The results suggest that Cl− acts in an allosteric manner to regulate VGLUT activity. If this were the case, other substances might also bind at the same site and affect VGLUT2 Cl−-dependence. We thus screened for such substances, and included 10 mM Cl− in the standard assay because this concentration both approximates physiological conditions and confers maximal activity (Figure 1D). Among more than 30 metabolic intermediates tested, we found that ketone bodies (acetoacetate and 3-hydroxybutyrate) inhibited glutamate transport (Figures 3A and S3). In addition, pyruvate, an intermediate in the glycolytic pathway also inhibited glutamate transport (Figure 3A and Figures S3 and S4). Of the compounds tested, acetoacetate had the strongest inhibitory effect, exhibiting a half maximal inhibitory concentration (IC50) of 0.2 mM (Figure 3A). The presence of 10 mM acetoacetate did not affect the formation of Δψ by the proteoliposomes, confirming that a constant force was established during the assay (Figure 3B).
Figure 3. Acetoacetate is a physiological modulator of VGLUT2 through competition with Cl−.
A. Dose-dependent inhibition of VGLUT2 by various compounds as indicated in the presence of 10 mM Cl−. For results of other substances, see Figure S4. Glutamate uptake at 1 min is shown. See also Figure S3 and S4. B. Acetoacetate did not affect Δψ. Val-evoked formation of Δψ in the absence or presence of 10 mM acetoacetate. Error bars represent mean ± SEM; n = 3. C. Glutamate uptake at 1 min at various [Cl−] in the presence of the indicated concentrations of acetoacetate. Glutamate uptake in the absence of acetoacetate (Figure 1D) was also shown as a dashed line for comparison. D. Hill plot of glutamate uptake at the indicated concentrations of acetoacetate. Data were taken from Figure 3C. E. Eadie-Hofstee plot of glutamate uptake in the presence or absence of 0.2 mM acetoacetate. F. Inhibition caused by 5 mM acetoacetate was fully reversed by washing the proteoliposomes. Error bars represent mean ± SEM; n = 3. G. Protection of glutamate uptake activity by Cl− or acetoacetate against DIDS inactivation. Proteoliposomes were incubated in the absence or presence of 1 μM DIDS (DIDS treated VGLUT2) and in two cases, in the presence of 5 mM acetoacetate (acetoacetate and DIDS treated VGLUT2) for 1 min. These proteoliposomes were then incubated in buffer containing 10 or 100 mM Cl− in the presence or absence of DIDS. After 1 min, Val-evoked uptake of glutamate was measured. Error bars represent mean ± SEM; n = 3.
As expected, acetoacetate changed the Cl−-dependence of VGLUT2, shifting it to higher concentrations (Figure 3C), and suggesting a competitive interaction. In 10 mM Cl−, 1 mM acetoacetate inhibited glutamate uptake without affecting the cooperativity for Cl− (Figure 3D). The effect of 1 mM acetoacetate was overcome by 100 mM Cl− (Figure 3C). In contrast, acetoacetate did not compete with glutamate (Figure 3E). The effect of acetoacetate was totally reversible and full activity was recovered by washing the compound out of the preparation (Figure 3F). Furthermore, we found that the effects of Cl− and acetoacetate were observed when these compounds were present outside vesicles: neither was effective from the luminal side of the proteoliposomes (Figure 3G). DIDS (1 μM) is a potent inhibitor for VGLUT but its inhibition was effectively blocked by 100 mM Cl− (Fig. 3G; Hartinger and Jahn, 1993; Moriyama and Yamamoto, 1995; Juge et al., 2006). Similarly, the presence of 5 mM acetoacetate prevented DIDS-evoked inactivation of VGLUT2 (Figure 3G). Essentially the same effects of Cl− and acetoacetate were observed in the ATP-dependent glutamate uptake by synaptic vesicles (Figure S5).
Other SLC17 members share the same Cl− dependence
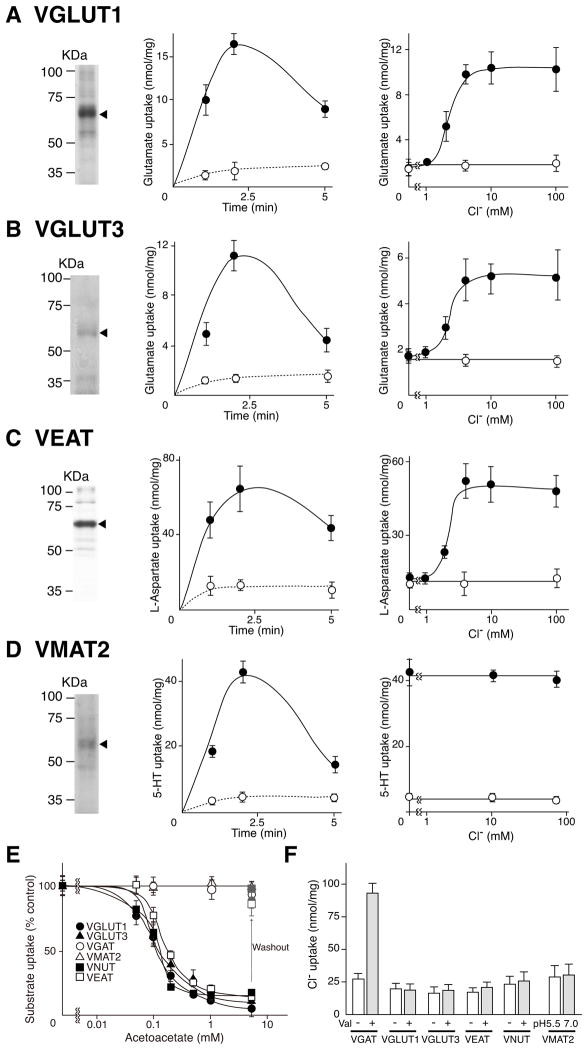
So far, six types of vesicular neurotransmitter transporters have been identified: the VGLUTs, VNUT (vesicular nucleotide transporter, SLC17A9), VEAT (vesicular excitatory amino acid transporter, SLC17A5), VGAT (SLC32A1), VMAT1-2 (vesicular monoamine transporter 1 and 2, SLC18A1-2), and VAChT (vesicular acetylcholine transporter, SLC18A3) (Schuldiner, 1994; Parsons, 2000; Eiden et al., 2004; Gasnier, 2004; Reimer and Edwards, 2004; Sawada et al., 2008; Miyaji et al., 2008; see also Figure S6). Since both Cl− dependence and acetoacetate sensitivity have been investigated for only VGLUT2, it was important to determine whether these characteristics were conserved in other VGLUTs and within the SLC17 family as a whole. We therefore tested the allosteric activation of these proteins by Cl− and the interaction of Cl− with acetoacetate.
VGLUT1, VGLUT3 and VEAT used Δψ as a driving force for transport and absolutely required Cl− with a dose-dependence similar to that of VGLUT2 (Figures 4A-4C). The effect of acetoacetate on Δψ and Cl−-dependent uptake in terms of dose dependence, protection by Cl− and reversibility was also similar to that of VGLUT2 (Figures 4E and S7). No radiolabeled Cl− was taken up by the proteoliposomes during transport of glutamate and aspartate (Figure 4F). We have already shown that VNUT requires Cl− for transport activity (Sawada et al., 2008). As expected, Δψ and Cl−-dependent uptake of ATP by VNUT was similarly inhibited by acetoacetate (Figure 4E). The inhibitory effect of acetoacetate was prevented by high concentrations of Cl− and was reversible (Figures 4E and S7). Cl− uptake was also not observed in VNUT (Figure 4F).
Figure 4. Cl− dependence and its modulation by acetoacetate is a specific property of SLC17 vesicular neurotransmitter transporters.
A. Val-evoked uptake of glutamate (0.1 mM) by purified, reconstituted VGLUT1 was assayed. (left) Coomassie brilliant blue-stained SDS gel of reconstituted proteoliposomes (10 μg protein each) showing the purified transporters. (center) Time course in buffer containing 10 mM Cl− (closed circles). (right) Val-evoked glutamate uptake at 1 min in the presence or absence of the indicated concentration of Cl−. B–D. Purification, time course, and Cl− dependence of VGLUT3 (B), VEAT (C) and VMAT2 (D). Glutamate (0.1 mM) and aspartate (0.1 mM) were used as substrates for VGLUT3 and VEAT, respectively. In the case of VMAT2, ΔpH-dependent uptake of serotonin (10 μM) was measured by the pH jump method. See also Figure S5. E. The effect of acetoacetate in the presence of 10 mM Cl−. In “washout” experiments, the proteoliposomes were washed with buffer lacking acetoacetate after incubation in 5 mM acetoacetate. Full activity (100%) of VNUT and VGAT was 16.0 ± 3.2 and 6.9 ± 1.0 n mol/min/mg protein, respectively. Full activity of VGLUT1, VGLUT3, VEAT and VMAT2 was as indicated above. Error bars represent mean ± SEM; n = 3. See also Figure S6. F. 36Cl− uptake at 1 min during the uptake of transport substrates by the respective transporters. Error bars represent mean ± SEM; n = 3.
VGAT activity also depends on Cl− (Juge et al., 2009). However, VGAT-mediated GABA uptake was actually accompanied by active Cl− flux (Figures 2 and 4F) (Juge et al., 2009) and was insensitive to acetoacetate up to at least 5 mM (Figure 4E). VMAT2 is a ΔpH-driven monoamine transporter (Schuldiner, 1994). The ΔpH-driven serotonin uptake by partially purified VMAT2 was found to be entirely independent of Cl− and acetoacetate (Figures 4D and 4E). No Cl− uptake by VMAT2 was observed (Figure 4F).
Acetoacetate modulates vesicular glutamate release
The fact that ketone bodies, in particular acetoacetate, modulate VGLUTs activity raises the possibility that ketone bodies modulate glutamatergic neurotransmission in vivo. We therefore investigated the effect of acetoacetate on vesicular storage and subsequent exocytotic release of glutamate.
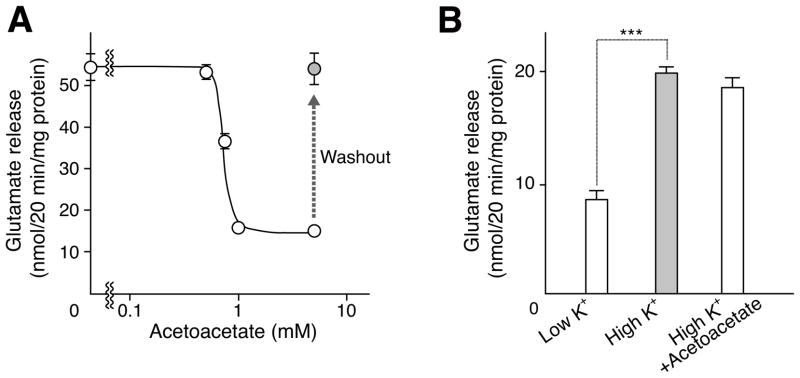
The effect of acetoacetate on the exocytosis of glutamate from neurons and astrocytes derived from hippocampus was studied first. Upon stimulation with 50 mM KCl, appreciable amounts of glutamate were released from neurons (Figure 5A). Acetoacetate inhibited glutamate exocytosis when added to culture medium at concentrations above 0.5 mM (Figure 5A). Maximum inhibition was observed at 1 mM acetoacetate. The inhibition was fully reversed by removal of acetoacetate from the culture medium (Figure 5A). Astrocytes also express VGLUT and are thus capable of storing glutamate in synaptic-like microvesicles and secreting it through exocytosis (Bezzi et al., 2004). Upon KCl stimulation, glutamate (19.8 ± 0.3 nmol/20 min/mg protein) was released from astrocytes. However, glutamate release was not affected by 5 mM acetoacetate (Figure 5B). Thus, acetoacetate reversibly inhibits glutamate exocytosis in neurons but not in astrocytes.
Figure 5. Acetoacetate modulates vesicular glutamate release from neurons but not from astrocytes.
A. Cultured hippocampal neurons were treated with the indicated concentrations of acetoacetate 20 min before KCl stimulation. For the “washout” experiment, acetoacetate-treated cells were washed with medium and incubated a further 1 h. KCl-dependent glutamate release was assessed for 20 min. Error bars represent mean ± SEM; n = 6. B. Hippocampal astrocytes were also treated with 5 mM acetoacetate. Glutamate secretion upon the addition of KCl was measured as described above. Error bars represent mean ± SEM; n = 6; asterisks indicate statistical significance, ***P<0.001.
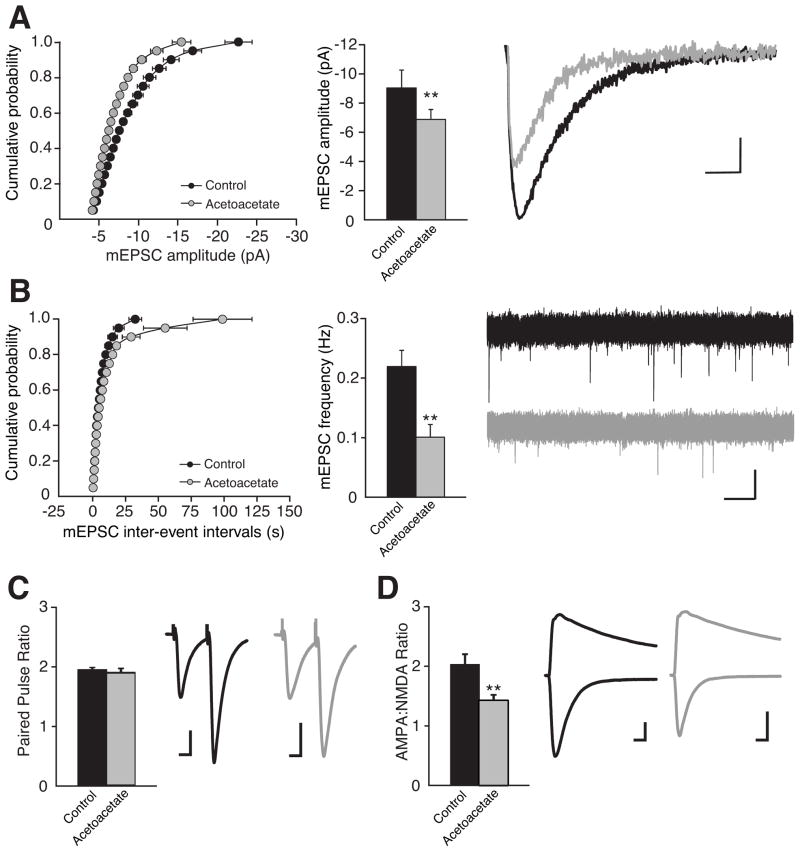
The effect of acetoacetate on quantal glutamate release was monitored by recording miniature excitatory postsynaptic currents (mEPSCs) from CA1 pyramidal cells in acute mouse hippocampal slices (Figure 6). The average amplitude of mEPSCs in slices with 10 mM acetoacetate (6.88 ± 0.69 pA; n = 15) was significantly smaller than in control slices (9.03 ± 1.23 pA; n = 15), reflecting a reduction in quantal size (Figure 6A). Acetoacetate also reduced the frequency of mEPSCs induced by hypertonic sucrose (Figure 6B), suggesting either a reduced likelihood of release or merely an increased proportion of events falling below the detection threshold of ~4 pA. However, there was no effect of acetoacetate on the paired-pulse ratio (Figure 6C) indicating no change in the probability of release. Interestingly, acetoacetate treatment resulted in a reduction in the AMPA:NMDA EPSC ratio (Figure 6D) suggesting that the synaptic glutamate concentration is non-saturating at AMPA receptors, a conclusion supported by other observations (Gray and Nicoll, unpublished data). In addition, acetoacetate had no effect on the amplitude or frequency of miniature inhibitory postsynaptic currents (mIPSCs) (Figure S8). Taken together, these results are consistent with the idea that acetoacetate suppresses the amount of glutamate stored per synaptic vesicle.
Figure 6. Acetoacetate reduces quantum glutamate release.
Whole-cell voltage clamp recordings from CA1 pyramidal neurons in acute mouse hippocampal slices incubated in either 10 mM LiCl or 10 mM lithium acetoacetate for >2h. A. mEPSC amplitude is significantly reduced following incubation with acetoacetate. Cumulative distribution and average mEPSC amplitudes (n=15) representative averaged traces; scale bars, 2 pA, 10 msec. B. mEPSC frequency is significantly reduced following incubation with acetoacetate. Cumulative distribution of mEPSC inter-event intervals (n=15), average mEPSC frequency, and sample traces; scale bars, 10 pA, 2 sec. C. Paired pulse ratio (PPR) is unchanged (n=16). Average PPR and representative traces; scale bars, 20 pA, 15 msec. D. AMPA:NMDA EPSC ratio is significantly reduced (n=16). Average ratio and representative traces; scale bars, 40 pA, 20 msec. Error bars = mean ± SEM; asterisks indicate statistical significance, **p<0.01.
Acetoacetate-mediated seizure control
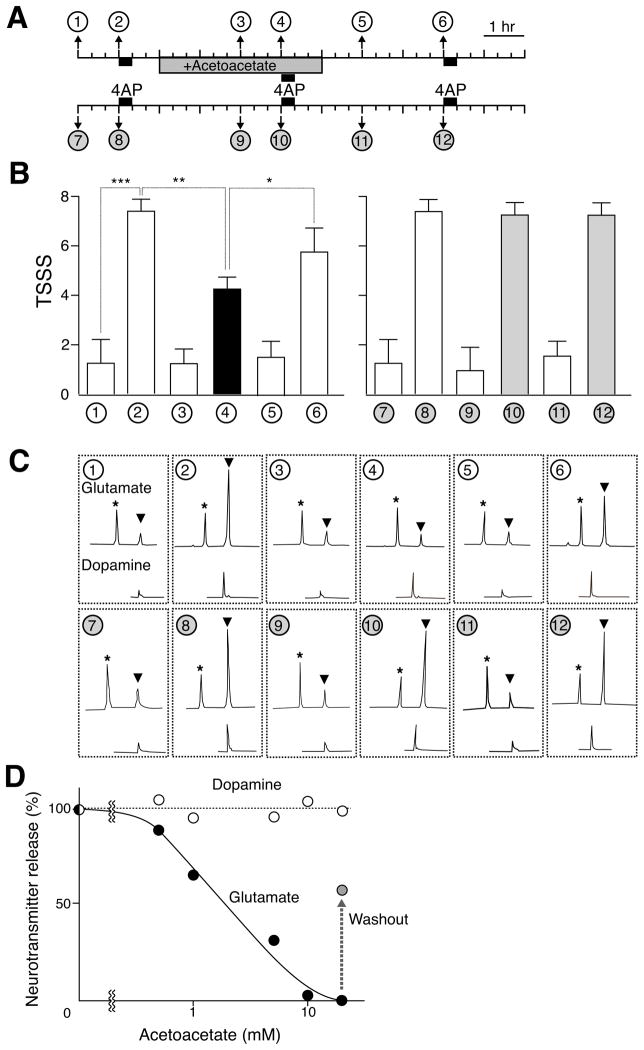
As the ability of acetoacetate to inhibit VGLUTs and transmitter release suggests that this may account for its anticonvulsant activity. (Freeman et al., 2006; Hartman et al., 2007). We therefore examined the effect of acetoacetate on 4-aminopyridine (4AP)-evoked glutamate secretion and seizures in the rat. 4AP is a K+-channel blocker with convulsant activity (Pena and Tapia, 2000), and was introduced through a micropipette directly into the rat brain. Extracellular levels of glutamate were monitored before, during and after the seizures, using dopamine as a control (Figure 7A). Administration of 4AP evoked seizures (Figure 7B) and led to the concomitant secretion of glutamate and dopamine as previously reported (Figures 7A and 7C; Pena and Tapia, 2000). Acetoacetate reduced the intensity of 4AP-evoked seizures (Figure 7B) and also decreased glutamate release in a dose-dependent manner (Figures 7C and 7D). In contrast, acetoacetate did not affect 4-AP-evoked dopamine secretion at all (Figures 7C and 7D). After removing acetoacetate from the dialysis solution, the intensity of 4AP-evoked seizures again increased (Figure 7B). The levels of glutamate secretion also partially recovered (Figures 7C and 7D).
Figure 7. Acetoacetate inhibited 4AP-evoked seizures and glutamate secretion.
A. Schematic diagram and typical protocol of 4AP-evoked seizures. At the times and duration indicated, 10 mM 4AP was added. Acetoacetate (10 mM) was added during the indicated period. B. 4AP-evoked seizures and the protective effect of 10 mM acetoacetate were observed for 1 h after each addition of 4AP and given a total seizure severity score (TSSS) based on behavioral analysis. Error bars represent mean ± SEM; n = 6. Asterisks indicate statistical significance (Student's t-test), *P<0.1, ** P<0.01, *** P<0.001. C. Typical HPLC chromatogram of glutamate and dopamine secreted at the indicated sampling times. * o-phospho-L-serine at 40 pmol as an internal marker. D. Dose dependence of acetoacetate on 4AP-evoked glutamate secretion.
DISCUSSION
Mechanism of Cl− dependence
The use of proteoliposomes containing purified VGLUT protein enabled us to separate the energetic coupling between primary proton pump and VGLUT, and to assess directly the interaction of Cl− with VGLUT. Using this assay, we presented evidence that extravesicular Cl− is absolutely necessary for glutamate transport, and acts as an allosteric effector. Strong positive cooperativity (~3) suggested that VGLUT-mediated glutamate transport is tightly regulated through Cl−. No active Cl− transport occurs during Δψ and Cl−-mediated glutamate transport through VGLUT. The rate of glutamate uptake is not affected by Cl− concentrations above 5 mM, indicating clearly that the decline in glutamate uptake by synaptic vesicles or proteoliposomes containing VGLUT and F-ATPase observed at higher Cl− concentrations in previous work (Naito and Ueda, 1985; Moriyama and Yamamoto, 1995; Juge et al., 2006; Schenck et al., 2009) is due to a secondary effect of the primary proton pump rather than VGLUT. Moreover, these results refute the possibility that the decline in uptake at Cl− concentrations above 10 mM results from competition with glutamate as recently proposed (Schenck et al., 2009). In further contrast to this report, we did not observe any stimulatory effect of intravesicular Cl−. At the present, this discrepancy is hard to explain. One possible explanation is that intravesicular Cl− activates the F-ATPase and thereby increases Δψ and extravesicular Cl− leaking from the lumen facilitates glutamate uptake. Such activation by internal Cl− has been observed for the V-ATPase (Moriyama and Nelson, 1987a, b).
We found that DIDS competes with Cl−. We also found that ketone bodies reversibly inhibited VGLUT2 through competition with Cl− and DIDS. These results suggested that Cl− and acetoacetate share the same binding site on VGLUT2 and that the occupation of this site by either Cl− or acetoacetate determines the activity status of VGLUT2, although a possibility that they bind at different sites that interact allosterically cannot be extruded. It is noteworthy that essentially the same results were observed with isolated synaptic vesicles (Figure S5), supporting the presence of extravesicular binding site(s) for Cl− and ketone bodies on VGLUT2. We further demonstrated that not only VGLUTs but also VNUT and VEAT exhibited similar Cl− dependence and lack of Cl− transport. NPT1, the first identified member of SLC17 transporter family, also exhibits similar Cl− dependence (Iharada et al., 2010). Furthermore, we recently found that SLC17 transporters in Drosophila melanogaster also retain similar Cl− dependence and lack of Cl− transport (Miyaji, Laridon, Dermaut, Omote and Moriyama, manuscript in preparation). Thus, the use of Cl− as an allosteric effector reflects a common mechanism for SLC17-type anion transporters.
At present, we are not able to identify the precise location of the putative Cl− binding site(s) on VGLUT2. Based on the 3D structure of the bacterial glycerol-3-phosphate transporter, we have proposed a structural model of VGLUT2 in which the amino acid residues essential for glutamate transport, Arg184, His128 and Glu191 are located close to the bottom of the protein (Juge et al., 2006; Almqvist et al., 2007). We assume that basic amino acid residue(s) in this area of the molecule are important for Cl− binding, and are now trying to identify the putative Cl− binding site(s) by extensive chemical modification combined with site-directed mutagenesis.
Metabolic control of glutamate release
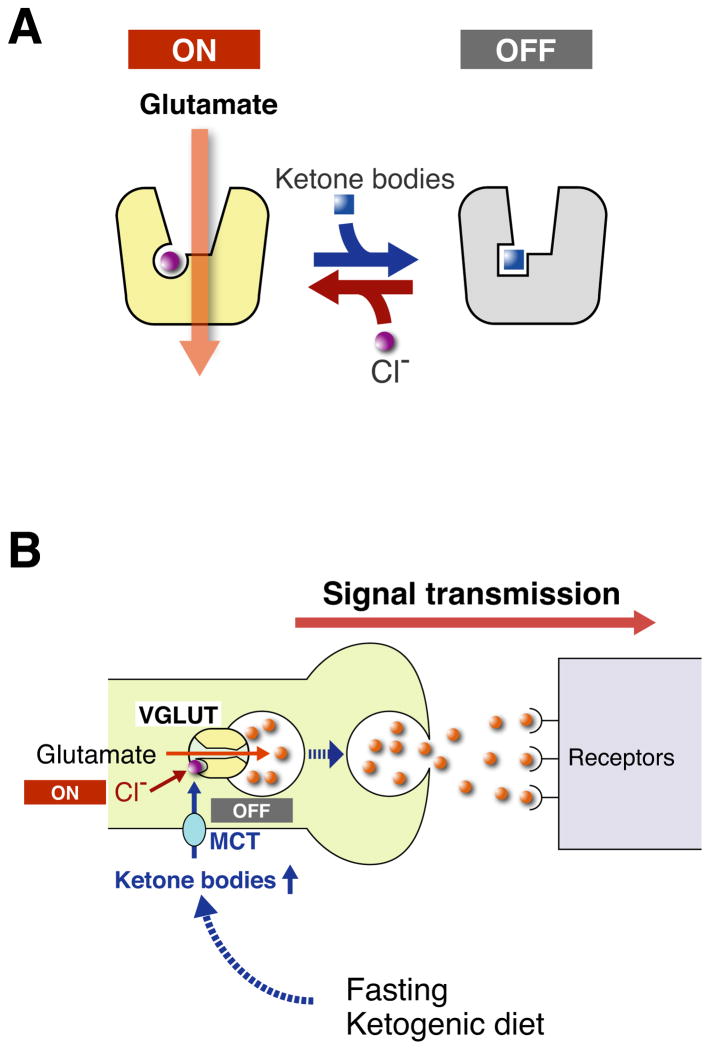
The unusually strong cooperativity of Cl− activation (~3) makes it possible for small changes in cytoplasmic [Cl−] to influence the activity of VGLUTs. When acetoacetate is present, the Cl− dependence of VGLUTs is modified and higher Cl− concentrations are needed to maintain transport activity. These observations suggest a novel mechanism to regulate glutamate release. The allosteric regulation of VGLUTs by Cl− functions as a switch to turn vesicular storage on or off, and influences the subsequent release of glutamate (Figure 8). Ketone bodies such as acetoacetate act as physiological, reversible and non-toxic modulators for this switch. Under normal conditions, the switch is continuously “on”, but when the concentration of ketone bodies rises sufficiently, the switch is turned off. Ketone bodies produced in the liver enter the circulatory system from which they eventually permeate the blood-brain barrier through a monocarboxylate transporter (MCT), and then serve as substrates for energy production in neurons and/or astrocytes (Figure S4; Laffel, 1999; Hartman et al., 2007; Freeman et al., 2006). The blood level of ketone bodies of ~10 mM upon either fasting or ingestion of a ketogenic diet appears easily high enough to modulate VGLUTs function and quantal glutamate release.
Figure 8. Proposed mode of action of ketone bodies on VGLUT-mediated suppression of glutamatergic neurotransmission.
A. The activity state of VGLUT is strongly dependent on the positive cooperativity of Cl− binding. Acetoacetate may replace Cl−, which may lead to inactivation of VGLUT, causing suppression of vesicular glutamate release. B. The concentration of circulating ketone bodies (acetoacetate) increases upon ketosis and can effectively suppress VGLUT activity leading to a shut down in excitatory neurotransmission. Once the state of ketosis is ended, for instance by eating carbohydrates, this suppression is readily released and excitatory neurotransmission recovers.
We found that this modulation of glutamate release by ketone bodies occurs in vivo. When applied to cultured neurons and sliced brain tissues, acetoacetate effectively and reversibly inhibited vesicular glutamate release. In addition, acetoacetate reduced quantal size as measured by mEPSCs in slices without affecting mIPSCs. Furthermore, acetoacetate suppresses the seizures and glutamate release evoked with 4AP. Since a ketogenic diet does not affect free glutamate levels in the brain nor the expression and activities of glutamate receptors or plasma membrane glutamate transporters, the present results support the idea that the target of this acetoacetate-evoked reduction in quantal size is VGLUT itself. It should be stressed that there are several seizure models and whether ketone bodies are effective in models other than 4AP-evoked seizures awaits further study.
It is noteworthy that acetoacetate-evoked modulation is neuron-specific. Astrocytes are glutamatergic in nature and secrete glutamate through exocytosis of VGLUT-containing secretory vesicles (Bezzi et al., 2004). Although all VGLUT isoforms exhibit similar affinity to acetoacetate, acetoacetate had no effect on vesicular glutamate release by astrocytes. At present, we do not know the reason for the lack of effect in astrocytes, but it is possible that ketone bodies are transported with different efficiencies due to the different transporters expressed in neurons and astrocytes and that this may lead to different accessibilities of acetoacetate to the Cl−-binding site on VGLUT. MCT is involved in the mobilization of ketone bodies into the brain. The isoform MCT2 is expressed in neurons, while isoforms MCT1 and MCT4 are present in astrocytes (Bergersen et al., 2002; Debernardi et al., 2003).
Since ketone bodies are metabolic intermediates connecting fatty acid oxidation, glycolysis and the TCA cycle, metabolic state may control glutamatergic neurotransmission by switching the VGLUTs between active and inactive states (Figure 8). Thus the metabolic control of vesicular glutamate release explains at least in part how ketone bodies help to control epilepsy. This mechanism also explains at least in part how ketone bodies exert neuroprotective effect (Zhao et al., 2006: Gasior et al., 2006; Hartman et al., 2007). It is well known that disruption of ketosis by the ingestion of sweets (carbohydrates) negatively and acutely affects the therapeutic efficacy of the ketogenic diet, but the mechanism remains unknown (Freeman et al., 2006). According to our model (Figure 8B), this phenomenon can be explained as follows: fasting and ketogenic diets cause ketosis, which in turn suppresses glutamatergic neurotransmission through the inhibition of vesicular glutamate storage. The ingestion of carbohydrates decreases levels of acetoacetate, which quickly turn VGLUT activity on, leading to an influx of glutamate into synaptic vesicles, and the restoration of glutamatergic neurotransmission. Thus, sugar-dependent breakdown of the therapeutic effect by the ketogenic diet may be consequence of metabolic control of vesicular release.
The switching of VGLUTs may also occur in pathological conditions, for example the maple syrup urine disease, a genetic disorder affecting the branched chain keto acid dehydrogenase complex. Toxic metabolites such as α-ketoisovaleric acid, α-keto-β-methylvaleric acid and α-ketoisocaproic acid accumulate and cause various neurological dysfunction. These compounds inhibit ATP-dependent glutamate uptake into isolated synaptic vesicles (Reis et al., 2000) and modulate Cl−-dependence of VGLUTs (Figure S3). Loss of vesicular glutamate release may be at least partly responsible for the various mental symptoms observed in these patients.
Conclusions and perspectives
The results show that anion-dependent switching of VGLUT activity plays a key role in the metabolic control of vesicular glutamate release. Cl− acts as an allosteric activator of VGLUT and triggers glutamate uptake upon binding. Ketone bodies compete for the putative Cl− binding site(s) and turn VGLUT activity off upon binding, causing a reduction in glutamatergic neurotransmission in vivo. The identification of ketone bodies as physiological modulators of VGLUTs opens the path for novel approaches in the development of drugs to treat neurological disorders caused by excessive glutamatergic neurotransmission. Drugs that interact with the putative Cl− binding site(s) would be expected to suppress excitatory neurotransmission. Elucidation of the molecular mechanism for allosteric regulation by Cl− and ketone bodies would greatly contribute to the understanding of the SLC17 family transport mechanism since the switching regulatory mechanism is conserved among all members, raising the possibility that purinergic and aspartergic neurotransmission could be also regulated by ketone bodies.
EXPERIMENTAL PROCEDURES
Expression of VGLUT2
Recombinant baculovirus containing rat VGLUT2 was described elsewhere (Juge et al., 2006). Recombinant baculovirus was constructed using the Bac-to-Bac baculovirus expression system (Invitrogen) according to the manufacturer s protocol. Briefly, cDNA was cloned into the pDEST10 vector to generate genes for N-terminal 6xHis-tag fusion proteins. DH10Bac cells carrying bacmid DNA were transformed with the resulting pDEST10 vector and recombinant bacmid was isolated from clones of these cells. Recombinant baculoviruses were obtained by infection of Sf9 cells with bacmid. High Five cells (5 × 106 cells/10 cm dish) were grown in Express Five medium (Gibco) supplemented with 2 mM L-glutamine and 10 μg/ml gentamycin at 27 °C. High Five cells were infected with recombinant baculoviruses at a multiplicity of infection of 2 and grown for an additional 48 hours.
Purification of VGLUT2
VGLUT2 was purified as described previously (Juge et al., 2006). Insect cells (1~2 × 108 cells) were suspended in buffer containing 20 mM Tris-HCl (pH 8.0), 0.1 M potassium acetate, 10% glycerol, 0.5 mM dithiothreitol, 1 μg/ml pepstatin A and 1 μg/ml leupeptin and disrupted by sonication with a TOMY UD200 tip sonifier. Cell lysates were centrifuged at 700 × g for 10 min to remove debris and the resultant supernatant was centrifuged at 160,000 × g for 1 h. The pellet (membrane fraction) was suspended in buffer containing 20 mM MOPS-Tris (pH 7.0), 10% glycerol, 1 μg/ml pepstatin A and 1 μg/ml leupeptin at approximately 3 mg protein/ml. The membrane fraction was solubilized with 2% octylglucoside. After centrifugation at 260,000 × g for 30 min, the supernatant was added to 1 ml of Ni-NTA Superflow resin (Qiagen) and incubated for 4 hours at 4 °C. The resin was washed with 10 ml of 20 mM MOPS-Tris (pH 7.0), 5 mM imidazole, 20% glycerol and 1% octylglucoside in a column. VGLUT2 was eluted from the resin with 3 ml of the same buffer containing 60 mM imidazole. The eluate containing purified VGLUT2 was stored at −80 °C where it was stable without loss of activity for at least a few months.
Reconstitution of VGLUT2
Reconstitution of purified recombinant VGLUT2 into liposomes was carried out by the freeze-thaw method described elsewhere (Juge et al., 2006). In brief, 10 μg VGLUT2 was mixed with 500 μg liposomes, frozen at −80 °C and left at this temperature for 15 min. The mixture was thawed quickly by holding the sample tube in the hand and diluted 60-fold with reconstitution buffer (20 mM MOPS-Tris (pH 7.0), 0.5 mM dithiothreitol, 0.15 M sodium acetate and 5 mM magnesium acetate). The buffer composition was changed as necessary. Reconstituted proteoliposomes were pelleted by centrifugation at 200,000 × g for 1 h at 4 °C and suspended in 0.2 ml of 20 mM MOPS-Tris (pH 7.0) containing 0.15 M sodium acetate and 5 mM magnesium acetate. Asolectin liposomes were prepared as follows. Soybean lecithin (20 mg; Sigma type IIS) was suspended in 2 ml of 20 mM MOPS-NaOH (pH 7.0) containing 1 mM dithiothreitol. The mixture was sonicated in a bath-type sonicator until clear, divided into small aliquots, and stored at −80 °C until use.
Expression, purification and reconstitution of VGLUT1, VGLUT3, VNUT, VGAT and VMAT2
The cDNAs of rat VGLUT1, human VGLUT3, human VNUT, mouse VEAT, rat VGAT and rat VMAT2 have been described previously (Bellocchio et al., 2000; Takamori et al., 2002; Sawada et al., 2008; Miyaji et al., 2008; Juge et al., 2009; Liu et al., 1992). These transporters were expressed in insect cells, purified, reconstituted into proteoliposomes and assayed in a manner analogous to that described for rat VGLUT2 above.
Uptake of neurotransmitters
Assays were carried out by the gel-permeation procedure as described previously (Juge et al., 2006). For Val-evoked glutamate uptake, a 500 μl of reaction mixture containing 20 mM MOPS-Tris (pH 7.0), 5 mM magnesium acetate, 10 mM KCl, 0.14 M potassium acetate, 2 μM Val and 100 μM [2,3-3H] L-glutamate (0.5 MBq/μmol, GE Health Care) was incubated for 3 min at 27 °C. Proteoliposomes containing VGLUT2 (0.5 μg protein per assay) were added to the mixture to initiate the reaction and incubated for a further 1 min. Aliquots (130 μl) were taken at the times indicated and centrifuged through a Sephadex G-50 (fine) spin column at 760 × g for 2 min. Radioactivity in the eluate was counted by liquid scintillation.
Essentially the same protocol was used for the Val-evoked uptake of aspartate and ATP. The assay mixture containing 100 μM [2,3-3H] aspartate (0.5 MBq/μmol) or 100 μM [α32P] ATP (3.7 MBq/μmol) was used instead of glutamate. For serotonin uptake by VMAT2, proteoliposomes were prepared as described above except for 20 mM MES-Tris (pH 5.5) was used. The assay was initiated by addition of proteoliposomes to assay mixture containing 20 mM MOPS-Tris (pH 7.0), 5 mM magnesium acetate, 10 mM KCl, 0.14 M potassium acetate, 10 μM [2-3H] serotonin (0.5 MBq/μmol) and incubated for a further 1 min at 27 °C. Aliquots (130 μl) were taken at the times indicated and centrifuged through a Sephadex G-50 (fine) spin column. Radioactivity of the eluate were measured.
Cl− transport
For Cl− transport, a 500 μl reaction mixture containing 20 mM MOPS-Tris (pH 7.0), 5 mM magnesium acetate, 5 mM glutamate, 0.14 M potassium acetate, 2 μM Val and 10 mM radioactive 36Cl− (740 MBq/g, ARC) was preincubated for 3 min at 27 °C. Reactions were started by adding the VGLUT2-containing proteoliposomes (0.5 μg protein per assay) and incubated for a further 1 min. Aliquots (130 μl) were taken at the indicated times and passed through a spin column as described above. Essentially the same protocol was used for Cl− transport by VGAT-containing proteoliposomes instead that GABA at 5 mM was included in the assay mixture (Juge et al., 2009). Nystatin was added to a final concentration of 250 μM to determine internal [Cl−] after equilibrium of Cl− was established across the proteoliposomal membrane.
Cl− transport was also monitored by SPQ fluorescence with excitation (Ex) and emission (Em) wavelengths of 344 nm and 443 nm, respectively, with a path length of 5 nm (Biwersi et al., 1994). The assay was carried out at 27 °C in 2 ml 20 mM MOPS-Tris (pH 7.0), 5 mM magnesium acetate, 10 mM KCl, 0.15 M potassium acetate and proteoliposomes containing 1 μg VGLUT2 or VGAT.
Measurement of Δψ
Δψ was measured by both fluorescence quenching of oxonol-V, as described (Juge et al., 2006) and radioisotope distribution (Juge et al., 2009). Proteoliposomes were incubated under standard assay conditions in the presence of 20 μM [14C] potassium SCN for 3 min, and the internal concentration of [14C]SCN was quantified as described. The internal volume of proteoliposomes was determined by measuring the exclusive volume with [14C]mannitol (Juge et al., 2006). Δψ was then calculated using following formula: Δψ = RT/F × ln{[internal SCN ]/[external SCN ]}, R; gas constant, T; absolute temperature, F; Faraday constant.
Cell culture and isolation of neurons and astrocytes
Rat fetal hippocampal neurons were isolated and cultured as described in a previously published report (Banker and Cowan, 1977). After isolation, the hippocampus was incubated in Hank's solution containing 0.25% trypsin, 0.01% DNase1 for 15 min at 37 °C. The cells were washed twice with DMEM and cultured at 4.0 × 105 cells/3.5 cm dish in Neurobasal medium (GIBCO) supplemented with 0.5 mM glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.25 mg/ml fungizone and B27 supplement (GIBCO). Hippocampal astrocytes were isolated using a procedure similar to that used for neurons, cultured in Dulbecco s modified Eagle s medium containing 10% fetal bovine serum in 5% CO2/95% air at 37 °C.
Determination of L-glutamate and dopamine
Cultured cells (4.0 × 106 cells/dish) were washed three times with Ringer s solution containing 128 mM NaCl, 1.9 mM KCl, 1.2mM KH2PO4, 2.4 mM CaCl2 1.3 mM MgSO4, 26 mM NaHCO3, 10 mM glucose, 0.2% BSA, 10 mM HEPES-Tris (pH 7.4) and then incubated in Ringer s solution and inhibitor for 1h at 37 °C. The cultured cells were transferred to 2 ml of the above Ringer s solution containing KCl at the specified concentrations. At the times indicated, samples (100 μl each) were taken and the amount of L-glutamate was determined by high pressure liquid chromatography (HPLC) on a COSMOSIL5 C18-ARII column (4.6 × 150 mm; Nacalai Tesque Inc.) and fluorescence detected as described previously (Nakatsuka et al., 2001). Dopamine contents were measured by HPLC combined with amperometric detection as described previously (Nakatsuka et al., 2001).
Electrophysiology
Electrophysiological assessment was performed as described previously (Elias et al., 2006). Briefly, transverse 300 μm hippocampal slices were cut from C57BL/6 mice on a vibrating microtome (D.S.K microslicer DTK-1000, Dosaka EM, Kyoto, Japan) in a solution containing: 2.5 mM KCl, 0.5 mM CaCl2, 7 mM MgCl2, 1.25 mM NaH2PO4, 25 mM NaHCO3, 7 mM glucose, 210 mM sucrose. Slices were recovered at 35 °C for ~1h in artificial cerebrospinal fluid (ACSF) containing: 110 mM NaCl, 2.5 mM KCl, 25 mM NaHCO3, 1 mM NaH2PO4, 10 mM glucose, 4 mM CaCl2, 4 mM MgCl2, and either 10 mM LiCl or 10 mM lithium acetoacetate depending on experimental condition. Slices were then maintained for at least 1h at room temperature, slices then transferred to a submersion chamber on an upright Olympus microscope, perfused in ACSF saturated with 95% O2/5% CO2. Whole cell recordings were obtained with 3 to 5 MΩ borosilicate glass pipettes filled with intracellular solution containing: 135 mM CsMeSO4, 8 mM NaCl, 10 mM HEPES (pH 7.3), 0.3 mM EGTA, 5 mM QX-314-Cl, 0.3 mM NaGTP, 4 mM MgATP, and 290 mOsm. For mIPSC experiments, 135 mM CsCl replaced CsMeSO4. mEPSCs were obtained at −70 mV in the presence of 0.5 μM TTX, 0.1 mM picrotoxin and 50 mM sucrose (to increase mEPSC frequency). mIPSCs were obtained at −70 mV in the presence of 0.5 μM TTX, 10 μM NBQX and 100 μM APV. Miniature currents were semiautomatically detected by offline analysis using in-house software in Igor Pro (Wavemetrics) with a detection threshold of 4 pA. For evoked responses, Schaffer collaterals were stimulated with a bipolar tungsten electrode at 0.1 Hz. Paired-pulse ratios were measured by giving two pulses at a 50 ms interval and taking the ratio of the two peaks of the EPSCs from an average of 40–50 sweeps. AMPA:NMDA ratios were measured by dividing the peak EPSC current at −70 mV by the EPSC current 100 msec after stimulation at +40 mV. Series resistance was monitored and not compensated and data from cells in which series resistance varied by >25% were discarded. Synaptic responses were collected with a Multiclamp 700B amplifier (Axon Instruments, Foster City, CA), filtered at 2 kHz and digitized at 10 kHz. For analysis of cumulative distributions, the D'Agostino-Pearson omnibus normality test was used. For all other analyses an unpaired t test was used. All errors bars represent standard error measurement.
Microdialysis
Microdialysis of rat brain hippocampus was carried out as previously described (Funada and Hara, 2001). Adult Wistar rats weighing 220–250 g each were group-housed under controlled conditions (24 °C with a 12-hour light-dark cycle). All procedures were carried out in accordance with the Guidelines for Animal Experiments at Okayama University. Rats were anesthetized with ketamine (60 mg/kg) and xylazine (10 mg/kg) and mounted on the stereotaxic frame. An intracranial guide (A-I-8, Eicom, Japan) was implanted in the left hippocampus (5.2 mm caudal to bregma, 4.6 mm lateral to bregma, and 4.6 mm ventral to dura) and was fixed to the skull with dental cement. The cannula was closed by insertion of a stainless steel obturator. Twenty-four hours after implantation of the guide cannula, A-I-8 microdialysis probes were inserted into the hippocampus and then connected to a microinjection pump at a rate of 2 μL/min. The probes were continuously perfused by microdialysis with modified Ringer s solution containing 147 mM NaCl, 2.3 mM CaCl2, 4 mM KCl. Seizure was induced by delivering 4AP (10 mM) through the microdialysis probe. Samples were collected every twenty minutes in a fraction collector and then analyzed by HPLC.
Behavioral assessment of seizure severity
Behavioral changes in each rat were recorded throughout the experiment. During each sample the rats were rated on a previously published seizure severity scale (Racine, 1972; Meurs et al., 2008) that corresponded to focal limbic seizure models: (0) normal and non-epileptic activity; (1) mouth and facial movements, hyperactivity, grooming, sniffing, scratching and wet dog snakes; (3) forelimb clonus, forelimb extension; (4) rearing, salivating, tonic-clonic activity; (5) falling, status epilepticus.
Miscellaneous procedures
Synaptic vesicles from the brain of rats (Wistar, male, about 200 g weight) were prepared by the published procedure without the last permeation step (Huttner et al., 1983). Transport assay by the synaptic vesicles, polyacrylamide gel electrophoresis in the presence of SDS and Western blotting were performed as described (Moriyama and Yamamoto, 1995). Protein concentration was assayed using bovine serum albumin as a standard (Schaffner and Weissmann, 1973).
Data analysis
All numerical values are shown as the mean ± SEM; n = 3–15, unless specified. Statistical significance was determined by the Student s t-test.
Supplementary Material
Acknowledgments
We thank Drs. Miki Hiasa and Masafumi Iharada for help in the immunohistochemical study and purification of VGLUT1 and VGLUT3. N.J. was supported by a Research Fellowship from the Japan Society for the Promotion of Science for Young Scientists. J.A.G. was supported by an NIH training grant. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan to Y.M., NIH 5R01MH080379 to R.A.N, NIMH, NIGMS and NIDA to R.H.E. The authors declare that they have no conflicting financial interests. Correspondence and reprints should be addressed to Y.M. (e-mail: moriyama@pharm.okayama-u.ac.jp). Y.M, T.I. H.O, H.U, C.H, R.H.E and R.A.N designed research, analyzed data and wrote the paper. N.J, J.A.G, T.M., H.O and T.I. performed research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature. 2004;427:803–807. doi: 10.1038/nature02314. [DOI] [PubMed] [Google Scholar]
- Almqvist J, Huang Y, Laaksonen A, Wang DN, Hovmoller S. Docking and homology modeling explain inhibition of the human vesicular glutamate transporters. Protein Sci. 2007;16:1819–1829. doi: 10.1110/ps.072944707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton DB, De Vivo DC. An animal model for the ketogenic diet. Epilepsia. 1974;15:211–227. doi: 10.1111/j.1528-1157.1974.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Bailey EE, Pfeifer HH, Thiele EA. The use of diet in the treatment of epilepsy. Epilepsy Behav. 2005;6:4–8. doi: 10.1016/j.yebeh.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–442. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RT, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Bergersen L, Rafiki A, Ottersen OP. Immunogold cytochemistry identifies specialized membrane domains for monocarboxylate transport in the central nervous system. Neurochem Res. 2002;27:89–96. doi: 10.1023/a:1014806723147. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Biwersi J, Tulk B, Verkman AS. Long-wavelength chloride-sensitive fluorescent indicators. Anal Biochem. 1994;219:139–143. doi: 10.1006/abio.1994.1242. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48:43–58. doi: 10.1111/j.1528-1167.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Paquet M, Pare JF, Hassel B, Smith Y, Hall RA, Dingledine R. Evidence against enhanced glutamate transport in the anticonvulsant mechanism of the ketogenic diet. Epilepsy Res. 2007;74:232–236. doi: 10.1016/j.eplepsyres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Debernardi R, Pierre K, Lengacher S, Magistretti PJ, Pellerin L. Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. J Neurosci Res. 2003;73:141–155. doi: 10.1002/jnr.10660. [DOI] [PubMed] [Google Scholar]
- Edwards RH. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–858. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Eiden LE, Schafer MK, Weihe E, Schutz B. The vesicular amine transporter family (SLC18): amine/ proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflugers Arch. 2004;447:636–640. doi: 10.1007/s00424-003-1100-5. [DOI] [PubMed] [Google Scholar]
- Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Freeman J, Veggiotti P, Lanzi G, Tagliabue A, Perucca E. The ketogenic diet: from molecular mechanisms to clinical effects. Epilepsy Res. 2006;68:145–180. doi: 10.1016/j.eplepsyres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004a;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004b;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Funada M, Hara C. Differential effects of psychological stress on activation of the 5-hydroxytryptamine- and dopamine-containing neurons in the brain of freely moving rats. Brain Res. 2001;901:247–251. doi: 10.1016/s0006-8993(01)02160-6. [DOI] [PubMed] [Google Scholar]
- Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Bahav Pharmacol. 2006;17:431–439. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasnier B. The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids. Pflugers Arch. 2004;447:756–759. doi: 10.1007/s00424-003-1091-2. [DOI] [PubMed] [Google Scholar]
- Gras C, Amilhon B, Lepicard EM, Poirel O, Vinatier J, Herbin M, Dumas S, Tzavara ET, Wade MR, Nomikos GG, et al. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nat Neurosci. 2008;11:292–300. doi: 10.1038/nn2052. [DOI] [PubMed] [Google Scholar]
- Hartinger J, Jahn R. An anion binding site that regulates the glutamate transporter of synaptic vesicles. J Biol Chem. 1993;268:23122–23127. [PubMed] [Google Scholar]
- Hartman AL, Gasior M, Vining EP, Rogawski MA. The neuropharmacology of the ketogenic diet. Pediatr Neurol. 2007;36:281–292. doi: 10.1016/j.pediatrneurol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phsphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iharada M, Miyaji T, Fujimoto T, Hiasa M, Anzai N, Omote H, Moriyama Y. Type 1 sodium dependent phosphate transporter (SLC17A1 protein) is a Cl−-dependent urate exporter. J Biol Chem. 2010;285 doi: 10.1074/jbc.M110.122721. an electric version. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N, Yoshida Y, Yatsushiro S, Omote H, Moriyama Y. Vesicular glutamate transporter contains two independent transport machineries. J Biol Chem. 2006;281:39499–39506. doi: 10.1074/jbc.M607670200. [DOI] [PubMed] [Google Scholar]
- Juge N, Muroyama A, Hiasa M, Omote H, Moriyama Y. Vesicular inhibitory amino acid transporter is a Cl−/γ-aminobutyrate co-transporter. J Biol Chem. 2009;284:35073–35078. doi: 10.1074/jbc.M109.062414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15:412–426. doi: 10.1002/(sici)1520-7560(199911/12)15:6<412::aid-dmrr72>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peter D, Roghani A, Schuldiner S, Prive GG, Eisenberg D, Brecha N, Edwards RH. A c DNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70:539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- Lund TM, Risa O, Sonnewald U, Schousboe A, Waagepetersen HS. Availability of neurotransmitter glutamate is diminished when beta-hydroxybutyrate replaces glucose in cultured neurons. J Neurochem. 2009;110:80–91. doi: 10.1111/j.1471-4159.2009.06115.x. [DOI] [PubMed] [Google Scholar]
- Ma W, Berg J, Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels. J Neurosci. 2007;27:3618–3625. doi: 10.1523/JNEUROSCI.0132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycox PR, Deckwerth T, Hell JW, Jahn R. Glutamate uptake by brain synaptic vesicles. Energy dependence of transport and functional reconstitution in proteoliposomes. J Biol Chem. 1988;263:15423–15428. [PubMed] [Google Scholar]
- Meldrum BS, Rogawski MA. Molecular targets for antiepileptic drug development. Neurotherapeutics. 2007;4:18–61. doi: 10.1016/j.nurt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs A, Clinckers R, Ebinger G, Michotte Y, Smolders I. Seizure activity and changes in hippocampal extracellular glutamate, GABA, dopamine and serotonin. Epilepsy Res. 2008;78:50–59. doi: 10.1016/j.eplepsyres.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Miyaji T, Echigo N, Hiasa M, Senoh S, Omote H, Moriyama Y. Identification of a vesicular aspartate transporter. Proc Natl Acad Sci USA. 2008;105:11720–11724. doi: 10.1073/pnas.0804015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moechars D, Weston MC, Leo S, Callaerts-Vegh Z, Goris I, Daneels G, Buist A, Cik M, van der Spek P, Kass S, et al. Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J Neurosci. 2006;26:12055–12066. doi: 10.1523/JNEUROSCI.2556-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama Y, Nelson N. The purified ATPase from chromaffin granule membranes is an-anion-dependent proton pump. J Biol Chem. 1987a;262:9175–9180. [PubMed] [Google Scholar]
- Moriyama Y, Nelson N. Internal anion binding site and membrane potential dominate the regulation of proton pumping by the chromaffin granule ATPase. Biochem Biophys Res Commun. 1987b;149:140–144. doi: 10.1016/0006-291x(87)91615-9. [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Yamamoto A. Vesicular L-glutamate transporter in microvesicles from bovine pineal glands. Driving force, mechanism of chloride anion activation, and substrate specificity. J Biol Chem. 1995;270:22314–22320. doi: 10.1074/jbc.270.38.22314. [DOI] [PubMed] [Google Scholar]
- Naito S, Ueda T. Characterization of glutamate uptake into synaptic vesicles. J Neurochem. 1985;44:99–109. doi: 10.1111/j.1471-4159.1985.tb07118.x. [DOI] [PubMed] [Google Scholar]
- Nakatsuka S, Hayashi M, Muroyama A, Otsuka M, Kozaki S, Yamada H, Moriyama Y. D-Aspartate is stored in secretory granules and released through a Ca2+-dependent pathway in a subset of rat pheochromocytoma PC12 cells. J Biol Chem. 2001;276:26589–26596. doi: 10.1074/jbc.M011754200. [DOI] [PubMed] [Google Scholar]
- Nakazawa M, Kodama S, Matsuo T. Effects of ketogenic diet on electroconvulsive threshold and brain contents of adenosine nucleotides. Brain, Dev. 1983;5:375–380. doi: 10.1016/s0387-7604(83)80042-4. [DOI] [PubMed] [Google Scholar]
- Noh HS, Lee HP, Kim DW, Kang SS, Cho GJ, Rho JM, Choi WS. A cDNA microarray analysis of gene expression profiles in rat hippocampus following a ketogenic diet. Brain Res Mol Brain Res. 2004;129:80–87. doi: 10.1016/j.molbrainres.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Nordli DR, De Vivo DC. The ketogenic diet revisited: back to the future. Epilepsia. 1997;38:743–749. doi: 10.1111/j.1528-1157.1997.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Parsons SM. Transport mechanisms in acetylcholine and monoamine storage. FASEB J. 2000;14:2423–2434. doi: 10.1096/fj.00-0203rev. [DOI] [PubMed] [Google Scholar]
- Pena F, Tapia R. Seizures and neurodegeneration induced by 4-aminopyridine in rat hippocampus in vivo: role of glutamate- and GABA-mediated neurotransmission and of ion channels. Neuroscience. 2000;101:547–561. doi: 10.1016/s0306-4522(00)00400-0. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Reimer RJ, Edwards RH. Organic anion transport is the primary function of the SLC17/type I phosphate transporter family. Pflugers Arch. 2004;447:629–635. doi: 10.1007/s00424-003-1087-y. [DOI] [PubMed] [Google Scholar]
- Reis M, Farage M, Wolosker H. Chloride-dependent inhibition of vesicular glutamate uptake by alpha-keto acids accumulated in maple syrup urine disease. Biochim Biophys Acta. 2000;1475:114–118. doi: 10.1016/s0304-4165(00)00069-6. [DOI] [PubMed] [Google Scholar]
- Russell JM, Eaton DC, Brodwick MS. Effects of nystatin on membrane conductance and internal ion activities in Aplysia neurons. J Membr Biol. 1977;37:137–156. doi: 10.1007/BF01940929. [DOI] [PubMed] [Google Scholar]
- Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W, Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973;56:502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Schenck S, Wojcik SM, Brose N, Takamori S. A chloride conductance in VGLUT1 underlies maximal glutamate loading into synaptic vesicles. Nat Neurosci. 2009;12:156–162. doi: 10.1038/nn.2248. [DOI] [PubMed] [Google Scholar]
- Schuldiner S. A molecular glimpse of vesicular monoamine transporters. J Neurochem. 1994;62:2067–2078. doi: 10.1046/j.1471-4159.1994.62062067.x. [DOI] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, et al. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–275. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smear MC, Tao HW, Staub W, Orger MB, Gosse NJ, Liu Y, Takahashi K, Poo MM, Baier H. Vesicular glutamate transport at a central synapse limits the acuity of visual perception in zebrafish. Neuron. 2007;53:65–77. doi: 10.1016/j.neuron.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Malherbe P, Broger C, Jahn R. Molecular cloning and functional characterization of human vesicular glutamate transporter 3. EMBO Rep. 2002;3:798–803. doi: 10.1093/embo-reports/kvf159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thio LL, Wong M, Yamada KA. Ketone bodies do not directly alter excitatory or inhibitory hippocampal synaptic transmission. Neurology. 2000;54:325–331. doi: 10.1212/wnl.54.2.325. [DOI] [PubMed] [Google Scholar]
- Vining EP. Clinical efficacy of the ketogenic diet. Epilepsy Res. 1999;37:181–190. doi: 10.1016/s0920-1211(99)00070-4. [DOI] [PubMed] [Google Scholar]
- Wallén-Mackenzie A, Gezelius H, Thoby-Brisson M, Nygård A, Enjin A, Fujiyama F, Fortin G, Kullander K. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J Neurosci. 2006;26:12294–122307. doi: 10.1523/JNEUROSCI.3855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci USA. 2004;101:7158–7163. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Horyn O, Nissim I, Nissim I. Ketosis and brain handling of glutamate, glutamine, and GABA. Epilepsia. 2008;49:73–75. doi: 10.1111/j.1528-1167.2008.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, Humala N, Thiyagarajan M, Wang J, Pasinetti GM. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006;7:29. doi: 10.1186/1471-2202-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupec-Kania BA, Spellman E. An overview of the ketogenic diet for pediatric epilepsy. Nutri Clin Prac. 2008;23:589–596. doi: 10.1177/0884533608326138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.