Abstract
H-NS is an abundant DNA-binding protein that has been implicated in the silencing of foreign DNA in several different bacteria. The ability of H-NS dimers to form higher-order oligomers is thought to aid the polymerization of the protein across AT-rich stretches of DNA and facilitate gene silencing. Although the oligomerization of H-NS from enteric bacteria has been the subject of intense investigation, little is known regarding the oligomerization of H-NS family members from bacteria outside of the enterobacteriaceae, many of which share little sequence similarity with their enteric counterparts. Here we show that MvaT, a member of the H-NS family of proteins from Pseudomonas aeruginosa, can form both dimers and higher order oligomers, and we identify a region within MvaT that mediates higher-order oligomer formation. Using genetic assays we identify mutants of MvaT that are defective for higher-order oligomer formation. We present evidence that these mutants are functionally impaired and exhibit DNA-binding defects because of their inability to form higher-order oligomers. Our findings support a model in which the ability of MvaT to bind efficiently to the DNA depends upon protein-protein interactions between MvaT dimers and suggest that the ability to form higher-order oligomers is a conserved and essential feature of H-NS family members.
Keywords: H-NS, nucleoid-associated protein, oligomerization, two-hybrid, chromatin immunoprecipitation
Introduction
H-NS, the histone-like nucleoid structuring protein, is an abundant DNA-binding protein of ~15 kDa in size that serves to repress the expression of hundreds of different genes in enteric bacteria including E. coli, Salmonella typhimurium and Shigella flexneri (Dorman, 2004; Fang and Rimsky, 2008). In addition to its regulatory role, H-NS plays a structural role and may promote the formation of large topologically insulated DNA-loops in the chromosome through its well-documented ability to bridge adjacent DNA molecules (Dame et al., 2006; Noom et al., 2007). Genome-wide location studies performed in enteric bacteria have revealed that the protein displays a distinct preference for binding AT-rich regions of DNA, which is thought to facilitate the silencing of DNA that originated from a foreign source, such as that acquired through horizontal transfer (Navarre et al., 2006; Lucchini et al., 2006, Grainger et al., 2006; Oshima et al., 2006). Accordingly, H-NS represses the expression of many virulence genes that are housed on pathogenicity islands, which often have AT-contents much higher than the genome in which they reside (Navarre et al., 2006; Navarre et al., 2007; Dorman, 2007). This repression is thought to limit any potentially adverse effects of inheriting such xenogeneic DNA species, and at the same time, facilitate the integration of the genes they contain into the existing regulatory networks of the cell (Navarre et al., 2007; Dorman, 2007).
In its role as a repressor H-NS is thought to bind a specific sequence on the DNA with high affinity and then oligomerize across adjacent AT-rich regions (Bouffartigues et al., 2007; Lang et al., 2007; Sette et al., 2009). The resulting nucleoprotein complex mediates gene silencing by occluding promoters, or possibly even by trapping RNA polymerase at the promoter (Dorman, 2004; Fang and Rimsky, 2008). This repression can be readily circumvented by other DNA-binding proteins that displace H-NS from the DNA through competition for overlapping binding sites (Stoebel et al., 2009). Structurally, H-NS consists of two domains separated by a flexible linker. The carboxy (C)-terminal domain contains the primary DNA-binding determinants (Dorman, 2004; Sette et al., 2009), whereas the amino (N)-terminal domain is involved in oligomerization and contains a coiled-coil that mediates formation of the H-NS dimer. A central portion of the protein, comprising part of the N-terminal domain and linker, is involved in mediating the formation of higher-order oligomers (i.e. multimers of a complexity higher than a dimer) and it has been argued that the tetrameric form of the protein represents the basic functional unit in vivo (Stella et al., 2005). Indeed, the ability of H-NS to form higher-order oligomers is key to the function of the protein because of the pronounced effect it has on DNA-binding (Badaut et al., 2002).
H-NS family members are found in organisms other than the enterobacteriacea (Tendeng and Bertin, 2003). In Pseudomonas aeruginosa, an opportunistic pathogen of humans, the H-NS family member MvaT controls the expression of at least 150 or so different genes, including many that encode surface structures or virulence factors that contribute to colonization of the host (Vallet et al., 2004). Indeed, MvaT was originally described in P. aeruginosa as a global regulator of virulence gene expression (Diggle et al., 2002). Like H-NS, MvaT displays a distinct preference for binding to AT-rich regions of the DNA, and ~110 different regions of the chromosome have been identified with which MvaT associates (Castang et al., 2008). MvaT appears to function as a repressor and one of the operons that is most tightly repressed by MvaT is the cupA operon, which encodes fimbriae that facilitate biofilm formation (Vallet et al., 2001; Vallet et al., 2004; Meissner et al., 2007). Although MvaT has been described as a member of the H-NS family of proteins, based on functional and predicted structural similarities (Tendeng and Bertin, 2003; Tendeng et al., 2003), MvaT shares very little sequence identity with H-NS. Thus, it was not known whether MvaT could form higher-order oligomers, or to what extent the oligomeric state of MvaT influenced the function of the protein.
Here we describe a region within MvaT that mediates higher-order oligomer formation and present evidence that the ability to form such oligomers is critical to the function of the protein. These findings suggest that the ability of H-NS family members to form higher-order oligomers is a conserved and essential feature of these proteins.
Results
Genetic assays for MvaT oligomer formation
We wished to determine whether MvaT, like H-NS from E. coli, is capable of forming dimers and higher-order oligomers. We therefore initially asked whether MvaT could functionally substitute for the dimerization domain of the CI protein from bacteriophage λ, a sequence-specific DNA binding protein that binds its recognition site as a dimer (Hu et al., 2000). Specifically, we fused the amino-terminal domain (NTD) and linker of CI (residues 1–132) to MvaT (residues 2–124). We then asked whether the resulting λCINTD-MvaT fusion protein could bind a λ operator in cells of an E. coli reporter strain in which the λ operator is positioned between the −10 and −35 elements of a test promoter (Fig. 1A). In reporter strain FW123 the test promoter drives expression of lacZ, and binding of CI to the λ operator represses lacZ expression by occluding the promoter (Whipple, 1998). The results depicted in Fig. 1B show that full-length λCI and the λCINTD-MvaT fusion protein, but not the λCI NTD and linker (depicted λCINTD), can efficiently repress expression of the lacZ reporter in strain FW123. MvaT can therefore functionally replace the dimerization domain of λCI, suggesting that MvaT is capable of forming a dimer or higher-order oligomer. H-NS from E. coli can also functionally substitute for the dimerization domain of λCI (Spurio et al., 1997; Stella et al., 2005).
Figure 1.
MvaT forms oligomers in vivo. (A) Schematic representation of the bacterial genetic assay used to study MvaT oligomerization in vivo. In this system MvaT functionally replaces the dimerization domain of the bacteriophage λ CI protein (λCI). Occupancy of the λ operator located between the promoter −35 and −10 elements) by λCI prevents RNAP from binding the promoter and represses transcription. (B) Transcription repression by λCI and the λCINTD-MvaT fusion protein. FW123 cells containing a plasmid directing the IPTG-dependent synthesis of either the N-terminal domain and linker of λCI (λCINTD), λCI, or the λCINTD-MvaT fusion protein were grown in the presence of 20 µM IPTG and assayed for β-galactosidase activity. (C) Schematic representation of the bacterial two-hybrid assay used to detect MvaT higher-order oligomer formation. Contact between the λCINTD-MvaT fusion protein and MvaT (residues 2–124) fused to the N-terminal domain and linker of the α subunit of E. coli RNAP (α-MvaT), activates transcription from the test promoter which contains the λ operator OR2 centered 42 bp upstream of the transcription start site of promoter PRM. Note that when the λCINTD-MvaT fusion protein is bound to OR2 at this position, the CI moiety of the fusion protein contacts region 4 of σ70 bound to the −35 element of the test promoter (interaction not depicted). In E. coli strain BN30, the PRM test promoter is linked to lacZ on an F′ episome. (D) Transcription activation by the λCINTD-MvaT fusion protein in the presence of the α-MvaT fusion protein. BN30 cells harboring compatible plasmids synthesizing the indicated proteins were grown and assayed for β-galactosidase activity.
To determine whether we could detect the formation of MvaT oligomers with a complexity higher than a dimer we used a bacterial two-hybrid system. In this assay, contact between a protein (or protein domain) fused to a subunit of E. coli RNA polymerase (RNAP) and a partner protein fused to a DNA-binding protein activates transcription from a test promoter harboring a recognition site for the DNA-binding protein (Dove et al., 1997; Dove and Hochschild, 1998). Reasoning that the λCINTD-MvaT fusion protein must be at least a dimer in order to bind a λ operator we asked whether the DNA-bound λCINTD-MvaT fusion protein could interact with MvaT when it is tethered to RNAP (Fig. 1C). We therefore fused MvaT (residues 2–124) to the NTD and linker of the α subunit of E. coli RNAP (residues 1–248), replacing the carboxy-terminal domain (CTD) of α with MvaT. We then tested whether the λCINTD-MvaT fusion protein could activate transcription from a suitable test promoter in cells of an E. coli reporter strain that synthesized the resulting α-MvaT fusion protein. These assays were performed in reporter strain BN30 in which the PRM promoter drives expression of lacZ and the λ operator OR2 is centered 42 bp upstream of the transcription start site of PRM. The results depicted in Fig. 1D show that in reporter strain BN30 the λCINTD-MvaT fusion protein strongly activated transcription from the test promoter only in the presence of the α-MvaT fusion protein. Additional controls revealed that the α-MvaT fusion protein did not activate transcription from the test promoter in the presence of just λCINTD. These findings suggest that MvaT is capable of forming oligomers with a complexity higher than a dimer.
Isolation of MvaT mutants specifically defective for higher-order oligomerization
To determine whether the ability of MvaT to form higher-order oligomers is important for its activity we first sought to identify MvaT mutants that were specifically defective for these interactions. Our strategy for isolating such mutants involved two sequential genetic screening steps. In the first screening step, we used our bacterial two-hybrid assay (Fig. 1C) to isolate mutants of the λCINTD-MvaT fusion protein that were defective for interaction with the α-MvaT fusion protein. In the second screening step, we used our DNA-binding assay (Fig. 1A) to identify mutant λCINTD-MvaT fusion proteins that could still form dimers and thus bind a λ operator. In this way we hoped to isolate MvaT mutants that could no longer form higher order oligomers but were still able to functionally replace the dimerization domain of λCI.
To identify amino acid substitutions in MvaT that disrupt higher order oligomer formation we mutagenized the DNA encoding the MvaT portion of the λCINTD-MvaT fusion protein. Plasmids encoding mutant λCINTD-MvaT fusion proteins that failed to activate transcription from the PRM-lacZ reporter in the presence of the α-MvaT fusion protein were isolated and pooled. We then screened for those plasmids encoding mutant λCINTD-MvaT fusion proteins that could still form dimers and thus repress transcription from the test promoter in reporter strain FW123. Using this approach we identified two substitutions in the MvaT moiety of the λCINTD-MvaT fusion protein that specifically disrupted higher order oligomer formation. The results depicted in Fig. 2 show that substitutions F36S and R41P render the corresponding mutant λCINTD-MvaT fusion proteins incapable of activating transcription from the PRM-lacZ reporter in the presence of the α-MvaT fusion protein (Fig. 2A), but have no effect on the abilities of these fusions to bind a λ operator (Fig. 2B). These findings suggest that substitutions F36S and R41P disrupt certain higher-order interactions of MvaT but have little effect on the ability of MvaT to functionally substitute for the dimerization domain of λCI.
Figure 2.
Amino acid substitutions F36S and R41P specifically interfere with the ability of MvaT to form higher-order oligomers. (A) Effect of λCINTD, λCINTD-MvaT, λCINTD-MvaT (F36S) or λCINTD-MvaT (R41P) proteins on transcription in vivo from PRM in the presence of α-MvaT. BN30 cells containing compatible plasmids directing the synthesis of the indicated proteins were grown and assayed for β-galactosidase activity. (B) Transcription repression by the λCINTD-MvaT, λCINTD-MvaT (F36S), and λCINTD-MvaT (R41P) fusion proteins. FW123 cells harboring plasmids directing the IPTG-dependent synthesis of the indicated proteins were grown in the presence of 20 µM IPTG and assayed for β-galactosidase activity.
Defining the region of MvaT that participates in higher-order oligomer formation
To better define which region of MvaT mediates higher-order oligomer formation we used our bacterial two-hybrid assay. We fused different portions of MvaT to λCINTD and then asked which of these fusions were able to activate transcription from the PRM-lacZ reporter specifically in the presence of the α-MvaT fusion protein. We found that λCINTD-MvaT fusion proteins harboring MvaT residues 2–62, or 2–84 activated transcription from the test promoter just as well as the full-length λCINTD-MvaT fusion protein (harboring residues 2–124 of MvaT) (Fig. 3A), suggesting that residues 2–62 suffice to mediate higher-order oligomer formation. Using our repression assay (Fig. 1A), we found that MvaT residues 2–35, 2–45, 2–55, 2–62 and 2–84 could functionally substitute for the dimerization domain of λCI (Fig. 3B). In combination with Western blotting to measure the abundance of each fusion protein (data not shown), these repression assays revealed that the λCINTD-MvaT fusion proteins containing MvaT residues 2–35, 2–45, 2–55, or 2–62, bound DNA with comparable efficiencies. This suggests that those fusion proteins containing MvaT residues 2–35, 2–45, or 2–55, were not defective for interacting with the α-MvaT fusion protein in our two-hybrid assay (Fig. 3A) simply because they could no longer bind the DNA. Furthermore, the finding that residues 2–35 of MvaT could functionally substitute for the dimerization domain of λCI suggest that this region of MvaT contains a protein-interaction surface that may be involved in mediating formation of the MvaT dimer. Taken together these findings suggest that the region of MvaT spanning residues 2–62 is involved in higher-order oligomer formation, and that residues 2–35 of MvaT may be involved in dimer formation (see below).
Figure 3.
The N-terminal region of MvaT is involved in higher-order oligomer formation. (A) Ability of λCINTD-MvaT fusion proteins containing different portions of MvaT to interact with the α-MvaT fusion protein [α-MvaT(2–124)]. BN30 cells harboring compatible plasmids directing the synthesis of the indicated proteins were grown and assayed for β-galactosidase activity. Cells contained either α-MvaT(2–124) or just wild-type α (indicated α), and either the λCINTD (indicated-), λCINTD-MvaT(2–124), λCINTD-MvaT(2–35), λCINTD-MvaT(2–45), λCINTD-MvaT(2–55), λCINTD-MvaT(2–62), or the λCINTD-MvaT(2–84) fusion protein. (B) Transcription repression by the λCINTD-fusion proteins. FW123 cells harboring plasmids directing the IPTG-dependent synthesis of the indicated proteins were grown in the presence of 20 µM IPTG and assayed for β-galactosidase activity. Cells contained either the λCINTD (indicated -), MvaT(2–124), λCINTD-MvaT(2–35), λCINTD-MvaT(2–45), λCINTD-MvaT(2–55), λCINTD-MvaT(2–62), λCINTD-MvaT(2–84), or λCINTD-MvaT(35–62).
To test the hypothesis that the region of MvaT spanning residues 35–62 is responsible for mediating higher-order oligomer formation we used a different configuration of our bacterial two-hybrid assay in which the DNA-binding protein and the RNAP subunit are monomeric. This assay is, therefore, especially suited to detecting interactions between two protein monomers (Vallet-Gely et al., 2005). In particular, we used the zinc-finger DNA-binding protein V-Zif to display one interaction partner and the ω subunit of E. coli RNAP to display the other interaction partner. Interaction between a protein fused to V-Zif and a partner protein fused to ω activates transcription from a test promoter harboring a V-Zif binding site (Fig. 4A). Using this system, we first tested whether residues 35–62 of MvaT constitute a functionally independent oligomerization determinant. To do this and to determine which portion(s) of MvaT might interact with residues 35–62, we fused the corresponding portion of MvaT to V-Zif and then asked whether the resulting fusion protein could interact with different portions of MvaT that had been fused to ω. The results depicted in Figure 4B show that the V-Zif-MvaT fusion protein harboring MvaT residues 35–62 [V-Zif-MvaT (35–62)] activated transcription from the test promoter in the presence of the ω-MvaT fusion proteins harboring MvaT residues 2–124 (i.e. full-length MvaT), residues 2–62, residues 35–124, and residues 35–62. These findings suggest that there is an interaction surface on MvaT spanning residues 35–62. In further support of this idea we also found that residues 35–62 of MvaT could functionally replace the dimerization domain of λCI (Fig. 3B).
Figure 4.
Residues 35–62 of MvaT constitute a higher-order oligomerization determinant. (A) Schematic representation of the bacterial two-hybrid system. Contact between protein domains X and Y fused, respectively, to the ω subunit of E. coli RNAP and to V-Zif activates transcription from the test promoter driving expression of lacZ. The diagram depicts test promoter placZif1–61, which bears a V-Zif-binding site centered 61 bp upstream of the transcription start site of the lac core promoter. In E. coli strain KDZif1ΔZ, this test promoter is linked to lacZ on an F′ episome. (B) Effect of different V-Zif-MvaT fusion proteins on transcription in vivo from promoter placZif 1–61 in the presence of either the ω-Gal11P fusion protein (indicated GP), the ω-MvaT 2–124) fusion protein (indicated 2–124), the ω-MvaT (2–62) fusion protein (indicated 2–62), the ω-MvaT (35–124) fusion protein (indicated 35–124), the ω-MvaT (35–62) fusion protein (indicated 35–62), or the ω-MvaT (63–124) fusion protein (indicated 63–124). KDZif1ΔZ cells harboring compatible plasmids directing the IPTG-inducible synthesis of the indicated proteins were grown in the presence of 5 µM IPTG and assayed for β-galactosidase activity.
We next asked whether the interaction surface located between residues 35–62 of MvaT is the same surface of MvaT that is involved in mediating higher-order oligomer formation. To address this question we first fused the MvaT mutants harboring substitutions F36S and R41P that disrupt higher-order oligomer formation to V-Zif. We then asked whether these mutant fusion proteins could interact with residues 35–62 of MvaT. The results depicted in Fig. 4B show that the V-Zif-MvaT (2–124) fusion proteins containing substitutions F36S or R41P failed to activate transcription from the test promoter in the presence of the ω-MvaT (35–62) fusion protein or in the presence of the ω-MvaT (35–124) fusion protein. These mutant V-Zif-MvaT fusions were however, still capable of interacting with the ω-MvaT (2–62) fusion protein and the ω-MvaT (2–124) fusion protein, suggesting that the mutant V-Zif-MvaT fusions are not defective for interaction with fusion proteins containing residues 35–62 of MvaT simply because they are misfolded. These findings, taken together with our other two-hybrid results, suggest that residues 35–62 of MvaT constitute a region of the protein that is involved in mediating higher-order oligomer formation, and furthermore that higher-order oligomer formation is mediated through interaction between residues 35–62 on one MvaT monomer, and residues 35–62 on another monomer.
The finding that the V-Zif-MvaT (2–124) fusion proteins containing substitutions F36S or R41P interacted with the ω-MvaT (2–62) fusion protein as well as the ω-MvaT (2–124) fusion protein, but not with the ω-MvaT (35–124), or the ω-MvaT (35–62) fusion protein (Fig. 4B) are consistent with their being a second interaction surface on MvaT, distinct from the one involved in mediating higher-order oligomer formation, which accounts for the activity of the mutant V-Zif-MvaT fusions. This second interaction surface appears to involve residues 2–35 (a region MvaT that can functionally substitute for the dimerization domain of λCI; Fig. 3B). In further support of this idea, we found that the V-Zif-MvaT (2–35) fusion protein activated transcription from the test promoter in the presence of the ω-MvaT (2–62) fusion protein, but not in the presence of the ω-MvaT (35–62) fusion protein (Fig. 4B). This second interaction surface involving residues 2–35 of MvaT contains most of a predicted coiled-coil (Vallet-Gely et al., 2005) and could be involved in mediating the formation of MvaT dimers.
MvaT oligomerization mutants are functionally impaired
Having identified a region of MvaT that is involved in mediating higher-order oligomer formation, and having isolated MvaT mutants that appear to be specifically defective for higher-order oligomer formation, we sought to determine whether the ability of MvaT to form higher-order oligomers is important for function. To do this we asked whether MvaT mutants containing substitutions F36S and R41P could functionally substitute for wild-type MvaT in cells of P. aeruginosa.
We have shown previously that in P. aeruginosa MvaT represses phase-variable expression of the cupA fimbrial genes. In particular, in a PAO1 ΔmvaT cupA lacZ reporter strain, in which lacZ is positioned downstream of cupA1 on the chromosome (Fig. 5A), the cupA genes are expressed in a phase-variable manner, as manifest by the appearance of blue and white colonies on LB agar plates containing the chromogenic substrate X-Gal (Vallet-Gely et al., 2005) (Fig. 5A). This reversible ON-OFF switching of cupA gene expression in the ΔmvaT mutant strain could be repressed by the mvaT gene when provided in trans, giving rise to white colonies on LB agar plates containing X-Gal (Vallet-Gely et al., 2005) (Fig. 5A). To test whether mutants MvaT(F36S) and MvaT(R41P) could repress phase-variable expression of the cupA fimbrial genes as efficiently as wild-type MvaT we introduced into reporter strain PAO1 ΔmvaT cupA lacZ plasmids directing the synthesis of versions of either wild-type MvaT, MvaT(F36S), or MvaT(R41P), each containing a vesicular stomatitis virus glycoprotein (VSV-G) epitope tag at its C-terminus. Unlike wild-type MvaT containing a C-terminal VSV-G epitope tag (MvaT-V), mutants MvaT(F36S)-V, and MvaT(R41P)-V, failed to repress phase-variable expression of the cupA lacZ reporter (Fig. 5A). Western blotting using an antibody against the VSV-G epitope tag revealed that all three proteins were made at comparable amounts (data not shown). These results suggest that the ability of MvaT to form high-order oligomers is essential in order for MvaT to repress phase-variable expression of the cupA fimbrial genes.
Figure 5.
MvaT oligomerization mutants are impaired for functionality in vivo. (A) Upper panel: Schematic of the ΔmvaT cupA1 lacZ reporter strain. Lower panel: Colony phenotypes of PAO1 ΔmvaT cupA1 lacZ cells (indicated ΔmvaT) carrying either the empty control vector pPSV37 (indicated -) or plasmids directing the IPTG-dependent synthesis of either MvaT-V (indicated MvaT), MvaT(F36S)-V [indicated MvaT(F36S)], or MvaT(R41P)-V [indicated MvaT(R41P)] grown overnight at 37°C on LB agar plates containing X-Gal and IPTG. The results show that phase-variable expression of the cupA genes that occurs in a ΔmvaT mutant strain is complemented by wild-type MvaT-V but not by MvaT-V mutants containing amino acid substitutions F36S or R41P. (B) ChIP experiment using wild-type and mutant forms of MvaT-V. PAO1 ΔmvaT cells carrying plasmids synthesizing MvaT-V (indicated WT) or MvaT(F36S)-V (indicated F36S) or MvaT(R41P)-V (indicated R41P) in an IPTG-inducible manner were grown to mid-log in LB in the presence of 1 mM IPTG, then cells were harvested for Western blot analysis and ChIP. Top panel: ChIP-enriched-DNA was measured by qPCR and fold enrichment determined by comparison to the PA2896 promoter as a non-binding control. Error bars represent 1 SD from the mean fold enrichment. (C) (Upper panel) Western blot analysis of MvaT-V (WT) (lanes 1–2) and mutant forms F36S (lanes 3–4) and R41P (lanes 5–6) probed with anti-VSV-G antibody. (Lower) Western blot analysis probed with antibody against the α subunit of RNAP serves as a control for sample loading.
MvaT oligomerization mutants exhibit DNA-binding defects
H-NS mutants that are impaired for higher-order oligomer formation exhibit DNA-binding defects in vitro (Badaut et al., 2002). To determine whether our MvaT oligomerization mutants might be functionally impaired because they are unable to bind DNA, we used chromatin immunoprecipitation (ChIP) to measure their ability to associate with specific regions of the DNA in cells of P. aeruginosa.
Cells of a PAO1 ΔmvaT mutant strain ectopically synthesizing either MvaT-V, MvaT(F36S)-V, or MvaT(R41P)-V were grown to the mid-logarithmic phase of growth. Following ChIP, the degree of occupancy of MvaT, or MvaT mutant, at a particular promoter was then measured by quantitative real time PCR (qPCR), with fold enrichment being determined by comparison to the PA2896 promoter which served as a non-binding control (Castang et al., 2008). Whereas the mvaU, cupA and lecA promoters were all enriched to greater than 150-fold by ChIP with wild-type MvaT-V, these same promoters were enriched only to ~10–15 fold by ChIP with the MvaT(F36S)-V or MvaT(R41P)-V mutant (Fig. 5B). Western blotting revealed that similar amounts of wild-type MvaT-V and the MvaT mutant derivates were present in the cells used for ChIP, suggesting that the reduced occupancy of MvaT(F36S)-V and MvaT(R41P)-V at the promoters tested was not due to differences in protein abundance (Fig. 5C). Thus, although MvaT mutants harboring substitutions F36S and R41P that impair high-order oligomerization can still associate with target promoters in vivo, they exhibit markedly reduced binding to the DNA. These findings suggest that the ability of MvaT to form high-order oligomers is important for DNA-binding.
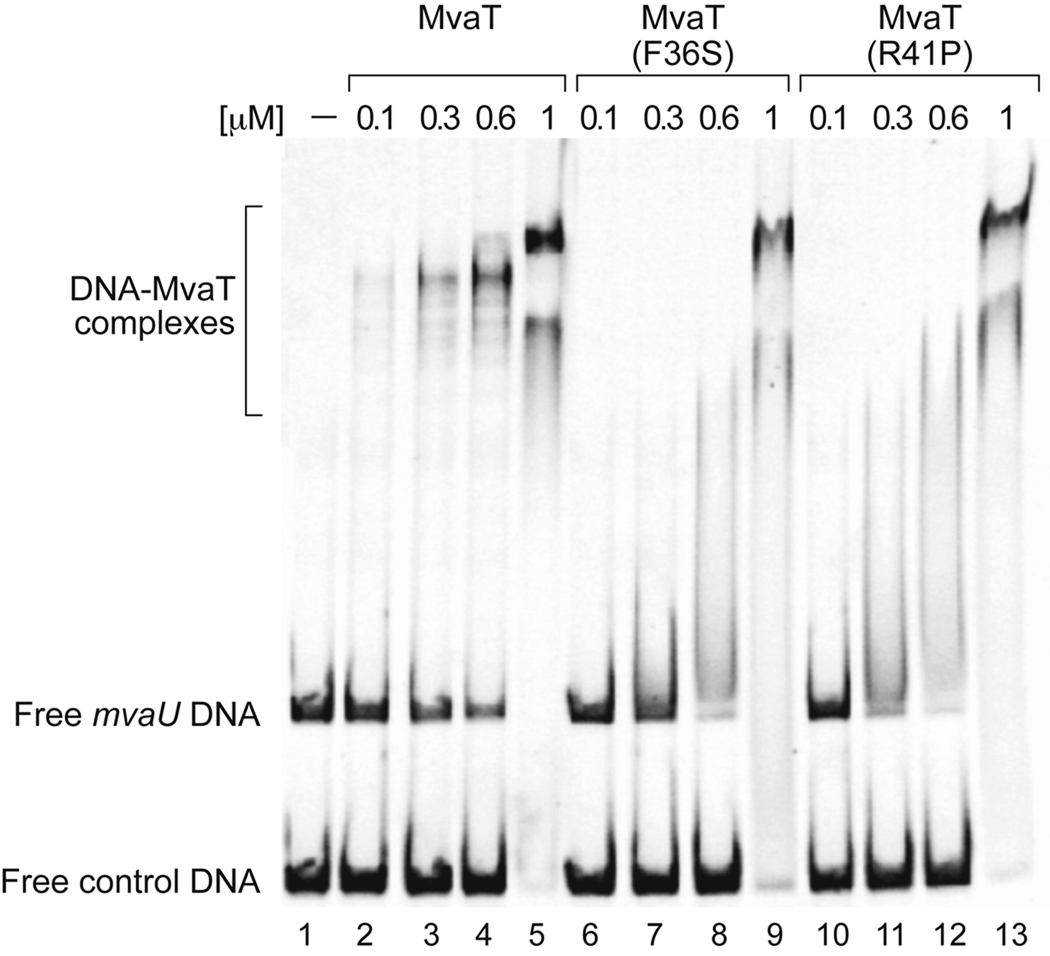
To directly test the effects of amino acid substitutions F36S and R41P on the ability of MvaT to bind DNA we purified wild-type MvaT together with the mutant derivatives and measured the ability of each of these proteins to bind DNA in vitro. To facilitate purification, six histidine residues were fused to the C-terminus of each protein; the His-tag did not impair the activity of MvaT as the his-tagged protein (MvaT-His6) repressed expression of the cupA lacZ reporter in cells of our PAO1 ΔmvaT cupA lacZ strain just as efficiently as the wild-type protein (data not shown). To measure DNA-binding, different concentrations of purified MvaT-His6, MvaT(F36S)-His6 and MvaT(R41P)-His6, were incubated with two fragments of DNA, one of 347 bp in length containing the mvaU promoter region, and another of 211 bp in length corresponding to a portion of the tex gene. Binding of wild-type MvaT-His6 and the MvaT mutants to these DNA species was then measured by electrophoretic mobility shift assay (EMSA). In vivo, MvaT is associated with the mvaU gene and promoter, but not the tex gene (Castang et al., 2008). The tex DNA fragment therefore served as a non-specific control in these assays. Wild-type MvaT preferentially bound the mvaU promoter fragment, giving rise to a variety of discrete stable complexes with different mobilities, which presumably correspond to DNA fragments containing different numbers of bound MvaT molecules (Fig. 6). Such discrete stable complexes were not formed by MvaT(F36S)-His6 or MvaT(R41P)-His6, which instead appeared to form complexes with the DNA that were unstable during electrophoresis. Note however, that like wild-type MvaT-His6, mutants MvaT(F36S)-His6 and MvaT(R41P)-His6 still appeared to bind preferentially to the mvaU promoter fragment. At sufficiently high protein concentrations, both MvaT(F36S)-His6 and MvaT(R41P)-His6 formed stable high molecular weight complexes with both DNA fragments, just like wild-type MvaT-His6 (Fig. 6). These findings show that mutants MvaT(F36S) and MvaT(R41P) exhibit a diminished capacity for binding DNA, but are not completely defective for DNA binding, consistent with our in vivo findings (Fig. 5).
Figure 6.
MvaT oligomerization mutants are defective for DNA binding in vitro. EMSA experiment using wild-type and mutant forms of MvaT (F36S and R41P). DNA fragment mvaU (~20 nM) and control DNA fragment tex (~23 nM) were incubated with the indicated concentration of purified C-terminal His-tagged protein, then analyzed on 5% polyacrylamide gel and stained with GelStar.
MvaT oligomerization mutants do not form higher-order oligomers in vitro
Our findings thus far suggest that MvaT can form oligomers of a complexity higher than a dimer and that the ability of MvaT to form certain high-order oligomeric species is essential in order for MvaT to bind DNA efficiently and function as a repressor. However, our genetic assays do not allow us to define precisely which oligomeric species MvaT can form, and therefore do not allow us to specify which oligomeric species can and cannot be formed by our oligomerization mutants. Therefore, to determine which oligomeric species MvaT is capable of forming, and determine which oligomeric state(s) of MvaT are inhibited by substitutions F36S and R41P we used in vitro cross-linking.
To trap and visualize the different oligomeric states of MvaT, MvaT(F36S) and MvaT(R41P) we incubated purified His-tagged proteins with glutaraldehyde for 30 minutes, removing cross-linked samples at different time-points. We then separated the different oligomeric species by SDS-PAGE and visualized them by Western blotting using an antibody against the His-tag present at the C-terminus of each protein. The results depicted in Fig. 7 show that wild-type MvaT is capable of forming dimers, trimers, tetramers, and higher-order species. Similar results have been reported for H-NS when in vitro cross-linking was used to assess oligomerization (Falconi et al., 1988; Badaut et al., 2002). Over the time-course of our experiment, the dimeric and tetrameric species of MvaT are the most prevalent suggesting that the trimeric form of MvaT may be a particularly short-lived or transient species (Fig. 7). It is also possible that the apparent trimeric MvaT species is an artifact resulting from the use of cross-linking to assess the oligomeric status of the protein; i.e. the MvaT trimer may simply represent a MvaT tetramer in which only three of the four monomers have been cross-linked to one another. Analysis of the cross-linked species formed by MvaT(F36S) and MvaT(R41P) suggest that these proteins cannot form tetramers, at least at the concentration of protein used in these experiments. Note that a faint band corresponding to an apparent trimer can be seen for both MvaT(F36S) and MvaT(R41P) (Fig. 7). Results similar to those shown in Fig. 7 were obtained when proteins were analyzed at a higher concentration (i.e. 12.4 µM; data not shown). Although comparison of the amount of cross-linked dimers formed by wild-type and mutant versions of MvaT makes it appear as if amino acid substitutions F36S and R41P impair the ability of MvaT to form a dimer (Fig. 7), this may simply reflect a shift in the monomer-dimer equilibrium that occurs as a consequence of the formation of covalently linked wild-type MvaT tetramers (see also Discussion).
Figure 7.
MvaT oligomerization mutants are impaired for higher-order oligomer formation in vitro. In vitro cross-linking experiment of wild-type and mutant forms of MvaT (F36S and R41P). Glutaraldehyde (0.04%) was added to purified C-terminal His-tagged proteins (1.85 µM). Aliquots were removed at the indicated time points and proteins analyzed by Western blotting using an antibody against the His tag on each protein. Different oligomeric species are indicated.
As an additional test of which oligomeric species can be formed by the wild-type and mutant versions of MvaT, we subjected purified proteins to size-exclusion chromatography. The results depicted in Fig. 8A indicate that wild-type MvaT formed a variety of species of different complexities at each concentration tested (as indicated by the asymmetric peak representing the elution profile of the protein). The complexity of the high-order oligomeric complexes formed by MvaT is concentration dependent; MvaT formed oligomeric species of up to ~31-mers, up to ~17-mers, and up to ~6-mers, at loading concentrations of 62, 42, and 14 µM, respectively. Thus, like H-NS, which can form a range of oligomeric species up to at least a 20-mer (Smyth et al., 2000), MvaT does not exist in a single defined oligomeric state. Size-exclusion chromatography revealed that both MvaT(F36S) and MvaT(R41P) appear to form a single oligomeric species that most closely resembles a dimer, or possibly a trimer, at the three different protein concentrations tested (Fig. 8B and C). Consistent with the results of our genetic assays, these findings suggest that amino acid substitutions F36S and R41P do not interfere with formation of the MvaT dimer. Furthermore, consistent with the results of our cross-linking experiments, these findings suggest that both MvaT(F36S) and MvaT(R41P) do not form tetramers, at least at the protein concentrations tested.
Figure 8.
Size-exclusion chromatography analysis of wild-type MvaT, MvaT(F36S) and MvaT(R41P). The figure presents protein absorbance at 280 nm as a function of the elution volume (ml). Purified His-tagged proteins (100 µl) were applied to a Superdex 200 HR10/300 GL column in 50 mM Tris-HCl pH 8 and 300 mM NaCl at the indicated concentrations. (A) Wild-type MvaT at 14 µM (pale grey line), 42 µM (dark grey line) and 62 µM (black line). (B) MvaT(F36S) at 6.5 µM (pale grey line), 16 µM (dark grey line) and 33 µM (black line). (C) MvaT(R41P) at 6 µM (pale grey line), 12 µM (dark grey line) and 31 µM (black line).
MvaT oligomerization mutants exert a dominant-negative phenotype
Next, we asked whether MvaT mutants F36S and R41P could exert dominant-negative effects in vivo, reasoning that if MvaT(F36S) and MvaT(R41P) were inactive because they failed to form tetramers then these proteins might be expected to sequester wild-type MvaT monomers into dimers that are incapable of forming higher-order oligomeric species. To test this prediction we ectopically expressed wild-type MvaT-V, MvaT(F36S)-V, and MvaT(R41P)-V in cells of our PAO1 cupA lacZ reporter strain in which expression of cupA is tightly repressed by wild-type MvaT (Vallet-Gely et al., 2005). The results presented in Fig. 9 show that ectopic expression of MvaT(F36S)-V and MvaT(R41P)-V, but not wild-type MvaT-V, resulted in an increase in cupA gene expression in our reporter strain. Mutants MvaT (F36S) and MvaT (R41P) therefore exert a dominant-negative effect in vivo, consistent with our initial interpretation that amino acid substitutions F36S and R41P influence the activity of MvaT by preventing higher-order oligomer formation.
Figure 9.
Dominant-negative effect of MvaT(F36S) and MvaT(R41P). PAO1 cupA1 lacZ cells harboring either the empty control vector pPSV37 (indicated -), pPSV-MvaT-V (indicated MvaT), pPSV-MvaT(F36S)-V (indicated F36S), or pPSV-R41P-V (indicated R41P), were grown at 37°C to mid-logarithmic phase in the presence of 1 mM IPTG and assayed for β-galactosidase activity.
In E. coli the ectopic synthesis of sufficiently high concentrations of H-NS can result in growth arrest (Spurio et al., 1992). Although the ectopic synthesis of high concentrations of wild-type MvaT-V (but not the MvaT mutants) can also inhibit cell growth (Fig. S1), at the level of induction used for the experiment of Fig. 9, excess wild-type MvaT-V did not interfere with cell growth, but did result in a further decrease in cupA gene expression. Taken together, our findings suggest that mutants MvaT(F36S) and MvaT(R41P) are functionally impaired because they fail to form high-order oligomers.
Discussion
We have identified an N-terminal region of MvaT, spanning residues 35–62, which appears to be involved in mediating the formation of higher-order oligomers. Amino acid substitutions within this region interfere with the formation of MvaT tetramers as well as oligomers of higher complexity. ChIP analysis reveals that the corresponding MvaT mutants fail to efficiently bind the DNA in vivo, and the purified mutant proteins exhibit DNA-binding defects in vitro. Furthermore, these MvaT mutants fail to repress expression of the cupA fimbrial genes in P. aeruginosa. Our findings reported here, taken together with earlier studies of H-NS from enteric bacteria, suggest that the ability of H-NS family members to form higher-order oligomers is a conserved and essential feature shared amongst even quite disparate members of this family of nucleoid-associated proteins.
Our findings suggest that the functional role of higher-order oligomer formation by MvaT is to facilitate DNA-binding. In support of this idea, we found that MvaT mutants with substitutions F36S and R41P, which have a particularly pronounced effect on the ability of MvaT to form tetramers, fail to functionally substitute for MvaT in vivo and exhibit DNA-binding defects both in vivo and in vitro. This is reminiscent of the effect amino acid substitution L26P has on the ability of H-NS to form higher-order oligomers and bind the DNA (Badaut et al., 2002). Our findings therefore support a model in which protein-protein interactions between adjacently (or non-adjacently) bound dimers of MvaT, mediated in whole or in part by interactions between residues within the region comprising residues 35–62, facilitate the cooperative binding of MvaT to the DNA.
We identified two amino acid substitutions in MvaT, F36S and R41P, which prevented the formation of higher-order oligomers, but had no effect on the ability of MvaT to functionally substitute for the dimerization domain of λCI. Nevertheless, chemical cross-linking studies with the corresponding mutant proteins suggest these substitutions might influence dimer formation in addition to tetramer and higher-order oligomer formation. However, size-exclusion chromatography analyses suggest that MvaT(F36S) and MvaT(R41P) are not defective for dimer formation, and ectopic synthesis of these oligomerization defective mutants resulted in a dominant-negative effect on cupA gene expression in P. aeruginosa, arguing that the observed in vivo defects of these mutants are a result of their inability to form higher-order oligomers. It is conceivable that the apparent dimerization defects of our MvaT mutants, observed when using cross-linking, simply reflect their inability to form tetramers. Indeed, the formation of covalently linked MvaT tetramers (by the wild-type protein) would be expected to shift the monomer-dimer equilibrium towards formation of the dimer. Furthermore, when MvaT is present as a tetramer, cross-links between two monomers may be more likely to occur than when MvaT is present as a dimer; a monomer in a dimer can only crosslink to the other available monomer, whereas a monomer in a tetramer could, at least in principle, crosslink to any one of three other available monomers.
The N-terminal region of H-NS appears to be involved in mediating both dimer formation and higher-order oligomer formation (Ueguchi et al., 1996; 1997; Badaut et al., 2002; Esposito et al., 2002; Dorman, 2004; Rimsky, 2004; Leonard et al., 2009). Structural studies with different N-terminal portions of H-NS suggest that residues 1–47 constitute the minimal dimerization domain, and that dimer formation might be mediated either by a parallel coiled-coil interface, or an anti-parallel coiled-coil interface, provided by a helix spanning residues 23–50 (Rimsky, 2004; Esposito et al., 2002; Bloch et al., 2003). Earlier work implicated a central region within H-NS, comprising residues 49–90, in mediating the formation of higher-order oligomers (Dorman, 2004; Badaut et al., 2002; Bloch et al., 2003). Recent biophysical studies indicate that the portion of H-NS comprising residues 1–77, which contains the N-terminal region and most of the linker, is sufficient for high-order oligomerization (Leonard et al., 2009). Bacterial two-hybrid analyses support the involvement of both the N-terminal and linker regions of H-NS in higher-order oligomer formation (Stella et al., 2005). There is also some evidence that the C-terminal region of H-NS might influence higher-order oligomer formation (Stella et al., 2005). Like H-NS, MvaT is predicted to consist of N and C-terminal structural modules separated by a flexible linker (Tendeng and Bertin, 2003; Tendeng et al., 2003). Our bacterial two-hybrid analyses indicate that the N-terminal region of MvaT contains two distinct interaction surfaces, one involving residues 2–35, and another contained within a region spanning residues 35–62. We have presented evidence that the interaction surface present on the 35–62 region is involved in mediating higher-order oligomer formation. The region spanning residues 2–35 contains most of the predicted coiled-coil region and, by analogy with H-NS, could mediate formation of the MvaT dimer (Vallet-Gely et al., 2005; Esposito et al., 2002). We did not detect any protein-protein interactions mediated by the C-terminal region of MvaT (data not shown), which is thought to be responsible for binding the DNA (Tendeng and Bertin, 2003). Nevertheless, our findings do not rule out the possibility that the C-terminal region of MvaT might influence oligomerization. Thus, although the N-terminal region of MvaT shares little to no sequence identity with the N-terminal region of H-NS from enteric bacteria, this region of the protein bears functional similarities to the corresponding region of H-NS. Moreover, given that the N-terminal region of MvaT is quite highly conserved amongst different members of the MvaT family (Baehler et al., 2006), we think it likely that the regions corresponding to the two interaction surfaces we have identified in MvaT from P. aeruginosa, will function analogously in other MvaT family members from other Pseudomonads (Baehler et al., 2006; Rescalli et al., 2004; Renzi et al., 2010).
In P. aeruginosa there is a second H-NS family member called MvaU that functions coordinately with MvaT (Vallet et al., 2004; Vallet-Gely et al., 2005; Castang et al., 2008; Li et al., 2009). MvaU interacts directly with MvaT, a situation that may have parallels with the interaction of H-NS with the H-NS-related StpA protein in enteric bacteria (Williams et al., 1996; Johansson et al., 1998; 1999; Lucchini et al., 2009). MvaT-MvaU heterodimers may therefore exist in the cell (Vallet-Gely et al., 2005). MvaU can also interact with itself, suggesting that MvaU homodimers might also be present (Vallet-Gely et al., 2005). The interaction between MvaT and MvaU has been shown to involve residues 1–62 of each protein (Vallet-Gely et al., 2005), but has not been defined further. If the region of MvaT responsible for mediating higher-order oligomer formation (comprising residues 35–62) were to interact with MvaU, the in vivo effects of substitutions F36S and R41P in MvaT could be attributed to their effects on this interaction. It is therefore possible that the in vivo effects of substitutions F36S and R41P in MvaT could be attributable to their influence on protein-protein interactions between different MvaT homodimers, between MvaT homodimers and MvaT-MvaU heterodimers, between MvaT homodimers and MvaU homodimers, or between MvaT-MvaU heterodimers and MvaU homodimers, or a combination of these interactions. In support of these possibilities, we have found that MvaT interacts with residues 35–62 of MvaU (the region of MvaU corresponding to residues 35–62 of MvaT), whereas our MvaT oligomerization mutants do not (data not shown).
Like H-NS from enteric bacteria, and the H-NS-like Lsr2 protein from Mycobacterium tuberculosis (Chen et al., 2008), MvaT has been shown to be able to bridge DNA duplexes (Dame et al., 2005). Certain biophysical studies have been interpreted to suggest that DNA-bridging does not involve protein-protein interactions between different DNA-bound dimers of H-NS, at least under the experimental conditions tested (Dame et al., 2006, Wiggins et al., 2009; see however Liu et al., 2010). Although our experiments do not address whether the protein-protein interactions that mediate higher-order oligomerization of MvaT influence the ability of the protein to bridge the DNA, the mutants we have identified may provide a useful tool for addressing this question. However, it should be noted that although the ability of H-NS family members to bridge DNA duplexes is well documented (Dame et al., 2005; 2006), and although this ability quite likely explains how H-NS exerts many of its functions (Noom et al., 2006; Stoebel et al., 2008; Dorman and Kane 2009; Dillon and Dorman, 2010), the role that DNA-bridging plays in mediating the activities of H-NS family members has yet to be clearly defined (Fang and Rimsky, 2008). Regardless of whether or not there is any connection between the higher-order oligomerization of MvaT and DNA-bridging, our studies suggest that higher-order oligomer formation by MvaT plays an obligatory role in mediating the effects of this protein on gene expression.
Experimental Procedures
Bacterial Strains, Media, and Chemicals
Pseudomonas aeruginosa strains PAO1 cupA lacZ, PAO1 ΔmvaT cupA lacZ, and PAO1 ΔmvaT have been described previously (Vallet-Gely et al., 2005). Escherichia coli DH5αF'IQ (Invitrogen) was used as the recipient strain for all plasmid constructions. E. coli strain FW123 (Whipple, 1998) was used as the reporter strain for the λCI dimerization assays. E. coli strains BN30 (provided by Bryce Nickels, Rutgers University), and KDZif1ΔZ (Vallet-Gely et al., 2005) were used as the reporter strains for the bacterial two-hybrid experiments. In reporter strain BN30 promoter PRM drives expression of lacZ on an F′ episome and the λ operator OR2 is centered 42 bp upstream of the transcription start site of PRM. E. coli strain BL21-CodonPlus(DE3)-RP (Stratagene) was used for protein purification.
When growing E. coli, antibiotics were used when necessary at the following concentrations: gentamicin (15 µg/ml), carbenicillin (100 µg/ml), tetracycline (10 µg/ml), kanamycin (50 µg/ml), and chloramphenicol (25 µg/ml). When growing P. aeruginosa, antibiotics were used when necessary at the following concentrations: gentamicin (25 µg/ml for liquid cultures and 30 µg/ml for solid media). Phase-ON and phase-OFF colonies of the PAO1 ΔmvaT cupA lacZ reporter strain were visualised following growth on LB agar containing X-Gal (75 µg/ml).
Plasmids for λCI Dimerization and Bacterial Two-Hybrid Assays
Plasmid pBRα-MvaT confers resistance to carbenicillin and encodes residues 1–248 of the α-subunit of E. coli RNA polymerase (RNAP) fused to residues 2–124 of P. aeruginosa MvaT via a linker of three alanine residues. The α-mvaT fusion gene is under the control of tandem lpp and lacUV5 promoters. Plasmid pBRα-MvaT was made by cloning an appropriate NotI/BamHI digested PCR product into NotI/BamHI-digested pBRαLN (Hu et al., 2006).
Plasmid pACλCILN confers resistance to chloramphenicol and encodes the N-terminal domain and linker (residues 1–132) of λCI followed by three alanine residues (λCINTD) under the control of the lacUV5 promoter. Plasmid pACλCILN was made by cloning an appropriate NsiI/BamHI-digested PCR product into NsiI/BstYI-digested pACλCI (Dove et al., 1997). Plasmids pACλCILN-MvaT, pACλCILN-MvaT(2–35), pACλCILN-MvaT(2–45), p A CλCILN-MvaT(2–55), pACλCILN-MvaT(2–62), pACλCILN-MvaT(2–84), and pACλCILN-MvaT(35–62), direct the synthesis of MvaT residues 2–124 (i.e. the full-length protein), residues 2–35, 2–45, 2–55, 2–62, 2–84, and 35–62, respectively, fused to the λCINTD. The pACλCILN-fusion plasmids were made by cloning the appropriate NotI/BamHI-digested PCR products into NotI/BstYI-digested pACλCILN. Plasmids pBRα and pACλCI encode full-length α and full-length λCI, respectively, and have been described previously (Dove et al., 1997).
Plasmid pBRωGP directs the synthesis of an ω-Gal11P fusion protein (Dove and Hochschild, 1998) and was made by first replacing a SalI-PvuII fragment of plasmid pBRω-Gal11P (Dove and Hochschild, 1998) with a similar SalI-PvuII fragment made by the PCR that contains restriction sites SalI-BamHI-AscI-StyI and PvuII. The resulting plasmid was then digested with NdeI and NotI and the region specifiying rpoZ (encoding ω) was replaced with a NdeI-NotI fragment containing an rpoZ gene in which the native BamHI site has been changed, resulting in pBRωGP. Plasmid pBRωGP can be used to create fusions to the C-terminus of the ω subunit of E. coli RNAP; proteins are fused to ω via a small linker composed of three alanine residues specified in part by a NotI restriction site. Plasmid pBRωGP confers resistance to carbenicillin, harbors a ColE1 origin of replication, and carries an IPTG-inducible lacUV5 promoter that drives expression of the ω fusion protein. Plasmid pBRω-MvaT directs the synthesis of E. coli ω (residues 1–90) fused to residues 2–124 (the full length protein) of P. aeruginosa MvaT. Plasmid pBRω-MvaT(2–62) directs the synthesis of ω (residues 1–90) fused to residues 2–62 of MvaT. Plasmid pBRω-MvaT (35–62) directs the synthesis of ω (residues 1–90) fused to residues 35–62 of MvaT. Plasmid pBRω-MvaT(35–124) directs the synthesis of ω (residues 1–90) fused to residues 35–124 of MvaT. Plasmid pBRω-MvaT(63–124) directs the synthesis of ω (residues 1–90) fused to residues 63–124 of MvaT. The pBRω-fusion plasmids were made by cloning the appropriate NotI/BamHI-digested PCR products into NotI/BamHI-digested pBRωGP. Each of the respective ω fusion proteins is therefore under the control of an IPTG-inducible lacUV5 promoter.
Plasmid pACTR-V-Zif-AP directs the synthesis of V-Zif, the zinc-finger DNA-binding domain of the murine Zif268 protein (Zif) containing a vesicular stomatitis virus glycoprotein (VSV-G) epitope tag fused to its N-terminus via a two amino acid linker (Gly-Ser) (to facilitate protein detection by Western blotting). Plasmid pACTR-V-Zif-AP was made by cloning an NdeI/BamHI-digested PCR product (containing a NdeI site, the coding sequence for a VSV-G epitope tag, a glycine and a serine residues, the Zif coding sequence, a NotI site, a stop codon and a BamHI site) into pACTR-Zif-AP (provided by Keith Joung, Harvard Medical School) cut with NdeI and BamHI. Plasmid pACTR-V-Zif-AP confers resistance to tetracycline and harbors a p15A origin of replication. This plasmid can be used to create fusions to the C-terminus of V-Zif. Proteins are fused to V-Zif via a four-amino acid linker (Val-Ala-Ala-Ala) specified in part by a NotI restriction site. Plasmid pACTR-V-Zif-MvaT directs the synthesis of full-length MvaT (residues 2–124) fused to the C-terminus of Zif. Plasmid pACTR-V-Zif-MvaT(F36S) directs the synthesis of MvaT(F36S) (residues 2–124) fused to the C-terminus of V-Zif. Plasmid pACTR-V-Zif-MvaT(R41P) directs the synthesis of MvaT(R41P) (residues 2–124) fused to the C-terminus of V-Zif. Plasmid pACTR-V-Zif-MvaT(2–35) directs the synthesis of MvaT (residues 2–35) fused to the C-terminus of V-Zif. Plasmid pACTR-V-Zif-MvaT(35–62) directs the synthesis of MvaT residues 35–62) fused to the C-terminus of V-Zif. The pACTR-V-Zif-fusion plasmids were made by cloning the appropriate NotI/BamHI digested PCR products into NotI/BamHI-digested pACTR-V-Zif-AP. Each of the respective V-Zif fusion proteins is therefore under the control of an IPTG-inducible lacUV5 promoter.
Genetic screen to isolate MvaT mutants specifically defective for higher-order oligomerization
The mvaT portion of the fusion gene encoded by pACλCILN-MvaT was mutagenized randomly by the PCR with Taq DNA polymerase. A pool of plasmids encoding the resulting λCINTD-MvaT mutants was transformed into BN30 cells containing plasmid pBRα-MvaT. Transformants were plated onto LB plates containing X-gal (40 µg/ml) and the β-galactosidase competitive inhibitor tPEG (250 µM). We screened for colonies exhibiting low lacZ expression relative to cells containing a plasmid encoding wild-type λCINTD-MvaT. About < 150 colonies were picked out of the < 10,000 colonies examined. Plasmids encoding the λCINTD-MvaT mutants were extracted and transformed into FW123 cells. Transformants were plated onto LB plates containing X-gal (60 µg/ml) and IPTG (20 µM). We then screened for colonies exhibiting as low lacZ expression as cells containing a plasmid encoding wild-type λCINTD-MvaT. The plasmids from the white colonies were then isolated, re-transformed into BN30 cells containing plasmid pBRα-MvaT and assayed for β-galactosidase activity to confirm the phenotypes observed on plates. Plasmids were then transformed into FW123 cells and assayed for β-galactosidase activity. Genes encoding the mutant MvaT proteins impaired for higher-order oligomerization but not for dimerization were then sequenced.
β-Galactosidase Assays
Cells were permeabilized with sodium dodecyl sulfate and CHCl3 and assayed for β-galactosidase activity as described previously (Dove and Hochschild, 2004). Assays were performed at least two times in triplicate on separate occasions. Representative data sets are shown. Values are averages based on three independent measurements from one experiment.
Complementation Vectors
Plasmids pPSV-MvaT-V, pPSV-MvaT(F36S)-V and pPSV-MvaT(R41P)-V direct the synthesis of MvaT, MvaT(F36S), and MvaT(R41P) respectively with a VSV-G epitope-tag fused to their C-terminus. On each of these vectors the mvaT-V fusion gene is under the control of the IPTG-inducible lacUV5 promoter. Site-directed mutagenesis of mvaT was carried out by the PCR to introduce mutations specifying amino acid substitutions F36S or R41P. mvaT or mvaT(F36S) or mvaT(R41P) coding sequences flanked 5′ by a SacI site and a consensus Shine-Dalgarno sequence followed by a NdeI site and flanked 3′ by a NotI site, and sequence ATATACAGATATTGAAATGAATAGATTAGGAAAA encoding the VSV-G epitope tag and a stop codon followed by a BamHI site were generated by the PCR, digested with SacI and BamHI and cloned into pPSV37 (Lee et al., 2010) cut with SacI and BamHI to generate plasmid pPSV-MvaT-V, pPSV-MvaT(F36S)-V and pPSV-MvaT(R41P)-V. Production of the MvaT-V, MvaT(F36S)-V and MvaT(R41P)-V proteins was confirmed by Western blotting with an anti-VSV-G antibody (Sigma-Aldrich).
Chromatin Immunoprecipitation (ChIP)
Cultures of PAO1 ΔmvaT transformed with either pPSV37, pPSV-MvaT-V, pPSV-MvaT(F36S)-V or pPSV-MvaT(R41P)-V were inoculated in duplicate at a starting OD600 of ~0.05 and grown with aeration to an OD600 of ~0.6 at 37 °C in LB supplemented with gentamicin. ChIP was then performed with 3 ml of culture by using anti-VSV-G agarose beads (BETHYL laboratories) and fold enrichment values were measured by quantitative PCR relative to the PA2896 promoter essentially as described previously (Castang et al., 2008).
Plasmids for protein purification
Plasmid pET24-MvaT-His6 encodes MvaT (residues 1–124) with a C-terminal hexa-histidine tag (MvaT-His6). pET24-MvaT-His6 was made by cloning an NdeI/XhoI-cut PCR product into pET24b (Novagen) digested with NdeI and XhoI. Plasmids pET24-MvaT(F36S)-His6 and pET24-MvaT(R41P)-His6 encode, respectively, MvaT(F36S) and MvaT(R41P) (residues 1–124) with a C-terminal hexa-histidine tag and were made by subcloning the appropriate NdeI/NotI digested fragment from pPSV-MvaT(F36S)-V and pPSV-MvaT(R41P)-V, respectively, into NdeI/NotI digested pET24b.
Protein purification
Plasmids pET24-MvaT-His6 or pET24-MvaT(F36S)-His6 or pET24-MvaT(R41P)-His6 were introduced into E. coli strain BL21 CodonPlus(DE3)-RP (Stratagene). Transformants were grown at 37 °C in LB supplemented with 50 µg/ml of kanamycin to an OD600 of ~0.6. Expression was induced by the addition of IPTG to a final concentration of 0.2 mM. Cells were harvested after a further 2 hours incubation at 37 °C and lysed by addition of 0.6 mg/ml lysozyme for 30 minutes on ice followed by sonication. MvaT-His6, MvaT(F36S)-His6 and MvaT(R41P)-His6 were purified by affinity purification using TALON resin (CLONTECH). His-tagged proteins were eluted in buffer containing 300 mM NaCl, 250 mM imidazole, 10 mM Tris (pH 8). To remove any bound DNA/RNA, proteins were first dialyzed against buffer A containing 100 mM NaCl, 1 mM EDTA, 5% glycerol and 20 mM Tris (pH 8) to decrease the salt concentration and then applied to an HiTrap Heparin HP 1 ml column (GE Healthcare). The column was washed extensively with buffer A and proteins were eluted using a linear NaCl gradient (0.1–1 M, 25 ml), at a flow rate of 1 ml/minute. Proteins were desalted and concentrated further using Amicon Ultra-4 centricon units (Millipore) with a molecular mass cut-off of 10 kDa according to the manufacturer's directions. Proteins were finally mixed with an equal volume of glycerol and stored at −80 °C.
Electrophoretic Mobility Shift Assays (EMSA)
A 347-bp PCR product carrying the mvaU promoter (~20 nM) and a 211-bp PCR product carrying a portion of the tex gene (~23 nM) (used as a control) were mixed together with varying concentrations of MvaT-His6, MvaT(F36S)-His6, or MvaT(R41P)-His6 in a total reaction volume of 20 µl and in a buffer containing 20 mM Tris-HCl (pH 7.5), 100 mM KCl, 1 mM DTT, 100 µg BSA and 10% glycerol. Reactions were incubated at 25°C for 10 minutes. The DNA-protein mix was then loaded onto a non-denaturing 5% polyacrylamide gel and run in 0.5x TBE buffer. The gel was stained with Gelstar nucleic acid gel stain (Fisher) in TBE, and DNA was visualized under UV illumination.
In vitro Protein Cross-linking
Proteins MvaT-His6, MvaT(F36S)-His6, and MvaT(R41P)-His6 were treated with the protein cross-linking agent glutaraldehyde in a buffer consisting of 40 mM Hepes pH 7.9 and 150 mM NaCl. The reaction mixture contained 1.85 µM of the purified protein and 0.04% glutaraldehyde (Sigma-Aldrich) in a total volume of 10 µl. Reactions were performed at 25°C and were quenched by the addition of 10 µl of 2X NuPAGE® LDS sample buffer (Invitrogen) supplemented with 25 mM DTT and incubated at 70°C for 10 minutes. Samples were separated on 4%-12% Bis-Tris NuPAGE®gels in MOPS running buffer (Invitrogen) and analyzed by Western blotting using an anti-His antibody (Novagen).
Size-exclusion Chromatography
Size-exclusion chromatography was performed on an AKTA Purifier UPC 10 using a Superdex 200 HR10/300 GL column (G.E. Healthcare) operated at 4°C. The column was equilibrated with 50 mM Tris-HCl pH 8 and 300 mM NaCl. After 10 min incubation at 25°C 100 µl of sample at the indicated concentration was loaded onto the column at a flow rate of 0.5 ml/minute and the elution monitored at 280 nm. The column was calibrated using a mix of molecular weight standards (100 µl) containing thyroglobulin (670 kDa), γ-globulin (158 kDa), ovalbumin (44 kDa), Myoglobin (17 kDa), and Vitamin B12 (1.35 kDa) (Biorad Gel Filtration Standard). The apparent molecular weights of MvaT, MvaT(F36S) and MvaT(R41P) were determined by comparing the relevant peak maxima to a standard curve obtained with the globular protein standards.
Supplementary Material
Acknowledgements
We thank Arne Rietsch, Talia Ramsdell and Keith Joung for plasmids, Bryce Nickels and Ann Hochschild for strains, Renate Hellmiss for artwork, and Ann Hochschild for comments on the manuscript. This work was supported by National Institutes of Health grant AI069007 (to S.L.D.).
References
- Badaut C, Williams R, Arluison V, Bouffartigues E, Robert B, Buc H, Rimsky S. The degree of oligomerization of the H-NS nucleoid structuring protein is related to specific binding to DNA. J. Biol. Chem. 2002;277:41657–41666. doi: 10.1074/jbc.M206037200. [DOI] [PubMed] [Google Scholar]
- Baehler E, de Werra P, Wick LY, Pechy-Tarr M, Mathys S, Maurhofer M, Keel C. Two novel MvaT-like global regulators control exoproduct formation and biocontrol activity in root-associated Pseudomonas fluorescens CHA0. Mol. Plant Microbe Interact. 2006;19:313–329. doi: 10.1094/MPMI-19-0313. [DOI] [PubMed] [Google Scholar]
- Bloch V, Yang Y, Margeat E, Chavanieu A, Auge MT, Robert B, et al. The H-NS dimerization domain defines a new fold contributing to DNA recognition. Nat. Struct. Biol. 2003;10:212–218. doi: 10.1038/nsb904. [DOI] [PubMed] [Google Scholar]
- Bouffartigues E, Buckle M, Badaut C, Travers A, Rimsky S. H-NS cooperative binding to high-affinity sites in a regulatory element results in transcriptional silencing. Nat. Struct. Mol. Biol. 2007;14:441–448. doi: 10.1038/nsmb1233. [DOI] [PubMed] [Google Scholar]
- Castang S, McManus HR, Turner KH, Dove SL. H-NS family members function coordinately in an opportunistic pathogen. Proc. Natl. Acad. Sci. USA. 2008;105:18947–18952. doi: 10.1073/pnas.0808215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JM, Ren H, Shaw JE, Jing Wang Y, Li M, Leung AS, et al. Lsr2 of Mycobacterium tuberculosis is a DNA-bridging protein. Nucleic Acids Res. 2008;36:2123–2135. doi: 10.1093/nar/gkm1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame RT, Luijsterburg MS, Krin E, Bertin PN, Wagner R, Wuite GJ. DNA bridging: a property shared among H-NS-like proteins. J. Bacteriol. 2005;187:1845–1848. doi: 10.1128/JB.187.5.1845-1848.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame RT, Noom MC, Wuite GJL. Bacterial chromatin organization by H-NS protein unravelled using dual DNA manipulation. Nature. 2006;444:387–390. doi: 10.1038/nature05283. [DOI] [PubMed] [Google Scholar]
- Diggle SP, Winzer K, Lazdunski A, Williams P, Camara P. Advancing the quorum in Pseudomonas aeruginosa: MvaT and the regulation of N-acylhomoserine lactone production and virulence gene expression. J. Bacteriol. 2002;184:2576–2586. doi: 10.1128/JB.184.10.2576-2586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SC, Dorman CJ. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat. Rev. Microbiol. 2010;8:185–195. doi: 10.1038/nrmicro2261. [DOI] [PubMed] [Google Scholar]
- Dorman CJ. H-NS: a universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2004;2:391–400. doi: 10.1038/nrmicro883. [DOI] [PubMed] [Google Scholar]
- Dorman CJ. H-NS, the genome sentinel. Nat. Rev. Microbiol. 2007;5:157–161. doi: 10.1038/nrmicro1598. [DOI] [PubMed] [Google Scholar]
- Dorman CJ, Kane KA. DNA bridging and antibridging: a role for bacterial nucleoid-associated proteins in regulating the expression of laterally acquired genes. FEMS Microbiol. Rev. 2009;33:587–592. doi: 10.1111/j.1574-6976.2008.00155.x. [DOI] [PubMed] [Google Scholar]
- Dove SL, Joung JK, Hochschild A. Activation of prokaryotic transcription through arbitrary protein-protein contacts. Nature. 1997;386:627–630. doi: 10.1038/386627a0. [DOI] [PubMed] [Google Scholar]
- Dove SL, Hochschild A. Conversion of the omega subunit of Escherichia coli RNA polymerase into a transcriptional activator or an activation target. Genes Dev. 1998;12:745–754. doi: 10.1101/gad.12.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove SL, Hochschild A. A bacterial two-hybrid system based on transcription activation. Methods Mol. Biol. 2004;261:231–246. doi: 10.1385/1-59259-762-9:231. [DOI] [PubMed] [Google Scholar]
- Esposito D, Petrovic A, Harris R, Ono S, Eccleston JF, Mbabaali A, et al. H-NS oligomerization domain structure reveals the mechanism for high order self-association of the intact protein. J. Mol. Biol. 2002;324:841–850. doi: 10.1016/s0022-2836(02)01141-5. [DOI] [PubMed] [Google Scholar]
- Falconi M, Gualtieri MT, La Teana A, Losso MA, Pon CL. Proteins from the prokaryotic nucleoid: primary and quaternary structure of the 15-kD Escherichia coli DNA binding protein H-NS. Mol. Microbiol. 1998;2:323–329. doi: 10.1111/j.1365-2958.1988.tb00035.x. [DOI] [PubMed] [Google Scholar]
- Fang FC, Rimsky S. New insights into transcriptional regulation by H-NS. Curr. Opin. Microbiol. 2008;11:113–120. doi: 10.1016/j.mib.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger DC, Hurd D, Goldberg MD, Busby SJW. Association of nucleoid proteins with coding and non-coding segments of the Escherichia coli genome. Nucleic Acids Res. 2006;34:4642–4652. doi: 10.1093/nar/gkl542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JC, Kornacker MG, Hochschild A. Escherichia coli one- and two-hybrid systems for the analysis and identification of protein-protein interactions. Methods. 2000;20:80–94. doi: 10.1006/meth.1999.0908. [DOI] [PubMed] [Google Scholar]
- Johansson J, Dagberg B, Richet E, Uhlin BE. H-NS and StpA proteins stimulate expression of the maltose regulon in Escherichia coli. J. Bacteriol. 1998;180:6117–6125. doi: 10.1128/jb.180.23.6117-6125.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson J, Uhlin BE. Differential protease-mediated turnover of H-NS and StpA revealed by a mutation altering protein stability and stationary-phase survival of Escherichia coli. Proc. Natl. Acad. Sci. USA. 1999;96:10776–10781. doi: 10.1073/pnas.96.19.10776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang B, Blot N, Bouffartigues E, Buckle M, Geertz M, Gualerzi CO, et al. High-affinity DNA binding sites for H-NS provide a molecular basis for selective silencing within proteobacterial genomes. Nucleic Acids Res. 2007;35:6330–6337. doi: 10.1093/nar/gkm712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P-C, Stopford CM, Svenson AG, Rietsch A. Control of effector export by the Pseudomonas aeruginosa type III secretion proteins PcrG and PcrV. Mol. Microbiol. 2010;75:924–941. doi: 10.1111/j.1365-2958.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard PG, Ono S, Gor J, Perkins SJ, Ladbury JE. Investigation of the self-association and hetero-association interactions of H-NS and StpA from Enterobacteria. Mol. Microbiol. 2009;73:165–179. doi: 10.1111/j.1365-2958.2009.06754.x. [DOI] [PubMed] [Google Scholar]
- Li C, Wally H, Miller SJ, Lu C-D. The multifaceted proteins MvaT and MvaU, members of the H-NS family, control arginine metabolism, pyocyanin synthesis, and prophage activation in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2009;191:6211–6218. doi: 10.1128/JB.00888-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen H, Kenney LJ, Yan J. A divalent switch drives H-NS/DNA-binding conformations between stiffening and bridging modes. Genes Dev. 2010;24:339–344. doi: 10.1101/gad.1883510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini S, McDermott P, Thompson A, Hinton JCD. The H-NS-like protein StpA represses the RpoS (σ38) regulon during exponential growth of Salmonella typhimurium. Mol. Microbiol. 2009;74:1169–1186. doi: 10.1111/j.1365-2958.2009.06929.x. [DOI] [PubMed] [Google Scholar]
- Lucchini S, Rowley G, Goldberg MD, Hurd D, Harrison M, Hinton JCD. H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog. 2006;2:e81. doi: 10.1371/journal.ppat.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner A, Wild V, Simm R, Rohde M, Erck C, Bredenbruch, et al. Pseudomonas aeruginosa cupA-encoded fimbriae expression is regulated by a GGDEF and EAL domain-dependent modulation of the intracellular level of cyclic diguanylate. Environ. Microbiol. 2007;9:2475–2485. doi: 10.1111/j.1462-2920.2007.01366.x. [DOI] [PubMed] [Google Scholar]
- Navarre WW, Porwollik S, Wang Y, McClelland M, Rosen H, Libby SJ, Fang FC. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science. 2006;313:236–238. doi: 10.1126/science.1128794. [DOI] [PubMed] [Google Scholar]
- Navarre WW, McClelland M, Libby SJ, Fang FC. Silencing of xenogeneic DNA by H-NS—facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes Dev. 2007;21:1456–1471. doi: 10.1101/gad.1543107. [DOI] [PubMed] [Google Scholar]
- Noom MC, Navarre WW, Oshima T, Wuite GJ, Dame RT. H-NS promotes looped domain formation in the bacterial chromosome. Curr. Biol. 2007;17:R913–R914. doi: 10.1016/j.cub.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Oshima T, Ishikawa S, Kurokawa K, Aiba H, Ogasawara N. Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA polymerase. DNA Res. 2006;13:141–153. doi: 10.1093/dnares/dsl009. [DOI] [PubMed] [Google Scholar]
- Renzi F, Rescalli E, Galli E, Bertoni G. Identification of genes regulated by the MvaT-like paralogues TurA and TurB of Pseudomonas putida KT2440. Environ. Microbiol. 2010;12:254–263. doi: 10.1111/j.1462-2920.2009.02064.x. [DOI] [PubMed] [Google Scholar]
- Rescalli E, Saini S, Bartocci C, Rychlewski L, de Lorenzo V, Bertoni G. Novel physiological modulation of the Pu promoter of TOL plasmid: negative regulatory role of the TurA protein of Pseudomonas putida in the response to suboptimal growth temperatures. J. Biol. Chem. 2004;279:7777–7784. doi: 10.1074/jbc.M310580200. [DOI] [PubMed] [Google Scholar]
- Rimsky S. Structure of the histone-like protein H-NS and its role in regulation and genome superstructure. Curr. Opin. Microbiol. 2004;7:109–114. doi: 10.1016/j.mib.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Sette M, Spurio R, Trotta E, Brandizi C, Brandi A, Pon CL, et al. Sequence-specific recognition of DNA by the C-terminal domain of nucleoid-associated protein H-NS. J. Biol. Chem. 2009;284:30453–30462. doi: 10.1074/jbc.M109.044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth CP, Lundbäck T, Renzoni D, Siligardi G, Beavil R, Layton M, et al. Oligomerization of the chromatin-structuring protein H-NS. Mol. Microbiol. 2000;36:962–972. doi: 10.1046/j.1365-2958.2000.01917.x. [DOI] [PubMed] [Google Scholar]
- Spurio R, Durrenberger M, Falconi M, La Teana A, Pon CL, Gualerzi CO. Lethal overproduction of the Escherichia coli nucleoid protein H-NS: ultramicroscopic and molecular autopsy. Mol. Gen. Genet. 1992;231:201–211. doi: 10.1007/BF00279792. [DOI] [PubMed] [Google Scholar]
- Spurio R, Falconi M, Brandi A, Pon CL, Gualerzi CO. The oligomeric structure of nucleoid protein H-NS is necessary for recognition of intrinsically curved DNA and for DNA bending. EMBO J. 1997;16:1795–1805. doi: 10.1093/emboj/16.7.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella S, Spurio R, Falconi M, Pon CL, Gualerzi CO. Nature and mechanism of the in vivo oligomerization of nucleoid protein H-NS. EMBO J. 2005;24:2896–2905. doi: 10.1038/sj.emboj.7600754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoebel DM, Free A, Dorman CJ. Anti-silencing: overcoming H-NS-mediated repression of transcription in Gram-negative enteric bacteria. Microbiology. 2008;154:2533–2545. doi: 10.1099/mic.0.2008/020693-0. [DOI] [PubMed] [Google Scholar]
- Tendeng C, Bertin PN. H-NS in Gram-negative bacteria: a family of multifaceted proteins. Trends Micriobiol. 2003;11:511–518. doi: 10.1016/j.tim.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Tendeng C, Soutourina OA, Danchin A, Bertin PN. MvaT proteins in Pseudomonas spp.: a novel class of H-NS-like proteins. Microbiology. 2003;149:3047–3050. doi: 10.1099/mic.0.C0125-0. [DOI] [PubMed] [Google Scholar]
- Ueguchi C, Seto C, Suzuki T, Mizuno T. Clarification of the dimerization domain and its functional significance for the Escherichia coli nucleoid protein H-NS. J. Mol. Biol. 1997;274:145–151. doi: 10.1006/jmbi.1997.1381. [DOI] [PubMed] [Google Scholar]
- Ueguchi C, Suzuki T, Yoshida T, Tanaka K, Mizuno T. Systematic mutational analysis revealing the functional domain organization of Escherichia coli nucleoid protein H-NS. J. Mol. Biol. 1996;263:149–162. doi: 10.1006/jmbi.1996.0566. [DOI] [PubMed] [Google Scholar]
- Vallet I, Diggle SP, Stacey RE, Camara M, Ventre I, Lory S, et al. Biofilm formation in Pseudomonas aeruginosa: fimbrial cup gene clusters are controlled by the transcriptional regulator MvaT. J. Bacteriol. 2004;186:2880–2890. doi: 10.1128/JB.186.9.2880-2890.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet-Gely I, Donovan KE, Fang R, Joung JK, Dove SL. Repression of phase-variable cup gene expression by H-NS-like proteins in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2005;102:11082–11087. doi: 10.1073/pnas.0502663102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet I, Olson JW, Lory S, Lazdunski A, Filloux A. The chaperone/usher pathways of Pseudomonas aeruginosa: identification of fimbrial gene clusters (cup) and their involvement in biofilm formation. Proc. Natl. Acad. Sci. USA. 2001;98:6911–6916. doi: 10.1073/pnas.111551898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipple FW. Genetic analysis of prokaryotic and eukaryotic DNA-binding proteins in Escherichia coli. Nucleic Acids Res. 1998;26:3700–3706. doi: 10.1093/nar/26.16.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RM, Rimsky S, Buc H. Probing the structure, function, and interactions of the Escherichia coli H-NS and StpA proteins by using dominant negative derivatives. J. Bacteriol. 1996;178:4335–4343. doi: 10.1128/jb.178.15.4335-4343.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins PA, Dame RT, Noom MC, Wuite GJL. Protein-mediated molecular bridging: a key mechanism in biopolymer organization. Biophys. J. 2009;97:1997–2003. doi: 10.1016/j.bpj.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.