Abstract
Background
Whole body vibration (WBV) is a novel modality of exercise shown to improve musculoskeletal function. This study aims to examine the effects of standing posture during low magnitude WBV training on muscle function and muscle morphology in older adults.
Methods
Nineteen men and women (50-80 years) were recruited to a three month randomised controlled trial and allocated to one of three groups: WBV with flexed knees (FK), WBV with locked knees (LK), or sham WBV with flexed knees (CON). Exposure was intermittent (1 min WBV:1 min rest) for 20 min, three times per week for 13 weeks. Measurements were taken at baseline and at three months. Primary outcomes included upper and lower body muscle function (strength, power and velocity). Secondary outcomes were muscle morphology, balance, habitual and maximal gait velocity, stair climb power, and chair stand performance.
Results
Sixteen subjects completed the study. Relative (%) upper body contraction velocity improved significantly after WBV with FK compared to LK (FK 16.0%, LK -7.6%, CON 4.7, p = 0.01). Relative upper body strength (LK 15.1%, p = 0.02; FK 12.1%, p = 0.04; CON 4.7%) increased significantly following WBV compared to control. Absolute (p = 0.05) and relative (p = 0.03) lower leg strength significantly improved with both standing postures (LK 14.4%; FK 10.7%; CON 1.3%). Only the LK group differed significantly from CON in relative leg strength gains (p = 0.02). Potentially clinically meaningful but statistically non-significant improvements in lower leg muscle cross-sectional area (LK 3.7 cm2, FK 2.4 cm2, CON 2.2 cm2 p = 0.13) were observed after WBV with LK compared to the other groups. No significant effects of WBV on any functional performance tests were observed.
Conclusions
Our results suggest that WBV may improve muscle strength and contraction velocity in some muscle groups in older adults. However, hypothesised differential adaptation to standing posture (FK > LK) was observed only for upper body contraction velocity, making recommendations regarding this prescriptive element inconclusive. The efficacy, mechanism of action and long term feasibility of WBV for musculoskeletal health in older adults warrants continued investigation in robustly designed, sufficiently powered future studies.
Trial Registration
ACTRN12609000353291.
Background
Age-related loses of skeletal muscle mass (sarcopenia) [1,2] and altered neuromuscular activation manifest as changes in muscle function in older adults [3,4]. Muscle weakness, reduced muscle power and slower contraction velocity are amongst these functional changes [5-9] and are important and prevalent risk factors for falls, frailty, disability and loss of functional independence in the aged [9-12]. Sarcopenia is common, being reported to occur in 40% of people aged 80 years and older [13], highlighting the risk of disability and loss of independence in this cohort.
Presently, pharmacological treatment [14-19], and resistance exercise [20,21] are available to alleviate age-related muscular deterioration [22]. However pharmacological methods have been variably successful, often resulting in unwanted side-effects [22,23]. Resistance training has been shown to be an effective method to counteract sarcopenia [21]; though for some older individuals with very advanced frailty or certain diseases, robust resistance exercise may be unavailable or precluded [5,24]. Whole Body Vibration (WBV) exposure has therefore been proposed as a potentially safe, low-intensity alternative to current modalities to combat sarcopenia in exercise-intolerant, exercise-aversive or mobility-limited individuals, without the potential risks or behavioural barriers associated with high intensity exercise.
WBV uses high-frequency mechanical stimuli generated by a vibrating platform which are transmitted through the body [25]. The mechanical stimuli produced are thought to use neural pathways, stimulating muscle spindles, the sensory receptors located within the belly of the muscle. The 1a afferent signals are transmitted monosynaptically to activate alpha-motoneurons, initiating muscle fibre contractions [26,27]. The vibration stimulus is thought to result in a tonic vibration reflex, or tonic contraction of the muscle [28].
Improvements from WBV exercise have been reported in muscle function (strength [2,29-33], power [2,32], velocity [32]), balance [29,34-37], a reduction of muscle spasticity in those with cerebral palsy [38], and postural control in those with Parkinson's Disease [39]. However, many studies have included resistance training with non-uniform resistance protocols between WBV and non-WBV groups [25,26,40,41], leaving the mechanism of improvements unclear. Few studies [32,42] have examined the effect of WBV alone (without concomitant exercise on the platform), and some trials [1,42] have reported non-significant musculoskeletal adaptations. Furthermore, the ideal vibration dose, time course, frequency and posture to elicit an optimal response remain uncertain.
Although the posture adopted during WBV exercise has been observed to significantly influence the transmission of vibration to the skeleton [2,32,43], no studies have specifically investigated posture and its influence on adaptation to WBV. Vibration has been observed as best transmitted up the skeleton when standing with a straight, erect posture [44]. Using transcutaneous pins in the spine and femur, Rubin et al [44] reported a higher transmissibility of vibration through the skeleton when participants stood with locked knees, compared to a relaxed or flexed knee posture. Performing static exercise concomitant with flexed knee condition during WBV training has been shown to significantly increase activation of leg muscles [45,46]. Significant improvements in muscle strength and power after WBV exposure have been reported when subjects stood with knees slightly flexed [2,32]. Russo et al [32] found a significant increase in leg muscle power and velocity of contraction, and Rees et al [2] reported increases in both ankle plantar-flexion torque and power when comparing WBV to a control group. Together these results suggest that knee flexion may facilitate muscle improvements during WBV exposure.
However, to our knowledge, no studies have directly examined the effect of knee posture adopted during WBV on muscle adaptations. Robust, well-designed studies, particularly in older adults at risk of sarcopenia, are required in order to determine the most effective vibration prescription to elicit muscle adaptations. The literature suggests that a single WBV dose may be differently transmitted to muscle tissue dependent on knee position. Therefore the aim of this study was to determine the effect of knee position adopted during WBV on adaptation to the vibration stimulus in muscle in older adults, in order to refine the WBV prescription for these important outcomes. Specifically, we hypothesised that: (1) WBV exposure would improve all outcomes relative to Control and (2) WBV exposure with flexed knees would dampen skeletal vibration transmission by absorbing energy into muscles, thereby enhancing muscle activation, contractile activity and adaptations.
Methods
Study Design
The study was a three-month double blind, randomised placebo-controlled clinical trial to investigate the effect of knee position during WBV exposure on muscle function, muscle morphology and physical performance in older adults. Outcome measures were assessed at baseline and three months. The primary analytic strategy was complete case analysis, without regard to intervention adherence, and without imputation for missing data.
Randomisation
Participants were randomised to one of three groups following the completion of baseline assessments by an investigator independent of the study via a computerised program [47] using the method of randomly permuted blocks. Strata used included gender, age and use of osteoporosis medication, and randomisation was performed in blocks of six. Allocation concealment was complete, as participants were notified of their group assignment via a sealed, opaque envelope distributed after the completion of all baseline assessments.
Participants
Participants were recruited by means of posters and articles in local medical, physiotherapy and dental practices, pharmacies, community businesses, Senior citizens, Bowling and Returned Soldiers' Leagues Clubs, senior websites, online forums, newsletters, newspapers, and via letterbox drops to houses. Recruitment occurred during March to June 2009.
Participant screening was conducted using a telephone questionnaire. Inclusion criteria were non-institutionalised adults aged 50 and older (women at least one year postmenopausal), no cognitive impairment, able to stand unaided for at least 20 minutes, perform a partial squat for 60 seconds, and be willing to participate in the study. If currently on medications for osteoporosis (e.g. bisphosphonates, Vitamin D, calcium), dosages had to be maintained for the duration of the study. Exclusion criteria included: contraindications to vibration exposure (pacemaker, current kidney or gall stones, acute lower back pain, blood clot or thrombosis within the last six months, fracture or joint replacement within the past 12 months, vibration-related injuries, amputation of lower extremities other than toes, Raynaud's disease), contraindications to strength testing, active malignancy or a terminal or rapidly progressive illness, and diseases related to bone metabolism other than osteoporosis, such as Paget's disease, end-stage renal failure, rheumatoid arthritis or multiple myeloma.
Written informed consent was obtained from all participants prior to enrolment. The study was approved by the University of Sydney Human Research Ethics Committee and registered under the Australia New Zealand Clinical Trials Registry (Number: ACTRN12609000353291).
Intervention
Whole Body Vibration Exposure
Participants were assigned to one of three groups (two experimental intervention groups and one sham group) for a three-month period. The WBV dose and posture paradigms are described in Table 1. WBV exposure for all groups was intermittent (1 min vibration:1 min rest) for 20 min, 3 days per week for 3 months (13 weeks). The total number of exercise sessions was 39. WBV was conducted standing position on a synchronous vibration platform engineered by Australian Catholic University (2004). The motor speed controller was calibrated to vibrate at a frequency of 12 Hz. This frequency setting was achieved by attaching a spring-loaded potentiometer underneath the platform base and measuring the vibration rate. The amplitude of the vibration (1 mm peak to peak) was determined by the size of the cam fitted to the motor shaft. All participants stood on the vibration platform with their feet shoulder-width apart, hands by their sides, and wore standardised thick cotton socks to prevent any dampening that might result from footwear [48].
Table 1.
Whole Body Vibration Groups and Protocols
| Whole Body Vibration Group | Vibration | Posture on Platform | Vibration Dose | ||
|---|---|---|---|---|---|
| Frequency (Hz) | Peak-to-Peak Displacement (mm) | Magnitude (Peak Acceleration) (g) | |||
| FK | Active | Flexed Knees (20°flexion) | 12 | 1 | 0.3 |
| LK | Active | Locked knees | 12 | 1 | 0.3 |
| CON | Sham Control | Flexed knees (20°flexion) | 12 | 0 | 0 |
FK: Flexed Knees Group
LK: Locked Knees Group
CON: Control Group
We recognise that a four group, fully-factorial design would have been optimal to test the main effects of vibration and knee position, and the interaction of vibration and knee position on study outcomes. However, due to the pilot nature of the study, we chose three groups that would allow us to separate the effects of attention, vibration and standing posture and investigate two primary questions: (1) What is the effect of WBV exposure added to a constant knee position/exposure volume (Control/Flexed (CON) vs. Vibration/Flexed (FK))? and (2) What is the effect of knee flexion on a constant WBV exposure volume (Vibration/Locked (LK) vs. FK)? Although examined, the comparison of CON to LK group was not of primary interest, as we recognised it would not be possible to isolate the effects of the two experimental condition changes (knee position and vibration exposure) from the control condition.
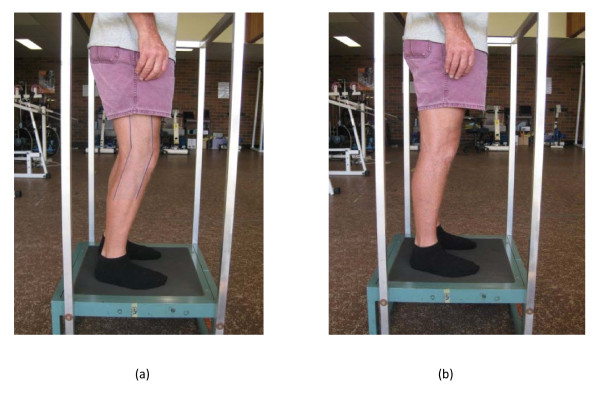
Each session was supervised by two trained research assistants in a University gymnasium. All participants were blinded as to which groups were hypothesised to be sham or active. Trainers by necessity were not blinded. The groups trained at separate times, to avoid unblinding and contamination. During CON training, the platform emitted a noise as the motor vibrated at a frequency of 12 Hz. However the amplitude was set to 0 mm, giving 0 g magnitude and providing a true sham control. During the FK conditions, a plastic sheet with the desired knee angle (20°) marked was taped to each participant's knee. During the LK condition, participants were instructed to lock knees but not to perform an isometric quadriceps contraction during the vibration exposure. The standing postures are depicted in Figure 1.
Figure 1.
Standing position on vibration platform. (a) Flexed knees with marker, the standing position for the FK (flexed knee) and CON (Control) groups during the vibration or sham stimulus respectively, and (b) Locked knees, the standing position for the LK (locked kness) group during the vibration stimulus.
Attendance and completion of the exposure dose was monitored by the instructor at each session. Participants were permitted an additional four weeks to make up any missed sessions to reach their target 39 sessions.
Outcomes
All outcomes were collected in double-blind fashion (both participants and assessors blinded). Baseline assessments were blinded (collected pre-randomisation) and measured by two assessors who also supervised the training sessions. Assessments at three months were measured by a different assessor blinded to the participants' group allocation and otherwise uninvolved in study procedures.
Primary Outcomes
Muscle Function (Strength, Power and Velocity)
Muscle function was assessed using Keiser pneumatic-resistance training equipment (Keiser Sports Health Equipment, Inc., Fresno, CA). Strength was measured using one repetition maximum (1RM) in two bilateral exercises: chest press and horizontal leg press. A 1RM was defined as the maximum load that can be lifted once while maintaining correct technique and reaching a full range of motion. Power and velocity were measured at 20, 30, 40, 50, 60, 70, 80, 90, and 100% of current 1RM and described in detail elsewhere [49]. The maximal explosive efforts were performed with 30-60 seconds rest in between [49].
Secondary Outcomes
Muscle Morphology
Total and regional (arms, legs and trunk) skeletal muscle mass and fat mass. Muscle-cross sectional area of mid-calf and mid-forearm were measured by Peripheral Quantitative Computed Tomography (pQCT) (Stratec XCT 2000; Medizintechnik, Pforzheim, Germany). The precision of repeat pQCT measurements was 0.7-1.4% (radius) and 0.8-2.9% (tibia) after repositioning in eight adults.
Physical Performance
Tests of physical performance included maximal (CV = 2.09%) and habitual (CV = 3.16%) gait speed over two meters, stair climb power, chair stand and six minute walk distance (CV = 5%) [50]. Balance, measured by balance index [49] was assessed on a computerised force platform (Chattecx Dynamic Balance System, Chattecx Corp, Chatanooga Group Inc, Hixson, TN; Software version 4.20). The mean of duplicate habitual gait velocity and maximum of duplicate maximal gait velocity and stair climb power measures were used. The remainder of the tests (balance, six minute walk, chair stand) were assessed only once at each time point.
Covariates
Anthropometrics
Height (cm) was measured using a wall-mounted stadiometer (Holtain stadiometer; Holtain Limited, Crymmych Pembs, UK), and body mass (kg) was measured using an electronic scale (HW-100k, A&D Bench Scales, Bradford, MA). These were measured in triplicate and the mean value used to calculate body mass index (BMI) (kg/m2). The CV of triplicate measurement on the same day in the whole sample was 0.14% for height and 0.04% for body mass.
Demographics and Health Questionnaires
Demographic characteristics and self-reported medications and health conditions were assessed using a questionnaire. Habitual physical activity level was estimated by the Physical Activity Scale for the Elderly (PASE) [51]. All questions covered a seven-day period prior to completing the questionnaire, using results to compare physical activity reported between the two time points. A weekly questionnaire was conducted to monitor health status and possible adverse effects of vibration exposure. These were defined a priori by review of existing literature, and included questions probing details of illnesses, symptoms or injuries subjects may have experienced in the past week, changes in medications, visits to health care professionals, any changes in their physical, mental or emotional health and reasons for any missed exercise sessions.
Statistical Analysis
Statistical analyses were performed using SPSS for Windows (Version 17.0). Data distributions were inspected visually and statistically for normality. Normally distributed data were reported as mean ± standard deviation (SD). Non-normally distributed data were normalised via log transformation, and if not possible, non-parametric statistics were used for these variables. Post hoc power analysis was calculated using the statistical program G Power (version 3.1.0) [52].
The primary analytic strategy compared the differences in primary and secondary outcomes between the locked knees, unlocked knees and sham WBV groups using all available data regardless of intervention compliance level. Analysis of covariance (ANCOVA) models of absolute change scores were constructed to compare the groups using the change score as the dependent variable and the baseline score as a covariate. Additional covariates considered for inclusion were characteristics that were different between the groups at baseline and related to the variable of interest (potential confounders). For muscle performance and body composition outcomes the month of each subject's baseline assessment was used as an additional covariate to control for the natural fluctuation of these parameters with changing seasons [53]. Fisher's post hoc Least Significant Difference (LSD) t tests were used for all pairwise comparisons whenever the f ratio in ANCOVA models was significant (P ≤ 0.05), to ascertain which groups were different from each other. Hedge's bias-corrected relative effect sizes (ES) and 95% confidence intervals were calculated for selected outcomes by COE's calculator [54] as:
Change in Treatment Group - Change in Control Group/pooled baseline SD A p-value of ≤0.05 and/or 95% CI exclusive of zero were accepted as statistically significant. Cohen's definition of effect size was used (Negligible = 0.2; Low = 0.2 - 0.49; Moderate = 0.5 - 0.79; Large = 0.8+) [55].
Results
Recruitment
From 154 persons assessed for eligibility, 19 were recruited and enrolled into the study (Figure 2). Two participants withdrew from the study (one after 14 weeks and the other following three sessions of exposure) due to personal reasons (LK = 1, CON = 1). One participant had not completed three months of exposure by the time of this analysis (FK = 1) and was not included in this report.
Figure 2.
Participant flow through the trial.
Participant Characteristics
The mean age of participants was 64.4 ± 8.1 (range 50-80) years. Participants were generally healthy but overweight with an average of 2.2 co-morbidities, most commonly osteoarthritis (63%), hypertension (47%) and lower back pain (47%) (Table 2). At baseline, no significant or clinically important differences were found between groups for any characteristics (Tables 2 and 3).
Table 2.
Baseline Participant Characteristics
| Variable | Total (n = 19) | FK (n = 6) | LK (n = 5) | CON (n = 8) | p-value* |
|---|---|---|---|---|---|
| Age (years) | 64.4 ± 8.1 | 63.3 ± 7.6 | 69.0 ± 6.9 | 62.3 ± 8.8 | 0.34 |
| 8/11 | 4/2 | 3/2 | 4/4 | ||
| Gender M/F, (%Female) | 0.47 | ||||
| (58%) | (69%) | (60%) | (50%) | ||
| Height (cm) | 166.7 ± 9.5 | 166.7 ± 9.1 | 164.1 ± 6.1 | 168.4 ± 12.0 | 0.76 |
| Body Mass (kg) | 79.5 ± 15.8 | 72.9 ± 10.4 | 81.1 ± 9.7 | 83.4 ± 121.2 | 0.48 |
| Body Mass Index (kg/m2)a | 28.5 ± 4.3 | 26.2 ± 2.8 | 30.0 ± 2.3 | 29.2 ± 5.7 | 0.30 |
| Co morbidities/conditions, n (%) | |||||
| - Osteoarthritis | 12 (63.2) | 4 | 3 | 5 | 0.97 |
| - Hypertension | 9 (47.4) | 1 | 3 | 5 | 0.19 |
| - Chronic Lower Back Pain | 9 (47.4) | 2 | 2 | 5 | 0.52 |
| - Osteoporosis | 5 (26.3) | 1 | 1 | 3 | 0.64 |
| Total Co morbidities (#) | 2.2 ± 1.2 | 1.3 ± 1.2 | 2.6 ± 1.1 | 2.5 ± 1.1 | 0.13 |
| Total Medications/day (#) | 4 ± 3.9 | 4.5 ± 4.1 | 3 ± 1.2 | 4.4 ± 5.3 | 0.81 |
| Habitual Physical Activity Levelb | 146.1 ± 44.1 | 137.3 ± 60.0 | 160.0 ± 49.9 | 145.8 ± 30.0 | 0.75 |
| Skeletal Muscle Cross Sectional Area (mm2)c | |||||
| - Mid-Calf | 67.8 ± 15.1 | 59.5 ± 12.9 | 72.6 ± 12.1 | 71.4 ± 17.6 | 0.27 |
| - Mid-Forearm | 33.5 ± 11.0 | 30.5 ± 11.3 | 35.0 ± 11.1 | 35.1 ± 11.8 | 0.728 |
All data presented as mean ± SD
Continuous variables analysed by ANOVA for normally distributed data
Categorical variables analysed by Chi square test
FK: Whole Body Vibration Flexed Knees Group
LK: Whole Body Vibration Locked Knees Group
CON: Control Group
a Body mass index: an indicator of body fat calculated by weight (kg)/height2 (m). Normal values range from 18.5 - 24.9 kg/m2.
Values ≥25 kg/m2 are considered overweight, and ≥30 kg/m2 are considered obese.
b The Physical Activity Scale for the Elderly (PASE) was used to monitor habitual physical activity over the preceding seven days. A higher score reflects more physical activity [51]
c measured by Peripheral Quantitative Computed Tomography ( pQCT)
Table 3.
Baseline Participant Functional Performance
| FK | LK | CON | |||
|---|---|---|---|---|---|
| Variable | Total (n = 19) | p-value | |||
| (n = 6) | (n = 5) | (n = 8) | |||
| Maximal Strength (N)a - | |||||
| - Leg Press | 1339.9 ± 416.1 | 1308.0 ± 579.2 | 1252.4 ± 289.6 | 1418.5 ± 380.0 | 0.78 |
| - Chest press | 327.1 ± 149.8 | 313.0 ± 190.9 | 293.0 ± 134.9 | 354.8 ± 137.8 | 0.79 |
| Relative Strength (kg/kg)b - | |||||
| - Leg Press | 8.9 ± 1.6 | 8.9 ± 2.1 | 8.4 ± 1.4 | 9.3 ± 1.5 | 0.38 |
| Peak Power (W) - | |||||
| - Leg Press | 652.3 ± 317.4 | 635.7 ± 438.3 | 637.6 ± 267.8 | 673.9 ± 282.6 | 0.97 |
| - Chest Press | 210.8 ± 108.4 | 191.3 ± 124.3 | 210.3 ± 69.4 | 225.6 ± 121.8 | 0.86 |
| Peak Velocity (cm/s) - | |||||
| - Leg Press | 94.5 ± 19.3 | 86.8 ± 21.8 | 89.3 ± 1.0 | 103.4 ± 20.1 | 0.23 |
| - Chest Press | 126.5 ± 32.0 | 115.1 ± 30.7 | 137.9 ± 16.8 | 129.4 ± 38.7 | 0.54 |
| Balance Indexc | 98.8 ± 25.2 | 98.0 ± 20.0 | 106.348 ± 30.2 | 94.8 ± 27.8 | 0.74 |
| 5 Chair rise time (s) | 10.1 ± 1.7 | 10.1 ± 1.8 | 10.1 ± 1.2 | 10.1 ± 2.0 | 1.0 |
| Stair Climb Power (W)d | 461.8 ± 195.9 | 420.2 ± 213.1 | 415.3 ± 92.4 | 530.6 ± 236.9 | 0.52 |
| Habitual Gait Velocity (m/s)e | 1.4 ± 0.2 | 1.3 ± 0.2 | 1.4 ± 0.2 | 1.3 ± 0.2 | 0.80 |
| Maximal Gait Velocity (m/s)e | 2.2 ± 0.4 | 2.2 ± 0.4 | 2.1 ± 0.3 | 2.3 ± 0.4 | 0.58 |
| Six Minute Walk (m) | 619.4 ± 77.0 | 612.5 ± 110.0 | 595.4 ± 50.3 | 639.5 ± 65.1 | 0.61 |
All data presented as mean ± SD
Continuous variables analysed by ANOVA for normally distributed data
FK: Whole Body Vibration Flexed Knees Group
LK: Whole Body Vibration Locked Knees Group
CON: Control Group
a Maximal Strength was measured via 1 repetition maximum (1RM) testing in Newtons
b Relative Strength (kg/kg): (leg press strength (kg)/leg Fat Free Mass (kg)) measured by Dual X-Ray absorptiometry (DXA)
c Balance Index : (Sum of 12 sway measures + (180 - sum of 6 time measures) [49]
dStair Climb power (W): (Body Mass (kg) × vertical height of the staircase (m)) × 9.8/time (s)
e Gait velocity (average of 2 trials for habitual gait; maximal of 2 trials for maximal gait) measured over 2 m using an Ultratimer
Compliance/Adverse Effects
No adverse events attributable to WBV exposure were reported. Compliance (number of vibration sessions completed divided by the 39 possible sessions available to each participant) for those completing the three months of WBV exposure was 98.8% with participants taking, on average, 42 days over 13-17 weeks to complete the 39 planned sessions.
Outcomes
Participants' baseline body composition, muscle function, and physical performance are presented in Tables 2 and 3. Between group comparisons of change scores are presented in Table 4.
Table 4.
Between Group Changes From Baseline to 3 Months
| Variable | FK | LK | CON | ||
|---|---|---|---|---|---|
| p value | Power | ||||
| (n = 5) | (n = 4) | (n = 7) | |||
| Muscle Function and Mass | |||||
| Maximal Strength (N)a - | |||||
| - Leg Press | 93.03 (-4.3, 190.37) | 202.32 (85.05, 319.59) | 9.59 (-75.11, 94.30) | 0.05* | 0.59 |
| - Chest press | -2.46 (-50.38, 45.50) | -3.72 (-98.91, 61.46) | -12.51 (-56.40, 31.38) | 0.93 | 0.06 |
| Peak Power (W)b - | |||||
| - Leg Press | 41.19 (-23.99, 106.37) | 95.73 (22.12, 169.35) | 119.19 (65.74, 172.63) | 0.18 | 0.29 |
| - Chest Press | 11.34 (-13.02, 35.70) | -0.09 (-47.04,47.02) | 3.08 (-26.43, 32.58) | 0.81 | 0.07 |
| Peak Velocity (cm/s)b- | |||||
| - Leg Press | -10.36 (-140.71, 119.99) | 43.02 (-104.10, 190.15) | 59.53 (-48.98, 168.05) | 0.68 | 0.10 |
| - Chest Press | 15.48 (3.59, 27.37) | -3.12 (-24.81, 18.58) | 0.33 (-13.42, 14.08) | 0.15 | 0.34 |
| Skeletal Muscle Cross - Sectional Area measured at 66% site (cm2)b,c | |||||
| - Mid-Calf | 2.43 (-2.02, 6.94) | 3.65 (-1.91, 9.20) | -2.2 (-6.043, 1.65) | 0.13 | 0.39 |
| - Mid-Forearm | -0.24 (-1.52, 1.04) | -0.48 (-2.18, 1.23) | 0.03 (-1.16, 1.22) | 0.87 | 0.07 |
| Functional Performance | |||||
| Balance Index d | 0.73 (-10.83, 12.28) | -7.19 (-20.42, 6.04) | -11.35 (-22.24, -0.47) | 0.28 | 0.24 |
| 5 Chair Rise Time (s) | -0.65 (-2.07, 0.78) | -1.29 (-2.87, 0.30) | -1.29 (-2.59, 0.01) | 0.73 | 0.09 |
| Stair Climb Power (W)e | 84.10 (-28.02, 196.30) | 63.68 (-55.17, 182.54) | 42.75 (-63.86, 149.35) | 0.86 | 0.07 |
| Habitual Gait Velocity (m/s)f | 0.40 (-0.02, 0.81) | 0.04 (-0.04, 0.50) | 0.07 (-0.30, 0.45) | 0.38 | 0.19 |
| Maximal Gait Velocity (m/s)f | 0.09 (-0.11, 0.30) | 0.01 (-0.22, 0.24) | 0.09 (-0.1, 0.28) | 0.81 | 0.75 |
| Six Minute Walk Distance (m) | 36.15 (3.18, 69.12) | 22.92 (-14.27, 60.11) | 31.58 (1.01, 62.15) | 0.84 | 0.07 |
Data presented as Estimated Marginal Means, adjusted mean difference (95% Confidence Intervals) after Analysis of Covariance (ANCOVA) testing using baseline values as the covariate
All data presented as mean ± SD
WBV FK: Whole Body Vibration Flexed Knees Group
WBV LK: Whole Body Vibration Locked Knees Group
CON: Control Group
* Indicates a significant difference between the three groups (p ≤ 0.05)
a Maximal strength was measured via 1 repetition maximum (1RM) testing in Newtons
b Estimated Marginal Means after Analysis of Covariance (ANCOVA) testing using baseline values and month of baseline pQCT scan as covariates
c Measured by Peripheral Quantitative Computed Tomography (pQCT)
d Balance index : (Sum of 12 sway measures + (180 - sum of 6 time measures) [49]
e Stair climb power (W): Body Mass (kg) × vertical height of the staircase (m) × 9.8/time (s)
f Gait velocity (average of 2 trials for habitual gait; maximal of 2 trials for maximal gait) measured over 2 m using an Ultratimer
Primary Outcomes
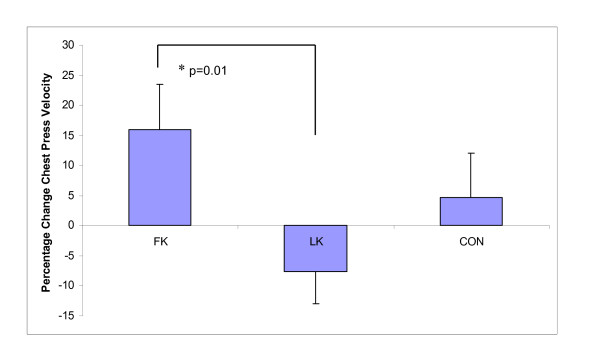
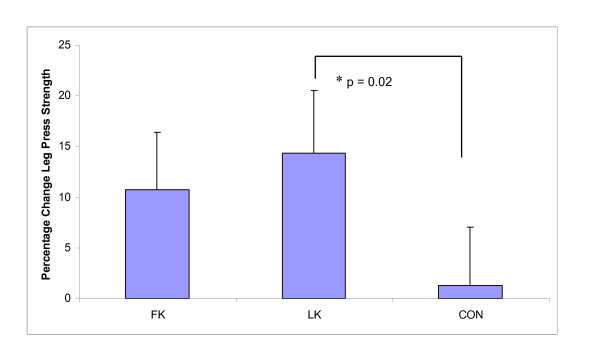
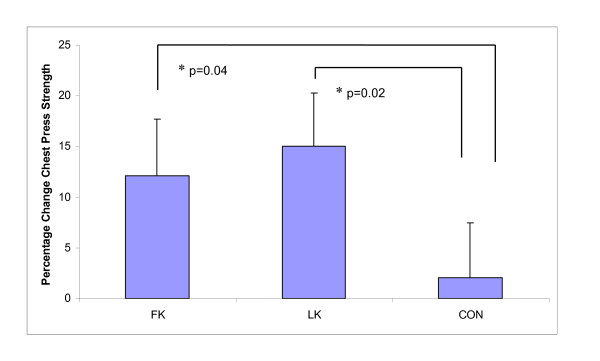
As hypothesised, relative (%) upper body (chest press) peak contraction velocity significantly improved after WBV exposure in the FK group compared to LK (CON 4.7%; FK 16.0% vs. LK - 7.6%, p = 0.01) (Figure 3), and similar trends were observed for changes in absolute upper body velocity (Table 4). This was supported by a large effect size (ES) (ES = 0.82, 95% Confidence Intervals (CI) (-0.87, 2.52)). Relative (p = 0.03) and absolute (p = 0.05) lower leg (leg press) strength (Figure 4) increased significantly with both standing postures during WBV exposure, (LK 14.4%; FK 10.7%; CON 1.3%; ES 0.12, 95% CI (-1.15, 1.39)), however, unexpectedly more so with LK than FK. Furthermore, only the LK group was significantly different from CON in relative strength gains (p = 0.02). Similarly, relative upper body strength improved significantly following WBV compared to the control group (LK 15.1%, p = 0.02; FK 12.1%, p = 0.04; CON 4.7%; ES 0.05, 95% CI (-1.34, 1.43)) (Figure 5), however, contrary to our hypothesis, greater improvements were observed with LK than with FK. No significant changes were observed in peak muscle power in any group (Table 4).
Figure 3.
Percentage change in chest press peak velocity following whole body vibration training. Data presented as Estimated Marginal Means, adjusted mean difference ± standard deviation after analysis of covariance (ANCOVA) testing using month of baseline assessment and baseline values as the covariates. FK = flexed knees group, LK = locked knees group, CON = control group * significant between group change.
Figure 4.
Percentage change in leg press strength following whole body vibration training. Data presented as Estimated Marginal Means, adjusted mean difference ± standard deviation after analysis of covariance (ANCOVA) testing using month of baseline assessment and baseline values as the covariates. FK = flexed knees group, LK = locked knees group, CON = control group * significant between group change.
Figure 5.
Percentage change in chest press strength following whole body vibration training. Data presented as Estimated Marginal Means, adjusted mean difference ± standard deviation after analysis of covariance (ANCOVA) testing using month of baseline assessment and baseline values as the covariates. FK = flexed knees group, LK = locked knees group, CON = control group * significant between group change.
Secondary Outcomes
Potentially clinically meaningful but statistically non-significant improvements in lower leg muscle cross-sectional area (LK 3.7 cm2, FK 2.4 cm2, CON 2.2 cm2 p = 0.13; ES 0.28, 95% CI (-0.91, 1.48)) were observed after WBV with LK compared to FK or CON exposures. There were no other significant or clinically meaningful changes observed in physical performance over the three months (Table 4).
Discussion
This is the first randomised controlled trial (RCT), to our knowledge, comparing different standing postures during WBV exposure in older adults. As hypothesised, three months of WBV exposure significantly improved absolute and relative lower body muscle strength and relative upper body strength and peak contraction velocity. The FK position was significantly better than LK for upper body muscle velocity improvement as hypothesised, but had no influence on muscle strength changes. A large calculated ES value for upper body contraction velocity adds support to the robustness of our findings for this outcome. This study is also to our knowledge, the first to report significant increases in upper body strength and contraction velocities after WBV. As expected, due to the pilot nature of this work (n = 16), type II errors likely contributed to the lack of significance for many of the other outcomes. The remainder of ESs were negligible to low, and all of the CIs included zero. Post-hoc power calculations of lower limb (leg press) strength indicated that a total of 78 subjects would be needed to demonstrate significance.
Chest press contraction velocity improved after WBV exposure, with an increase of 15.2% observed when standing in the FK position. Changes in contraction velocity may be the primary mechanism by which to improve peak power in older adults [56]. Because muscle power declines faster in older adults than strength does [57] and is more closely related to physical performance, functional independence and mobility than muscle strength [58,59] this finding may be clinically relevant. Muscle power has also been observed to improve with low, moderate and high load, high velocity power training [56]. Low load power training is most similar to the low-loading conditions during WBV exposure, although the frequency and number of contractions induced with the WBV would be far greater. Thus, WBV may provide an alternative exercise modality for those who cannot undertake power training. This, however, remains to be shown in future studies directly comparing the physiological and clinical benefits of power training vs. WBV, as no changes in muscle power itself were observed in this study. It is likely that greater improvements in muscle strength and/or velocity would have been necessary to improve power output itself [60].
Muscle Strength
We observed significant differences in strength between WBV and CON participants after three months, as hypothesised. Despite statistical significance of the increase in upper and lower body strength, the increases are smaller than those typically observed following resistance training in older adults [61]. Thus, the clinical relevance and long-term benefits of strength changes associated with WBV remain to be demonstrated. Although we anticipated more robust changes in lower body muscle function than upper body changes, due to dissipation of vibration transmission over the length of the body, this was true for muscle strength, but not contraction velocity. With no previous literature available for comparison, these findings require further investigation through more robust studies, testing specifically for the influence of knee position during WBV exposure on upper and lower body muscle strength, power and contraction velocity.
Effect of Standing Posture on Muscle Adaptations
The greater increase in upper body muscle velocity in the FK group over LK vs. CON supports our hypothesis that standing with flexed knees during WBV exposure facilitates adaptations in muscle. A similar vibration protocol was applied to investigate the transmissibility of vibration through to different areas of the skeleton when subjects adopted various knee positions [44]. The findings suggest that a flexed knee position may dampen vibrations to the skeleton. Similarly, our hypothesis proposed that this damping could actually facilitate the vibration adaptation muscle, resulting in better muscle function or morphology gains. Our results, however, provide inconsistent support for this hypothesis. Lower limb (leg press) strength improved significantly in both absolute and relative terms after three months of WBV exposure, however a greater increase was observed in the LK group than the FK group.
There are several explanations for these unexpected findings. Although all training was fully supervised and specific directions for standing position was given by the instructor, subjects could have unconsciously performed an isometric contraction of the quadriceps intermittently to stabilise the LK position during the WBV exposure. This may have inadvertently made the LK position more effective for muscle than the FK position, not due to dampening of vibrations, rather as a result of unintended concomitant isometric exercise. Many studies show that isometric contractions increase muscle strength, often to the same extent as dynamic contractions [62] which could explain the greater improvement with the LK position compared to FK for leg strength outcomes in our study. Such isometric contractions of leg muscles would not have influenced upper body strength, and this is supported by the similar strength changes observed for the LK and FK groups in the upper body. Further studies using electromyography (EMG) analysis to measure muscle activation of the upper and lower body during WBV, as well as accelerometer recordings over various body segments to measure vibration transmission with both locked knees and flexed knees posture may assist in determining the effect of stance on muscle contraction and transmission, and thereby refine the WBV prescription to include optimal stance to facilitate vibration to muscle.
Muscle Morphology
While there were no significant improvements in muscle cross sectional area of the upper or lower limbs, the FK group showed potentially clinically relevant muscle increases at the mid calf compared to the CON group. The lack of significance is likely partially due to a type II error given that our pilot study was inadequately powered for the ES of 0.28 noted in this secondary outcome. Calf muscle strength and size has been associated with gait and balance in older adults [63], and is therefore a potentially important clinical outcome. There are no other studies to our knowledge that have examined changes in muscle cross sectional area using pQCT after WBV exposure in older adults. The need for more robust, long term and sufficiently powered studies is emphasised by our findings.
There are several limitations in our study design. The study was underpowered for the secondary outcomes. Three months of WBV exposure may not have been sufficient to stimulate any musculoskeletal adaptations, particularly in muscle morphology. A four group fully-factorial design would be the optimal design to test the interaction of vibration and knee position on study outcomes. A lack of familiarisation tests prior to baseline assessment, as well as different assessors for baseline and three month testing may have led to results confounded by learning effects and differences due to assessor encouragement and testing experience. We have only tested one vibration magnitude (0.3g), the dose recommended by Rubin et al [44] to be beneficial to bone and not destructive to osteoblasts. It is possible that a different vibration magnitude is needed for optimal muscle adaptations, and in fact, many other studies have used higher g forces with success for such outcomes [2,32,33]. Specific dose-response studies varying vibration magnitude and total dose are required to refine this aspect of the prescription.
Conclusions
The results of this pilot RCT have provided some support for the efficacy of WBV exposure as a potential alternative to existing exercise modalities in increasing upper and lower body muscle strength and upper body contraction velocity in healthy older adults. With the aging population leading to a rise in the incidence of musculoskeletal disorders, the efficacy of WBV as a treatment modality warrants further investigation, particularly in mobility-limited cohorts, to assess whether WBV exposure can alleviate muscle wasting due to disease and inactivity. Following the novel and significant changes to upper body muscle function reported, future WBV research should include additional measures of upper body muscle function and morphology to determine the accuracy and reproducibility of our findings. Most importantly, future research must also establish whether improvements from WBV are retained once the vibration stimulus is withdrawn, and whether any meaningful clinical benefits ensue.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MM and FA carried out the recruitment, baseline testing, training and data entry, MM performed the statistical analyses and drafted the manuscript, FA performed the pQCT scans and analyses, RO and MFS conceived of the study, participated in its design, co-ordination, recruitment and statistical analyses, and revised the manuscript, DG taught the pQCT technique and analysed the pQCT scans. All authors read and approved the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Monica Mikhael, Email: mmik4082@uni.sydney.edu.au.
Rhonda Orr, Email: rhonda.orr@sydney.edu.au.
Fleur Amsen, Email: fleuramsen@gmail.com.
David Greene, Email: david.greene@acu.edu.au.
Maria A Fiatarone Singh, Email: maria.singh@sydney.edu.au.
Acknowledgements
We would like to thank Prof. Marcus Siebel and Beverly White (Dept. Endocrinology and Metabolism, Concord Repatriation and General Hospital) for DXA scans; Dr. Margaret Janu (Diagnostic Pathology Unit Concord Repatriation and General Hospital) for biochemical analyses; Prof Jeri Naughton for providing the vibration platforms; Ray Patton and Kylie Simpson for technical assistance throughout the study; and Michael Baker for the randomisation of participants and his comments on this manuscript.
References
- Bautmans I, Van Hees E, Lemper JC, Mets T, Bautmans I, Van Hees E, Lemper JC, Mets T. The feasibility of Whole Body Vibration in institutionalised elderly persons and its influence on muscle performance, balance and mobility: a randomised controlled trial [ISRCTN62535013] BMC Geriatr. 2005;5:17. doi: 10.1186/1471-2318-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees SS, Murphy AJ, Watsford ML. Effects of whole-body vibration exercise on lower-extremity muscle strength and power in an older population: a randomized clinical trial. Phys Ther. 2008;88(4):462–470. doi: 10.2522/ptj.20070027. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71(2):644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- Reed RL, Pearlmutter L, Yochum K, Meredith KE, Mooradian AD. The relationship between muscle mass and muscle strength in the elderly. [see comment] J Am Geriatr Soc. 1991;39(6):555–561. doi: 10.1111/j.1532-5415.1991.tb03592.x. [DOI] [PubMed] [Google Scholar]
- Fjeldstad C, Palmer IJ, Bemben MG, Bemben DA. Whole-body vibration augments resistance training effects on body composition in postmenopausal women. Maturitas. 2009;63(1):79–83. doi: 10.1016/j.maturitas.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Stauber PM, McHugh D, Koehler KM, Garry PJ. Cross-sectional age differences in body composition in persons 60+ years of age. J Gerontol A Biol Sci Med Sci. 1995;50(6):M307–316. doi: 10.1093/gerona/50a.6.m307. [DOI] [PubMed] [Google Scholar]
- Going S, Williams D, Lohman T. Aging and body composition: biological changes and methodological issues. Exerc Sport Sci Rev. 1995;23:411–458. doi: 10.1249/00003677-199500230-00016. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55(12):M716–724. doi: 10.1093/gerona/55.12.m716. [DOI] [PubMed] [Google Scholar]
- Kirkendall DT, Garrett WE Jr. The effects of aging and training on skeletal muscle. Am J Sports Med. 1998;26(4):598–602. doi: 10.1177/03635465980260042401. [DOI] [PubMed] [Google Scholar]
- Taaffe DR, Marcus R. Musculoskeletal health and the older adult. J Rehabil Res Dev. 2000;37(2):245–254. [PubMed] [Google Scholar]
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Eng J Med. 1988;319(26):1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci. 1995;50 Spec No:64–67. doi: 10.1093/gerona/50a.special_issue.64. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Khoeler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lendeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Bakhshi V, Elliott M, Gentili A, Godschalk M, Mulligan T. Testosterone improves rehabilitation outcomes in ill older men. [see comment] J Am Geriatr Soc. 2000;48(5):550–553. doi: 10.1111/j.1532-5415.2000.tb05002.x. [DOI] [PubMed] [Google Scholar]
- Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269(5 Pt 1):E820–826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels.[see comment] J Gerontol A Biol Sci Med Sci. 2001;56(5):M266–272. doi: 10.1093/gerona/56.5.m266. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ. et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84(8):2647–2653. doi: 10.1210/jc.84.8.2647. [DOI] [PubMed] [Google Scholar]
- Sih R, Morley JE, Kaiser FE, Perry HM, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial.[see comment] J Clin Endocrinol Metab. 1997;82(6):1661–1667. doi: 10.1210/jc.82.6.1661. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282(3):E601–607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Schimke JC, Ades P, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle MH C and response to resistance exercise. Am J. Physiol Endocrinol Metab. 2001;280(2):E203–208. doi: 10.1152/ajpendo.2001.280.2.E203. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. [see comment] N Eng J Med. 1994;330(25):1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- Borst SE, Borst SE. Interventions for sarcopenia and muscle weakness in older people. [see comment] Age & Ageing. 2004;33(6):548–555. doi: 10.1093/ageing/afh201. [DOI] [PubMed] [Google Scholar]
- Papadakis MA, Grady D, Black D, Tierney MJ, Gooding GA, Schambelan M, Grunfeld C. Growth hormone replacement in healthy older men improves body composition but not functional ability.[see comment] Ann Intern Med. 1996;124(8):708–716. doi: 10.7326/0003-4819-124-8-199604150-00002. [DOI] [PubMed] [Google Scholar]
- Schroeder ET, Terk M, Sattler FR. Androgen therapy improves muscle mass and strength but not muscle quality: results from two studies. Am J Physiol Endocrinol Metab. 2003;285(1):E16–24. doi: 10.1152/ajpendo.00032.2003. [DOI] [PubMed] [Google Scholar]
- Verschueren SMP, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res. 2004;19(3):352–359. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- Delecluse C, Roelants M, Verschueren S. Strength increase after whole-body vibration compared with resistance training. Med Sci Sport Exerc. 2003;35(6):1033–1041. doi: 10.1249/01.MSS.0000069752.96438.B0. [DOI] [PubMed] [Google Scholar]
- Cardinale M, Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev. 2003;31(1):3–7. doi: 10.1097/00003677-200301000-00002. [DOI] [PubMed] [Google Scholar]
- Roelants M, Delecluse C, Verschueren SM. Whole-body-vibration training increases knee-extension strength and speed of movement in older women. J Am Geriatr Soc. 2004;52(6):901–908. doi: 10.1111/j.1532-5415.2004.52256.x. [DOI] [PubMed] [Google Scholar]
- Rehn B, Lidstrom J, Skoglund J, Lindstrom B. Effects on leg muscular performance from whole-body vibration exercise: a systematic review. Scand J Med Sci Sports. 2007;17(1):2–11. doi: 10.1111/j.1600-0838.2006.00578.x. [DOI] [PubMed] [Google Scholar]
- Torvinen S, Kannus P, Sievanen H, Jarvinen TAH, Pasanen M, Kontulainen S, Jarvinen TLN, Jarvinen M, Oja P, Vuori I. Effect of four-month vertical whole body vibration on performance and balance. Med Sci Sport Exerc. 2002;34(9):1523–1528. doi: 10.1097/00005768-200209000-00020. [DOI] [PubMed] [Google Scholar]
- Mahieu NN, Witvrouw E, Van de Voorde D, Michilsens D, Arbyn V, Van den Broecke W. Improving strength and postural control in young skiers: whole-body vibration versus equivalent resistance training. J Athlet Train. 2006;41(3):286–293. [PMC free article] [PubMed] [Google Scholar]
- Torvinen S, Kannu P, Sievanen H, Jarvinen TAH, Pasanen M, Kontulainen S, Jarvine TLN, Jarvinen M, Oja P, Vuori I. Effect of a vibration exposure on muscular performance and body balance. Randomized cross-over study. Clin Physiol Funct Imaging. 2002;22(2):145–152. doi: 10.1046/j.1365-2281.2002.00410.x. [DOI] [PubMed] [Google Scholar]
- Russo C, Lauretani F, Bandinelli S, Bartali B, Cavazzini C, Guralnik J, Ferrucci L. High-frequency vibration training increases muscle power in postmenopausal women. Arch Phys Med Rehabil. 2003;84(12):1854–1857. doi: 10.1016/S0003-9993(03)00357-5. [DOI] [PubMed] [Google Scholar]
- Rees S, Murphy A, Watsford M. Effects of vibration exercise on muscle performance and mobility in an older population. J Aging Phys Act. 2007;15(4):367–381. doi: 10.1123/japa.15.4.367. [DOI] [PubMed] [Google Scholar]
- Fagnani F, Giombini A, Di CA, Pigozzi F, Di SV. The effects of a whole-body vibration program on muscle performance and flexibility in female athletes. Am J Phys Med Rehabil. 2006;85(12):956–962. doi: 10.1097/01.phm.0000247652.94486.92. [DOI] [PubMed] [Google Scholar]
- Kawanabe K, Kawashima A, Sashimoto I, Takeda T, Sato Y, Iwamoto J. Effect of whole-body vibration exercise and muscle strengthening, balance, and walking exercises on walking ability in the elderly. Keio J Med. 2007;56(1):28–33. doi: 10.2302/kjm.56.28. [DOI] [PubMed] [Google Scholar]
- Cheung W, Mok H, Qin L, Sze P, Lee K, Leung K. High-frequency whole-body vibration improves balancing ability in elderly women. Arch Phys Med Rehabi. 2007;88(7):852–857. doi: 10.1016/j.apmr.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Bruyere O, Wuidart MA, Di Palma E, Gourlay M, Ethgen O, Richy F, Reginster JY. Controlled whole body vibration to decrease fall risk and improve health-related quality of life of nursing home residents. Arch Phys Med Rehabil. 2005;86(2):303–307. doi: 10.1016/j.apmr.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Ahlborg L, Andersson C, Julin P. Whole-body vibration training compared with resistance training: effect on spasticity, muscle strength and motor performance in adults with cerebral palsy. J Rehabil Med. 2006;38(5):302–308. doi: 10.1080/16501970600680262. [DOI] [PubMed] [Google Scholar]
- Turbanski S, Haas CT, Schmidtbleicher D, Friedrich A, Duisberg P, Turbanski S, Haas CT, Schmidtbleicher D, Friedrich A, Duisberg P. Effects of random whole-body vibration on postural control in Parkinson's disease. Res Sports Med. 2005;13(3):243–256. doi: 10.1080/15438620500222588. [DOI] [PubMed] [Google Scholar]
- Roelants M, Delecluse C, Goris M, Verschueren S. Effects of 24 weeks of whole body vibration training on body composition and muscle strength in untrained females. Int J Sports Med. 2004;25(1):1–5. doi: 10.1055/s-2003-45238. [DOI] [PubMed] [Google Scholar]
- Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19(3):343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- Jordan MJ. et al. "Vibration Training: an Overview of the Area, Training Consequences, and future considerations". J Strength Cond Res. 2005;19(2):459–466. doi: 10.1519/13293.1. [DOI] [PubMed] [Google Scholar]
- Rubin C, Pope M, Fritton JC, Magnusson M, Hansson T, McLeod K. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine. 2003;28(23):2621–2627. doi: 10.1097/01.BRS.0000102682.61791.C9. [DOI] [PubMed] [Google Scholar]
- Hazell T, Jakobi J, Kenno K. The effects of whole-body vibration on upper- and lower-body EMG during static and dynamic contractions. Appl Physiol Nutr Metab. 2007;32(6):1156–63. doi: 10.1139/H07-116. [DOI] [PubMed] [Google Scholar]
- Roelants M, Verschueren SMP, Delecluse C, Levin O, Stijnen V. Whole-body-vibration-induced increase in leg muscle activity during different squat exercises. J Strength Cond Res. 2006;20(1):124–9. doi: 10.1519/R-16674.1. [DOI] [PubMed] [Google Scholar]
- Dallal GE. 2009. http://www.randomization.com
- Kiiski J, Heinonen A, Jaervinen TL, Kannus P, Sievanen H. Transmission of vertical whole body vibration to the human body. J Bone Miner Res. 2008;23(8):1318–1325. doi: 10.1359/jbmr.080315. [DOI] [PubMed] [Google Scholar]
- Orr R, de Vos NJ, Singh NA, Ross DA, Stavrinos TM, Singh Fiatarone MA. Power Training Improves Balance in Healthy Older Adults. J Gerontol A Biol Sci Med Sci. 2006;61A(1):78–85. doi: 10.1093/gerona/61.1.78. [DOI] [PubMed] [Google Scholar]
- Kervio G, Carre F, Ville NS, Kervio G, Carre F, Ville NS. Reliability and intensity of the six-minute walk test in healthy elderly subjects. Med Sci Sport Exerc. 2003;35(1):169–174. doi: 10.1097/00005768-200301000-00025. [DOI] [PubMed] [Google Scholar]
- Washburn RSK, Jetter A, Janney C. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Mayr S, Errdfelder E, Buchner A, Faul F. A short tutorial of G Power. Tutorials Quant Method Psychol. 2007;3:51–59. [Google Scholar]
- Dawson-Hughes B, Harris S. Regional changes in body composition by time of year in healthy postmenopausal women. Am J Clin Nutr. 1992;56(2):307–313. doi: 10.1093/ajcn/56.2.307. [DOI] [PubMed] [Google Scholar]
- Effect Size Calculator: a user guide to using the spreadsheet. http://www.pipsproject.org/RenderPagePrint.asp?LinkID=30325017
- Coe R. It's the Effect Size, Stupid. British Educational Research Association Annual Conference, Exeter. 2002.
- de Vos NJ, Singh NA, Ross DA, Stavrinos TM, Orr R, Fiatarone Singh MA. Optimal load for increasing muscle power during explosive resistance training in older adults. J Gerontol A Biol Sci Med Sci. 2005;60(5):638–647. doi: 10.1093/gerona/60.5.638. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Conwit R, Tobin J, Fozard J. Age-Associated Loss of Power and Strength in the Upper Extremities in Women and Men. J Gerontol A Biol Sci Med Sci. 1997;52(B2):67–76. doi: 10.1093/gerona/52a.5.b267. [DOI] [PubMed] [Google Scholar]
- Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65-89 years. Age & Ageing. 1994;23(5):371–377. doi: 10.1093/ageing/23.5.371. [DOI] [PubMed] [Google Scholar]
- Bassey EJ, Fiatarone MA, O'Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci. 1992;82(3):321–327. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- de Vos NJ, Singh NA, Ross D, Stavrinos TM, Orr R, Fiatarone Singh MA. Effect of Explosive Resistance Training Intensity on the Contribution of Force and Velocity to Peak Power Generation in Older Adults: A Randomized Controlled Trial. J Aging & Phys Act. 2008;16(4):393–407. doi: 10.1123/japa.16.4.393. [DOI] [PubMed] [Google Scholar]
- Salem GJ, Skinner JS, Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR. Exercise and Physical Activity for Older Adults. Med Sci Sport Exerc. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- Symons TB, Vandervoort AA, Rice CL, Overend TJ, Marsh GD, Symons TB, Vandervoort AA, Rice CL, Overend TJ, Marsh GD. Effects of maximal isometric and isokinetic resistance training on strength and functional mobility in older adults. J Gerontol A Biol Sci Med Sci. 2005;60(6):777–781. doi: 10.1093/gerona/60.6.777. [DOI] [PubMed] [Google Scholar]
- Chandler JM, Duncan PW, Kochersberger G, Studenski S. Is lower extremity strength gain associated with improvement in physical performance and disability in frail community-dwelling elders? Arch Phys Med Rehabil. 1998;79(1):24–30. doi: 10.1016/S0003-9993(98)90202-7. [DOI] [PubMed] [Google Scholar]