Abstract
Expansion of the genetic alphabet with a third base pair would lay the foundation of a semi-synthetic organism with an expanded genetic code and also have immediate in vitro applications. Previously, the unnatural base pairs formed between d5SICS and either dNaM or dMMO2 were shown to be well replicated by DNA polymerases under steady-state conditions and also transcribed by T7 RNA polymerase efficiently in either direction. We now demonstrate that DNA containing either the d5SICS-dNaM or d5SICS-dMMO2 unnatural base pair may be PCR amplified with fidelities and efficiencies that approach those of fully natural DNA. These results further demonstrate that the determinants of a functional unnatural base pair may be designed into predominantly hydrophobic nucleobases with no structural similarity to the natural purines or pyrimidines. Importantly, the results reveal that the unnatural base pairs may function within an expanded genetic alphabet and make possible many in vitro applications.
The genetic alphabet is constrained by the efficient polymerase-mediated replication of DNA and RNA containing the two natural base pairs. In addition to laying the foundation for a semi-synthetic organism with an expanded genetic code, an efficiently and selectively replicated and transcribed unnatural base pair would dramatically increase the potential of the already ubiquitous in vitro methodologies based on DNA and RNA, and their sequence specific amplification. PCR amplification of DNA containing unnatural base pairs was first reported by Benner and co-workers, using pairs with orthogonal H-bonding complementarity,1 followed by Hirao and co-workers,2 using a pair formed between substituted pyrimidine and pyrrole nucleotide analogs. These studies represent landmarks in the effort to expand the genetic alphabet, but the unnatural pairs used are limited by strong sequence dependencies and/or inefficient transcription into RNA. This may not limit their use in some in vitro applications, but does reduce their generality and preclude their eventual use in vivo as part of a semi-synthetic organism.
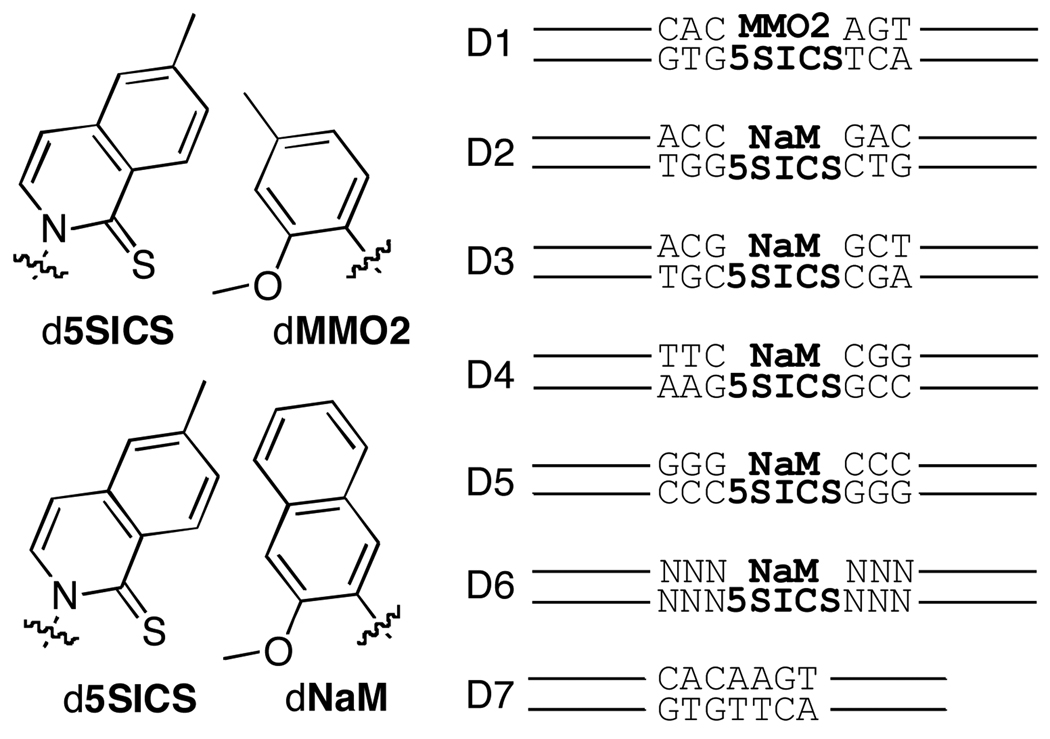
We have focused on developing unnatural base pairs formed between predominantly hydrophobic nucleobases that have no structural homology to the natural nucleobases, and that pair based on hydrophobic and packing forces. Screening of a library of nucleotides, followed by hit optimization, identified the pair formed between d5SICS and dMMO2 (Figure 1), which is relatively well recognized by different DNA polymerases under steady-state, single nucleotide incorporation conditions.3a Further optimization identified dNaM, which pairs with d5SICS to form an unnatural pair that is even better replicated under the same steady state conditions.3b Importantly, d5SICS-dMMO2, and especially d5SICS-dNaM, are also efficiently transcribed in both directions,3c suggesting that they might have immediate practical applications. Here, we examine whether these unnatural base pairs, which are much less natural-like than those previously examined, are also sufficiently well amplified for practical use.
Figure 1.
Unnatural base pairs (with sugar and phosphate backbone omitted for clarity) and duplexes employed in this study.
Nucleosides were synthesized and converted to the corresponding triphosphates or phosphoramidites, and the phosphoramidites were incorporated into DNA duplexes D1– D6 using automated DNA synthesis (Figure 1 and Supporting Information). The duplexes are 134 to 149 nucleotides in length with a single, centrally positioned d5SICS-dMMO2 (D1) or d5SICS-dNaM (D2–D6) pair. D1 is similar to the duplex used by Hirao et al.,2 while D2–D5 systematically vary the flanking dG-dC base pairs to probe for sequence dependencies among sequences that are relatively challenging to PCR amplify.4 D6 contains randomized nucleotides to further explore sequence-specific effects and D7 is identical to D1 but with the unnatural base pair replaced by a natural dA-dT. Using a gel-based assay, we first explored PCR amplification of D1 using exonuclease proficient DeepVent DNA polymerase in the presence and absence of d5SICSTP and dNaMTP (note that this results in the replacement of dMMO2 with dNaM during the first round of replication). Promisingly, PCR conditions were easily identified where DNA was amplified only when both natural and unnatural triphosphates were present (Supporting Information).
To better characterize amplification, we determined the yields of PCR product after 14 cycles, starting with 1 ng of D1–D6, dNTPs, d5SICSTP, and dNaMTP (Table 1). The 424-fold amplification of D1 was the highest and compares favorably with the 556-fold amplification of the control D7. Amplification levels of the other duplexes were slightly lower, likely due to their GC-content, but remained greater than 100-fold, except for D3 and D5, which were amplified 74- and 35-fold, respectively. The lower efficiency observed with D3 and D5 is not surprising considering that they position the unnatural base pair within a dG:dC run, which is particularly challenging to amplify by PCR.4 While amplification levels may be increased with additional rounds of PCR (see below), the data suggest that DNA containing the unnatural base pairs may be amplified with an efficiency that is sufficient for in vitro applications.
Table 1.
Efficiencies and fidelities of PCR amplification. a
| Template | dXTPs incorporated | Enzyme | Amplification | Fidelityb |
|---|---|---|---|---|
| D1 | dNaM, d5SICS | DeepVent | 424 | 99.7 |
| D2 | dNaM, d5SICS | DeepVent | 118 | 99.0 |
| D3 | dNaM, d5SICS | DeepVent | 74 | 98.5 |
| D4 | dNaM, d5SICS | DeepVent | 150 | 99.2 |
| D5 | dNaM, d5SICS | DeepVent | 35 | 98.0 |
| D6 | dNaM, d5SICS | DeepVent | 121 | 99.5 |
| D7 | - | DeepVent | 556 | - |
| D1c | dNaM, d5SICS | DeepVent | 2.7 × 106 | 99.8 |
| D6d | dNaM, d5SICS | DeepVent | 1.9 × 104 | 98.2 |
| D1 | dMMO2, d5SICS | DeepVent | 224 | 99.4 |
| D5 | dMMO2, d5SICS | DeepVent | 25 | 97.1 |
| D6 | dMMO2, d5SICS | DeepVent | 52 | 92.9 |
| D1 | dNaM, d5SICS | Taq | 159 | 98.7 |
| D5 | dNaM, d5SICS | Taq | 83 | 99.1 |
| D6 | dNaM, d5SICS | Taq | 104 | 92.7 |
| D1 | dNaM, d5SICS | Phusion | 257 | 99.7 |
| D5 | dNaM, d5SICS | Phusion | 28 | 85.7 |
| D6 | dNaM, d5SICS | Phusion | 82 | 95.9 |
Conditions: 1 ng DNA template; dNTPs/dXTP = 600/400 µM, 6 mM MgSO4, 0.03 U/µL enzyme, 8 min extension, 14 cycles.
Calculated as average fidelity for unnatural base pair replication in both directions, except with template D5, where it was calculated in one direction (see text).
100 fg DNA template, 30 cycles.
1 pg DNA template, 30 cycles.
To better characterize fidelity, the amplicons were sequenced (Table 1). In most cases, standard sequencing reactions (ABI 3730 DNA Analyzer) lacking unnatural triphosphates terminated at the unnatural nucleotide. In these cases, the fidelities (i.e. the percentage of unnatural base pair retention per doubling) are reported as the average determined by sequencing both amplified strands (Supporting Information). For D5, significant read-through was observed in one direction. However, because read-through was also observed with chemically synthesized control strands, we conclude that it results from sequencing and not from PCR amplification. In this case, the reported fidelity was determined from one strand context. Remarkably, in four of the six sequence contexts examined the average fidelity is at least 99%. It is slightly lower in D3 (98.5%) and D5 (98.0%), which again likely results from their particularly difficult, GC-rich sequence context. Despite the expected challenges associated with replicating the GC-rich sequences, the 99.5% fidelity observed with the random sequence of D6 suggests that in general, most sequences are compatible with high fidelity amplification of the unnatural base pair.
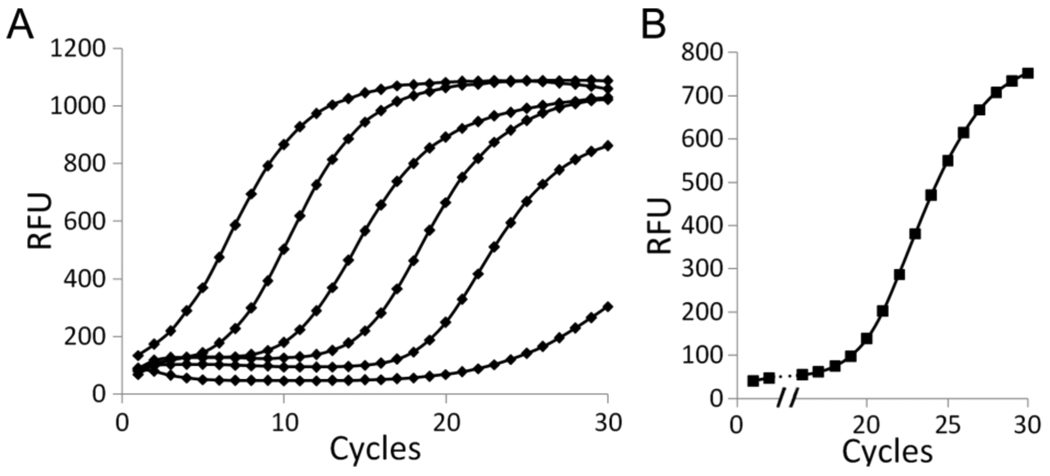
Many in vitro applications rely on the efficient amplification of minuscule quantities of a template. To further explore the potential utility of the unnatural base pair, we examined PCR amplification via quantitative real time PCR with decreasing amounts of D1 and D6 (Table 1 and Figure 2). After 30 cycles of PCR, we found that D1 amplification remains efficient (2.7 × 106) and accurate (99.8% fidelity) even with only 100 fg template. D6 was examined down to 1 pg, where amplification also remained efficient (1.9 × 104) and accurate (98.2% fidelity). The efficient and high fidelity amplification of D6 is particularly noteworthy given its randomized sequence. Moreover, sequencing revealed no major differences before and after amplification of D6 (Supporting Information), further suggesting that amplification is general and not strongly sequence-dependent.
Figure 2.
Quantitative real time PCR analysis. (A) 30 cycle amplification of (from left to right) 1 ng, 100 pg, 10 pg, 1 pg, and 0.1 pg of D1. The rightmost curve corresponds to no template (negative control) where signal results from primer-dimer formation. Each curve represents the average of two independent experiments. (B) 30 cycle amplification of 1 pg of D6. See Supporting Information for details.
To explore the determinants of efficient unnatural base pair amplification, we compared the amplification of DNA using d5SICSTP and dMMO2TP with that described above using d5SICSTP and dNaMTP. We examined amplification with duplexes D1 and D5, which correspond to the best and worst sequence contexts, respectively, for d5SICS-dNaM amplification, as well as with D6 (Table 1). The two unnatural pairs are replicated with similar efficiencies and fidelities in D1, reinforcing the idea that their incorporation into DNA does not rely solely on hydrophobicity, since dNaM is much more hydrophobic than dMMO2, and also that the intrabase packing interactions, which are similar in the two base pairs, make an important contribution. However, the data also reveal that d5SICS-dMMO2 is replicated with lower fidelity and efficiency in D5 and D6, suggesting that its recognition is more sequence context dependent than that of d5SICS-dNaM.
We next examined amplification with two other thermostable polymerases commonly used for PCR, Taq and Phusion™, using templates D1, D5, or D6, and dNTPs as well as d5SICSTP and dNaMTP (Table 1). Relative to DeepVent, Taq recognizes the unnatural base pair in D1 and D5 with similar efficiency and fidelity, but the fidelity is somewhat reduced with D6. Relative to DeepVent, Phusion™ polymerase amplifies d5SICS-dNaM in D1 with generally similar efficiency and fidelity, but in this case the fidelities are somewhat lower with both D5 and D6. The data suggest that the different polymerases behave similarly, except with increasing GC content, where divergent behavior is also observed with fully natural sequences,4b further demonstrating that the determinants of replication are contained within the base pairs and are not specific for a given polymerase.
We have now demonstrated not only that d5SICS-dNaM and d5SICS-dMMO2 are efficiently transcribed in either direction,3c but also that they may be amplified by PCR, and are thus the first pairs to fulfill all of the primary requirements of a fully functional unnatural base pair. From a theoretical perspective, the data reveal that all of the properties required of a functional unnatural base pair may be optimized within predominantly hydrophobic nucleobases that bear no homology to the natural nucleobases. From a practical perspective, the data suggest that d5SICS-dMMO2 and especially d5SICS-dNaM are sufficiently optimized for use as part of an in vitro expanded genetic alphabet. This should enable a variety of unprecedented applications,5 which are currently being explored.
Supplementary Material
Acknowledgment
Funding provided by NIH GM060005.
Footnotes
Supporting Information Available: Details of template synthesis and PCR analysis. This information is available free of charge via the Internet at http://www.pubs.acs.org.
References
- 1.(a) Sismour AM, Lutz S, Park JH, J LM, Boyer PL, Hughes SH, Benner SA. Nucleic Acids Res. 2004;32:728–735. doi: 10.1093/nar/gkh241. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yang Z, Sismour AM, Sheng P, Puskar NL, Benner SA. Nucleic Acids Res. 2007;35:4238–4249. doi: 10.1093/nar/gkm395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Hirao I, Kimoto M, Mitsui T, Fujiwara T, Kawai R, Sato A, Harada Y, Yokoyama S. Nat. Methods. 2006;3:729–735. doi: 10.1038/nmeth915. [DOI] [PubMed] [Google Scholar]; (b) Kimoto M, Kawai R, Mitsui T, Yokoyama S, Hirao I. Nucleic Acids Res. 2009;37:e14. doi: 10.1093/nar/gkn956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Leconte AM, Hwang GT, Matsuda S, Capek P, Hari Y, Romesberg FE. J. Am. Chem. Soc. 2008;130:2336–2343. doi: 10.1021/ja078223d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Seo YJ, Hwang GT, Ordoukhanian P, Romesberg FE. J. Am. Chem. Soc. 2009;131:3246–3252. doi: 10.1021/ja807853m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Seo YJ, Matsuda S, Romesberg FE. J. Am. Chem. Soc. 2009;131:5046–5047. doi: 10.1021/ja9006996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Hansen LL, Justensen J. In: PCR Primer: A Laboratory Manual. Diffenbach CW, Dveksler GS, editors. Woodbury, NY: Cold Spring Harbor Laboratory Press; 2003. pp. 43–62. [Google Scholar]; (b) Arezi B, Xing W, Sorge JA, Hogrefe HH. Anal. Biochem. 2003;321:226–235. doi: 10.1016/s0003-2697(03)00465-2. [DOI] [PubMed] [Google Scholar]
- 5.Klussmann S, editor. The Aptamer Handbook: Functional Oligonucleotides and Their Applications. Weinheim: Wiley-VCH; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.