Abstract
Objectives. We sought to investigate the structure of the genetic and environmental influences on 3 measures of mental well-being.
Methods. Analyses focused on the subsample of 349 monozygotic and 321 dizygotic same-sex twin pairs from a nationally representative sample of twins who completed self-report measures of emotional, psychological, and social well-being.
Results. The best-fit model contained a common pathway to all 3 measures of well-being, no shared environmental effects, and 1 set of parameters for men and women. Heritability for the latent “mental well-being” factor was high (72%) and best indexed by psychological well-being. Moderate trait-specific genetic effects were seen for emotional and social well-being. Nonshared environmental effects for all measures were mostly trait specific.
Conclusions. Genetic influences on the measures of mental well-being reflect a single, highly heritable genetic factor, although some trait-specific genetic influences were seen for emotional and social well-being. Moderate proportions of environmental influences were also shared, but the majority of unique environment was trait-specific.
Historically, mental health has been viewed as the absence of mental disorder, despite concepts that health in general is something positive1 or well-being,2 and not merely the absence of illness. Mental well-being (i.e., positive mental health) is now a focus of policy and science. The 1999 US Surgeon General's report was devoted to mental health, defined as “a state of successful performance of mental function, resulting in productive activities, fulfilling relationships with people, and the ability to adapt to change and to cope with adversity.”3(p4) In 2004, the World Health Organization highlighted the need to promote good mental health, defined as
a state of well-being in which the individual realizes his or her own abilities, can cope with the normal stresses of life, can work productively and fruitfully, and is able to make a contribution to his or her community.2(p12)
Mental health has been operationalized under the rubric of subjective well-being, or individuals’ evaluations of the quality of their lives. The nature of subjective well-being has been divided into 2 streams of research. The first of these streams equates well-being with happiness or feeling good. The second approach to well-being focuses on human potential that, when cultivated, results in functioning well in life. These 2 streams of subjective well-being research grew from 2 distinct philosophical viewpoints on happiness—1 reflecting the hedonic tradition, which championed positive emotions, and the other reflecting eudaemonic tradition, which championed striving toward excellence in functioning as an individual and a citizen.
The hedonic tradition is reflected in research on emotional well-being, where scholars use measures of satisfaction with life and positive affect (e.g., cheerfulness, happiness, and contentment).4 The tradition of eudaemonia is reflected in research on psychological5 and social6 well-being. Here, scholars use multidimensional scales that ask individuals to evaluate how well they see themselves functioning in life as they strive to achieve secular standards of purpose, contribution, integration, autonomy, intimacy, acceptance, and mastery in life. When subjective well-being is measured comprehensively, studies support the tripartite model consisting of emotional, psychological, and social well-being among US adults,7 college students,8 and adolescents.9
Existing literature on the genetic and environmental etiology of subjective well-being has focused on the emotional well-being components of satisfaction with life,10 positive affect,11–15 or both aspects, with evidence suggesting that a common set of genes underlies both life satisfaction and positive affect.16 Broad heritability estimates in those studies have ranged from 36% to 56%; no study has found evidence for strong effects of the family environment. Only 1 study14 found support for sex-specific effects, with females reporting a slightly (8%) higher heritability estimate than that reported by males.
Our purpose was to extend previous studies by investigating the genetic and environmental influences on the 3 components of subjective well-being that comprise the assessment of the mental health continuum.17 We also examined whether the structures of the genetic and environmental influences on the components of mental well-being are the same for men and women. We conclude by discussing the public health implications of the heritability of mental well-being, or positive mental health, for prevention of mental illness.
METHODS
Approximately 50 000 households that were representative of the US population were screened by telephone as part of the Midlife in the US (MIDUS) study to determine if any immediate relatives who were members of intact twin pairs resided there. The original MIDUS study took place in 1995; the follow-up MIDUS II survey took place in 2005. Inclusion criteria for MIDUS I included being first-degree relatives of the original contact or the contact's partner, being aged between 25 and 74 years at the time of recruitment, living in the continental United States, being reachable by telephone, and being able to speak English. A total of 14.8% of screened households had twin pairs, of which 60% of this group gave permission for the twins to be contacted for study recruitment—a response rate comparable to that of other twin studies.18,19 Twins participating in the MIDUS I survey completed self-report measures of emotional, psychological, and social well-being. Zygosity was determined via self-report questions about physical similarity and confusion in identifying the twins by family members, friends, and strangers. Previous studies have indicated that such measures have greater than 90% accuracy in identifying the zygosity of twin pairs.20
Our study examined self-report measures from a subsample of 1386 twins from same-sex twin pairs originally contacted as part of the MIDUS I survey. Given the low power to detect qualitative sex effects,21 we excluded opposite-sex dizygotic twins from our analysis. With this sample size, we had no realistic chance of detecting qualitative sex effects had they been present, as has been suggested in much larger samples for major depression.22 Including opposite-sex dizygotic twins in the presence of such effects can cause biases in estimation, particularly an inflation of heritability.22 Our resulting subsample contained 670 complete pairs (plus 46 individual twins without their co-twin). The complete same-sex twin pairs were divided into the following groups: 186 female monozygotic, 198 female dizygotic, 163 male monozygotic, and 123 male dizygotic pairs. Compared with the national MIDUS random digit dialing (RDD) 1995 sample, the composition of our same-sex twin subsample was 57% female (RDD = 44%), 93% White (RDD = 84%), 72% married (RDD = 68%), and 57% reporting 13 or more years of formal schooling (RDD = 49%). The mean age of our subsample was 44.6 years (RDD = 45.3 years).
Measures
We utilized 3 measures of mental well-being, employing the terminology developed by Keyes based on his and others’ studies6–9 of the structure of well-being: emotional, psychological, and social well-being. The well-being literature does not employ a common set of terms for describing these dimensions. Although many psychologists tend to refer to emotional well-being as “subjective well-being,” Keyes argues that all 3 dimensions of mental well-being are subjective assessments of well-being that are more clearly identified by whether the measures focus on feeling states (i.e., hedonia) toward one's life or how well individuals see themselves functioning in their lives (i.e., eudaemonia), with the latter being distinguished in terms of whether the functioning is psychological (i.e., more private and personal) or social (i.e., more public and communal). For consistency with the past research, which involves the same model in this article we employ Keyes’ terminology.
Factor analyses (principal components extraction and oblimin rotation) replicated in the same-sex twins the 3-factor structure; all items that were used in previous studies to measure each component of well-being in the national RDD sample also loaded most strongly (0.40 or higher) on its own factor as described next (results not shown).
We assessed emotional well-being with a 6-item scale of positive affect (e.g., “During the past 30 days, how much of the time did you feel calm and peaceful?”) and a single item measuring overall life satisfaction that reflects emotional well-being. As part of the self-administered questionnaire, respondents indicated how much of the time during the past 30 days—“none of the time,” “a little,” “some,” “most,” or “all of the time” (scored from 1 to 5, respectively)—they felt 6 symptoms of positive affect. The signs of positive affect are (1) cheerfulness, (2) being in good spirits, (3) extreme happiness, (4) calmness and peacefulness, (5) satisfaction, and (6) being full of life.23–25 Respondents also were asked to “rate your life overall these days” on a scale from zero to 10, where zero meant “the worst possible life overall” and 10 meant “the best possible life overall.”26,27 The 6 items of positive affect were summed together along with the single item measuring best possible life to form the scale of emotional well-being.
We assessed psychological well-being with 6 scales of 3 items each28 that asked respondents to indicate how well each item described how they generally functioned. The subscales included self-acceptance (e.g., “I like most parts of my personality”), positive relations with others (e.g., “Maintaining close relationships has been difficult and frustrating for me”), personal growth (e.g., “For me, life has been a continual process of learning, changing, and growth”), purpose in life (e.g., “When I look at the story of my life, I am pleased with how things have turned out so far”), environmental mastery (e.g., “I am good at managing the responsibilities of daily life”), and autonomy (e.g., “I have confidence in my own opinions, even if they are different from the way most other people think”). Psychological well-being items were based on a response scale from 1 to 7 (with 4 as a middle category of “neither agree nor disagree”), respondents indicated whether they “agreed” or “disagreed” “strongly,” “moderately,” or “slightly” that an item described how they functioned. Negative items were reverse-coded, and all items were summed together to form the scale.
We assessed social well-being with 5 scales of 3 items each6 that asked respondents to indicate how well each item described how they generally functioned. The subscales included social acceptance (e.g., “I believe that people are kind”), social growth (e.g., “Society is becoming a better place for everyone”), social contribution (e.g., “I have something valuable to give the world”), social coherence (e.g., “I try to think about and understand what could happen next in our country”), and social integration (e.g., “I feel close to other people in my community”). Social well-being items were based on a response scale from 1 to 7 (with 4 as a middle category of “neither agree nor disagree”), and respondents indicated whether they “agreed” or “disagreed” “strongly,” “moderately,” or “slightly” that an item described how they functioned. Negative items were reverse-coded, and all items were summed together to form the scale.
Statistical Methods
Our models divided the sources of individual differences in the 3 forms of well-being into 3 classes: additive genetic effects (A), shared family environment (C), and unique environment (E).29 Shared environment effects emanate from family and community experiences that increase similarity in twins who are raised together. Unique environment effects include conditions and experiences not shared by members of a twin pair (as well as measurement error) that increase individual differences between twins.
The multivariate twin model was designed to examine the amount of shared genetic and shared environmental influences on emotional, social, and psychological well-being. We began with an independent pathway model,29 which allowed the patterns of covariance resulting from genetic and environmental factors to differ. We then tested whether the common pathway model,29 in which the genetic and environmental influences on the 3 dimensions of well-being emanate from a single latent “mental well-being” factor, provided a better fit. The full model divided the genetic and environmental influences on emotional, social, and psychological well-being into those influences resulting from a common factor that influences all forms of mental well-being versus influences that are specific to each kind of mental well-being. (In this model, measurement error would appear largely in the trait-specific unique environmental effects.)
By studying only the same-sex male and female twins from the MIDUS subsample, we could test for quantitative sex effects, allowing us to determine if the magnitude of the genetic and environmental parameters in our structural model differed between sexes. Twin model fitting was performed by using the Mx software package version 1.54a (Department of Psychiatry, Virginia Commonwealth University Medical School, Richmond, VA). The goal of model fitting is to achieve an optimal balance between explanatory power and simplicity. This goal is best operationalized by the use of Akaike's information criterion (AIC),30–32 which equals χ2–2(df ), where df is the model's degrees of freedom. We sought to minimize the AIC value, because a lower AIC value reflects a model that optimizes the balance of complexity (i.e., simplicity) and accuracy (i.e., explanatory power). The model with the lowest AIC is the best-fitting model.
RESULTS
Table 1 presents the phenotypic correlations, internal reliabilities, and means of the 3 measures of well-being by gender. Correlations between the well-being scales ranged from 0.36 to 0.52 for female twins and from 0.43 to 0.59 for male twins, revealing comparable correlational structure by gender. Estimates of scale reliabilities of well-being were nearly identical for men and women, and all estimates were above 0.77. Mean levels of all 3 types of well-being were also the same for men and women as determined by nonsignificant F tests.
TABLE 1.
Descriptive Statistics of Mental Well-Being Scales, by Gender of the Same-Sex Twin: Midlife in the US Survey, 1995
| Mental Well-Being Scales | Emotional Well-Being | Psychological Well-Being | Social Well-Being | Meanwomen (SE) |
| Emotional well-being | 0.88 (0.89) | 0.52 | 0.36 | 11.5 (0.08) |
| Psychological well-being | 0.59 | 0.77 (0.77) | 0.48 | 33.7 (0.19) |
| Social well-being | 0.43 | 0.53 | 0.79 (0.82) | 23.2 (0.19) |
| Scale score range | 1–15 | 16–42 | 9–35 | |
| Meanmen (SE) | 11.4 (0.08) | 33.3 (0.18) | 23.4 (0.19) |
Note. Women's correlations in the upper diagonal, men's in the lower. The women's Cronbach α is the nonparenthetical value in the diagonal, the men's is in parentheses.
Our full independent pathway model (model 1 in Table 2) contained separately estimated parameters among both men and women for class A, C, and E effects shared between the 3 measures of emotional, social, and psychological well-being and those specific to each measure. In model 2, we simplified the independent pathway model by constraining to equality all these parameter estimates among men and women. As seen in Table 2, this constraint resulted in a substantial improvement in the AIC value (–15.1). In model 3, we set to zero all of the class C parameters in model 2, which further improved the AIC value (–19.9). Model 4 set to zero all the class A parameters in model 2, which produced an AIC value inferior to that seen for model 3 (–15.4). We then imposed on model 3 a common pathway, constraining the genetic and environmental pathways to the 3 forms of well-being to pass through a latent construct of mental well-being. Model 5 fit moderately better than model 3 (–20.8), making it the best-fitting model.
TABLE 2.
Model Fitting Results for the 3 Measures of Mental Well-Being: Midlife in the US Survey, 1995
| Description | Change in χ2 | Change in df | Change in AIC | |
| Model 1 | Full-independent pathway | … | … | … |
| Model 2 | All parameters equated in men and women | 14.9 | 15 | −15.1 |
| Model 3 | Model 2 plus all class C parametersa set to 0 | 22.1 | 21 | −19.9 |
| Model 4 | Model 2 plus all class A parametersb set to 0 | 26.6 | 21 | −15.4 |
| Model 5c | Model 3 constrained to common pathways | 25.2 | 23 | −20.8 |
Note. AIC = Akaike's information criterion; df = degrees of freedom.
Class C parameters are shared family environments.
Class A parameters are additive genetic effects.
The best-fit model.
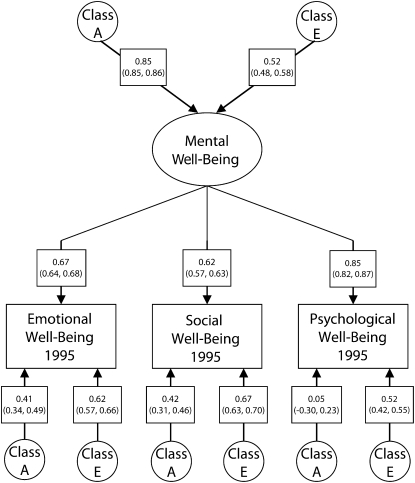
The parameter estimates with their 95% confidence intervals (CIs) from this best-fit model are shown in Figure 1; the results are summarized in Table 3. The heritability of the latent mental well-being factor was quite high at 72.3%. The heritability estimates were similar for the 3 kinds of mental well-being, ranging from a low of 45.6% for social well-being to a high of 52.3% for psychological well-being. The 3 well-being measures differed in the degree to which each measure reflected the common genetic factor as its primary source. Nearly all (i.e., 99%) of the genetic influences on psychological well-being originated from the common factor, compared with 65% for emotional and 61% for social well-being. In turn, trait-specific genetic influences (i.e., the amount of genetic influences attributable to other sources besides the common factor) were low and about equally important for social and emotional well-being (39% and 35%, respectively) and nearly nonexistent for psychological well-being (< 1%).
FIGURE 1.
Parameter estimates of the additive genetic (class A) and unique environmental (class E) influences on the common latent factor of mental well-being and on the 3 specific measures of well-being: MIDUS, 1995.
Note. MIDUS = Midlife in the US Survey. 95% confidence intervals in parentheses.
TABLE 3.
Decomposition of the Sources of Genetic and Environmental Variance in the Three Measures of Mental Well-Being: Midlife in the US Survey, 1995
| Genetic Effects |
Unique Environmental Effects |
|||||
| Mental Well-Being | Total Genetic Effect | % Common | % Specific | Total Unique Environmental Effect | % Common | % Specific |
| Emotional | 49.5 | 65 | 35 | 50.5 | 24 | 76 |
| Social | 45.6 | 61 | 39 | 55.4 | 19 | 81 |
| Psychological | 52.3 | 99 | 1 | 46.7 | 42 | 58 |
As seen in Table 3, three quarters or more of the environmental influences—which included measurement error—were trait specific for social and emotional well-being. Thus, most of the environmental influences on social and emotional well-being were attributable to sources other than the latent common factor. For psychological well-being, by contrast, more than 40% of the environmental variance resulted from the common factor rather than from trait-specific effects.
DISCUSSION
Our goal was to understand the interrelationships of the genetic and environmental factors that have an impact on the 3 dimensions of mental well-being: emotional, social, and psychological. We emphasize 5 major findings from our analyses.
First, we found that a common pathway model fit our data best, suggesting the existence of a latent propensity to mental well-being. This latent factor was quite heritable at 72% among the population. However, estimates of genetic effects of latent factors in such models are not directly comparable with estimates obtained from single scales; latent factor estimates are nearly always higher because errors of measurement are nearly all contained in the trait-specific environmental variance. Second, our measures for the 3 individual components of well-being—emotional, social, and psychological—were all moderately heritable, with estimates around 50%. Third, and consistent with the previous studies,11–16 we found no evidence for shared (familial) environmental influences on the measures of well-being.
Fourth, consistent with all but 1 previous investigation,14 we found no evidence that the magnitude of genetic and environmental effects on any kind of well-being differed for men and women. Fifth, all 3 measures were good indices of the latent propensity to mental well-being; psychological well-being, however, loaded particularly highly on the common factor and was therefore the best index of the 3 measures of the propensity for mental well-being. Whereas social and emotional well-being had considerable trait-specific genetic effects in our best-fit model, the genetic influence on psychological well-being was virtually all attributable to the common factor of mental well-being. This feature of the model also explains why a greater proportion of the environmental effects on psychological well-being was shared with the common factor, compared with environmental effects on social and emotional well-being.
Limitations
The number of twins in the MIDUS study is modest by modern standards and provides limited power to detect differences between the sexes or to discriminate between genetic and environmental sources of twin resemblance differences.29 Nonetheless, our model-fitting results were quite clear, with at least moderate and sometimes substantial differences in fit between competing models. The sample size was not sufficient to attempt to discriminate realistically between additive and nonadditive genetic effects.29
Conclusions
Compared with the independent pathways model, the common pathway twin model is more stringent because the covariation among the 3 kinds of well-being is by definition mediated by a single latent variable that in turn results from genetic and environmental factors. This latent variable in our model reflects mental well-being (i.e., the presence and relative absence of good mental health). At the phenotypic level, mental well-being is a syndrome, or a combination, of measures of positive feelings toward life (i.e., emotional well-being) and positive functioning in life (i.e., psychological and social well-being). At the genetic level, the latent construct of mental well-being contributes strongly to each of the 3 forms of subjective well-being.
Our findings supporting the common pathway model imply that the tripartite structure of well-being observed at the phenotypic level is caused by the latent, higher-order variable of mental well-being that has its own genetic and environmental influences. Because of the high loadings of the individual forms of well-being on the common factor, the common pathway model suggests that genetic influences operating on the latent variable will have a strong influence on each kind of well-being. Although emotional, social, and psychological well-being share a common set of genes, the 3 types of well-being are modestly correlated at the phenotypic level in large part because of environmental influences unique to each measure.7,8 Our findings support the validity of the diagnostic model of positive mental health—the “mental health continuum”—proposed by Keyes,17 where mental health, like mental illness, is best indicated by a syndrome of positive feelings toward life—i.e., emotional well-being—and functioning well in life in terms of psychological and social well-being.
Our findings raise the empirical question as to how mental well-being—the latent variable—differs from the lower-order facets of emotional, social, and psychological well-being. Moreover, support for the common pathway model in this study raises the empirical question as to how the higher-order construct of mental well-being creates the tripartite structure of subjective well-being.
Our model suggests 2 major processes. First, trait-specific genetic effects have moderate impacts on emotional and social well-being. Second, relatively substantial environmental influences unique to those 2 forms of well-being also have an impact on individuals. Quite likely, such genetic and environmental influences act over developmental time and involve effects of gene–environment correlations. For example, individuals with a strong genetic propensity for high levels of social well-being may create for themselves positive social environments that feed back, sustaining and further increasing their sense of social well-being. Future research should seek to identify individual traits and qualities associated with the genetic propensity for social well-being (e.g., compassion, altruism, and extraversion) that lead to the creation of positive social environments. In turn, future research should seek to identify the environmental qualities (e.g., trust, cooperation, and openness) that are conducive to well-being and that use this knowledge to promote the well-being of those with a lower innate propensity for well-being.
An important further research question is the degree to which genetic and environmental influences on well-being involve gene-by-environment interactions. For example, might a high genetic propensity for social or emotional well-being buffer the pathogenic effects of adverse environments (e.g., familial neglect) or exposure to high levels of stress and trauma? Conversely, might a low genetic predisposition to social and emotional well-being make individuals vulnerable to develop psychopathology in the face of mildly pathogenic or stressful environments? Understanding of how the genetic propensities to, and environmental influences on, mental well-being interrelate with each other and over developmental time can lead us to knowledge about how to create more mentally healthy environments and how to develop preventive efforts through promotion of protective aspects of well-being.
Acknowledgments
This research was supported by a grant from the National Institute on Aging (P01-AG020166) to conduct a longitudinal follow-up of the Midlife in the United States (MIDUS) investigation. The original MIDUS study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development.
Human Participant Protection
The MIDUS survey complied with institutional review board standards of the University of Wisconsin and of the Harvard Medical School, and interviewers read to the interviewees a standard informed consent protocol at the beginning of the telephone interview, which preceded the self-administered questionnaires.
References
- 1.Sigerist HE. Medicine and Human Welfare. New Haven, CT: Yale University Press; 1941 [Google Scholar]
- 2.World Health Organization Constitution Geneva, Switzerland: World Health Organization; 1948 [Google Scholar]
- 3.Mental Health: A Report of the Surgeon General. Rockville, MD: US Public Health Service; 1999 [Google Scholar]
- 4.Kahneman D, Diener E, Schwarz N, Well-Being: The Foundations of Hedonic Psychology. New York, NY: Russell Sage Foundation; 1999 [Google Scholar]
- 5.Ryff CD. Happiness is everything, or is it? Explorations on the meaning of psychological well-being. J Pers Soc Psychol. 1989;57:1069–1081 [Google Scholar]
- 6.Keyes CLM. Social well-being. Soc Psychol Q. 1998;61:121–140 [Google Scholar]
- 7.Gallagher MW, Lopez SJ, Preacher KJ. The hierarchical structure of well-being. J Pers. 2009;77(4):1025–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robitschek C, Keyes CLM. The structure of Keyes’ model of mental health and the role of personal growth initiative as a parsimonious predictor. J Couns Psychol. 2009;56:321–329 [Google Scholar]
- 9.Keyes CLM. Mental illness and/or mental health? Investigating axioms of the complete state model of health. J Consult Clin Psychol. 2005;73(3):539–548 [DOI] [PubMed] [Google Scholar]
- 10.Stubbe JH, Posthuma D, Boomsma DI, de Geus EJ. Heritability of life satisfaction in adults: a twin-family study. Psychol Med. 2005;35(11):1581–1588 [DOI] [PubMed] [Google Scholar]
- 11.Tellegen A, Lykken DT, Bouchard TJ, Wilcox KJ, Rich S, Segal NL. Personality similarity in twins reared apart and together. J Pers Soc Psychol. 1988;54(6):1031–1039 [DOI] [PubMed] [Google Scholar]
- 12.Lykken D, Tellegen A. Happiness is a stochastic phenomenon. Psychol Sci. 1996;7:186–189 [Google Scholar]
- 13.Røysamb E, Harris JR, Magnus P, Vitterso J, Tambs K. Subjective well-being: sex-specific effects of genetic and environmental factors. Pers Individ Dif. 2002;32:211–223 [Google Scholar]
- 14.Røysamb E, Tambs K, Reichborn-Kjennerud T, Neale MC, Harris JR. Happiness and health: environmental and genetic contributions to the relationship between subjective well-being, perceived health, and somatic illness. J Pers Soc Psychol. 2003;85:1136–1146 [DOI] [PubMed] [Google Scholar]
- 15.Nes RB, Røysamb E, Tambs K, Harris JR, Reichborn-Kjennerud T. Subjective well-being: genetic and environmental contributions to stability and change. Psychol Med. 2006;36:1033–1042 [DOI] [PubMed] [Google Scholar]
- 16.Bartels M, Boomsma DI. Born to be happy? The etiology of subjective well-being. Behav Genet. 2009;39(6):605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keyes CLM. The mental health continuum: from languishing to flourishing in life. J Health Soc Behav. 2002;43(2):207–222 [PubMed] [Google Scholar]
- 18.Kendler KS, Thornton LM, Gilman SE, Kessler RC. Sexual orientation in a US national sample of twin and sibling pairs. Am J Psychiatry. 2000;157(11):1843–1846 [DOI] [PubMed] [Google Scholar]
- 19.Kessler RC, Gilman SE, Thornton LM, Kendler KS. Health, wellbeing, and social responsibility in the MIDUS twin and sibling subsamples. : Brim OG, Ryff CD, Kessler RC, How Healthy Are We? A National Study of Wellbeing at Midlife. Chicago, IL: University of Chicago Press; 2004 [Google Scholar]
- 20.Lykken DT, Bouchard TJ, McGue M, Tellegen A. The Minnesota twin family registry: some initial findings. Acta Genet Med Gemellol (Roma). 1990;39(1):35–70 [DOI] [PubMed] [Google Scholar]
- 21.Prescott C.A., Gottesman I. Power limitations in detecting heterogeneity of genetic effects: the case of sex differences in alcoholism. Presented at the annual meeting of the Society for Research on Psychopathology; October 23–26, 1993; Chicago, IL [Google Scholar]
- 22.Kendler KS, Gatz M, Gardner C, Pedersen NA. Swedish national twin study of lifetime major depression. Am J Psychol. 2006;163:109–114 [DOI] [PubMed] [Google Scholar]
- 23.Bradburn NM. The Structure of Psychological Well-Being. Chicago, IL: Aldine; 1969 [Google Scholar]
- 24.Gurin G, Veroff J, Feld S. Americans View Their Mental Health. New York, NY: Basic Books; 1960 [Google Scholar]
- 25.Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: a developmental perspective on happiness. J Pers Soc Psychol. 1998;75(5):1333–1349 [DOI] [PubMed] [Google Scholar]
- 26.Andrews FM, Withey SB. Social Indicators of Well-Being: Americans’ Perceptions of Life Quality. New York, NY: Plenum; 1976 [Google Scholar]
- 27.Cantril H. The Pattern of Human Concerns. New Brunswick, NJ: Rutgers University Press; 1965 [Google Scholar]
- 28.Ryff CD, Keyes CLM. The structure of psychological well-being revisited. J Pers Soc Psychol. 1995;69(4):719–727 [DOI] [PubMed] [Google Scholar]
- 29.Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. New York, NY: Guilford Press; 2006 [Google Scholar]
- 30.Neale MC, Eaves LJ, Kendler KS. The power of the classical twin study to resolve variation in threshold traits. Behav Genet. 1994;24(3):239–258 [DOI] [PubMed] [Google Scholar]
- 31.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52(3):317–332 [Google Scholar]
- 32.Williams LJ, Holahan PJ. Parsimony-based fit indices for multiple-indicator models: do they work? Struct Equ Modeling. 1994;1(2):161–189 [Google Scholar]