Abstract
We evaluated longitudinal rates of Kaposi sarcoma (KS) and trends in CD4 counts at the time of KS diagnosis during the HIV epidemic (1985–2008). Although rates of KS have decreased, cases are now occurring at higher CD4 counts over time, with more than a third of cases diagnosed in 2002–2008 occurring at CD4 counts ≥350 cells/mm3. These data support future studies evaluating the impact of HAART initiation at higher CD4 counts to further reduce KS.
During the HIV epidemic, the types and presentations of cancers have dramatically changed [1–4]. As an AIDS-defining cancer, most Kaposi’s sarcoma (KS) cases have traditionally occurred at low CD4 counts (<200 cells/mm3) [5, 6]. Although KS rates have decreased [7], it is unknown whether KS will now be observed at higher CD4 counts.
We evaluated KS rates and trends in CD4 counts at KS diagnosis among HIV-infected persons using the U.S. Military HIV Natural History Study (NHS) [3, 8]. The diagnosis of KS was based on medical record review using standardized criteria [3]. Rates and rate ratios (overall and for time spent with CD4 cell count < 350 and ≥ 350 cells/mm3) with 95% confidence intervals (CI) were calculated with Poisson regression models for four a priori defined calendar periods (1985–1990, 1991–1995, 1996–2001, and 2002–2008). Participants contributed follow-up time to all possible calendar periods from baseline (six months prior to HIV diagnosis) to the event or censoring time (last study visit). Among those with KS and a proximal CD4 cell count (within one year prior to KS diagnosis), participants were compared by proximal CD4 count category (<350 versus ≥350 cells/mm3) with descriptive statistics (chi-squared and Wilcoxon tests) as appropriate. Medians are presented with interquartile ranges (IQR). We also evaluated factors associated with KS during the HAART era (the latest of 01 January 1996 or HIV diagnosis date) with time-updated proportional hazards models.
There were 5,067 participants with 39,522 PY of follow-up between 1985 and 2008. At HIV diagnosis, the median age was 28 (IQR 24–34) years; 92% were male; 45% were African American and 43% Caucasian. Median CD4 count was 504 (IQR 350–672) cells/mm3) and median HIV RNA level (available for 38% of the cohort) was 4.4 (IQR 3.7–4.9) log10 copies/ml.
Of the 247 KS events during the study period, there were 52, 138, 38, and 19 during the four calendar periods, respectively. The rates of KS decreased over time (Table 1). Compared to 1985–1990, HIV-infected persons in 2002–2008 had a 72% lower rate of KS (RR=0.28; 95% CI 0.16–0.47; p<0.001). Within each calendar period the rates were higher for time spent with CD4 <350 versus to ≥350 cells/mm3, although the rate ratios for those comparisons fell from 9.1 (95% CI 3.7–22.0) in 1985–1990 to 6.2 (95% CI 2.3–16.6) in 2002–2008.
Table 1.
Rates1 (Overall and by Time Spent in CD4 Cell Count Categories) of Kaposi’s Sarcoma by Calendar Period.
| Overall | By Time Spent in CD4 Cell Count Categories | ||||
|---|---|---|---|---|---|
| CD4 <350 | CD4 ≥350 | ||||
| Calendar Period |
Rate (95% CI) |
Rate (95% CI) |
Rate (95% CI) |
Rate Ratio 2 (95% CI) |
P-value |
| 1985–1990 | 6.5 (5.0 – 8.5) |
18.6 (11.6 – 25.6) |
2.0 (0.4 – 3.7) |
9.1 (3.7 – 22.0) |
<0.001 |
| 1991–1995 | 12.6 (10.7 – 14.9) |
21.5 (17.1 – 25.9) |
1.4 (0.4 – 2.4) |
15.4 (7.1 – 33.1) |
<0.001 |
| 1996–2001 | 3.8 (2.8 – 5.3) |
5.3 (2.7 – 7.7) |
0.7 (0.0 – 1.3) |
7.9 (2.7 – 23.5) |
<0.001 |
| 2002–2008 | 1.8 (1.1 – 2.8) |
4.6 (1.9 – 7.4) |
0.8 (0.2 – 1.3) |
6.2 (2.3 – 16.6) |
<0.001 |
Rates are per 1000 person years of follow-up and are given with 95% confidence intervals
Comparing rates for time spent with CD4 cell count <350 versus ≥350 cells/mm3
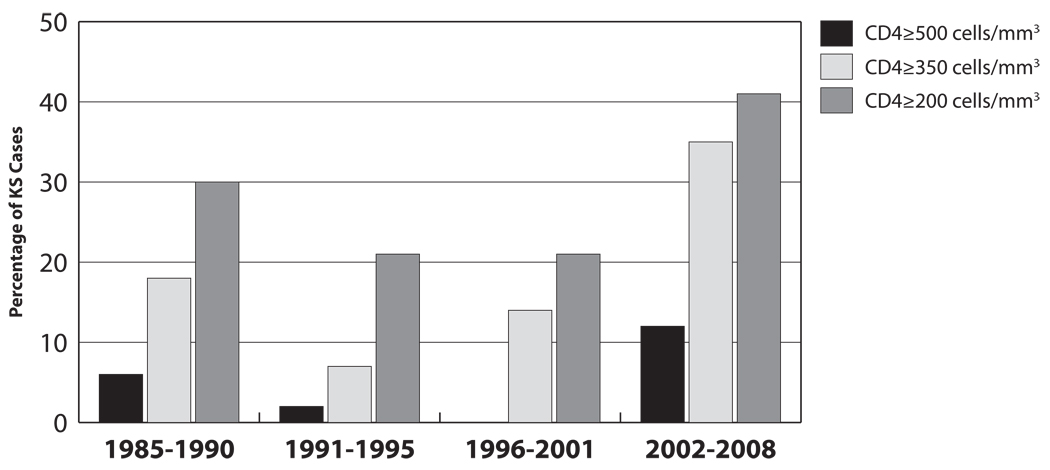
Among the 247 KS cases, 179 (72%) had a proximal CD4 count available. For the four calendar periods, the proximal CD4 count at KS diagnosis was ≥350 cells/mm3 for 18%, 7%, 14%, and 35%, respectively (p=0.01; Figure 1). Participants with proximal CD4 count <350 compared to ≥350 cells/mm3 at KS diagnosis were more likely to have a prior non-KS AIDS event (47% vs. 9%; p<0.001), diagnosed with HIV in the pre-HAART era (97% vs. 83%; p=0.001), and spent a smaller percentage of time on antiretroviral therapy (median of 49% versus 62%; p=0.09); the two groups did not differ by demographics or HIV duration at time of KS.
Figure 1.
CD4 Cell Count at Diagnosis of Kaposi’s Sarcoma during the Course of the HIV Epidemic (1985–2008)
Among the 3,422 participants with 20,263 PY of follow-up since availability of HAART in 1996, 45 had KS and a proximal CD4 count. From proportional hazards model considering only time-updated CD4 count, each incremental increase of 50 cells/mm3 decreased the risk of KS by 30% (HR 0.70, 95% CI 0.64–0.76, p<0.001). In a model with both CD4 category and HAART use as time-updated covariates: compared to those with CD4 count ≥350 cells/mm3 and on HAART, those with CD4 count ≥350 cells/mm3 but not on HAART had an increased risk of KS (HR 2.0; 95% CI 0.7–6.30; p=0.22) that did not reach statistical significance, while those with CD4 count <350 cells/mm3 (regardless of HAART use) had an increased risk (HR 8.3; 95% CI 3.4–20.2; p<0.001).
Our study demonstrates that although the KS rates have declined during the HAART era and lower CD4 counts remain an important risk factor, a greater proportion of KS cases are now occurring at higher CD4 counts. During the late HAART period, over a third of KS cases occurred at CD4 counts ≥350 cells/mm3. Clinicians should be aware of these trends and watchful for the occurrence of KS despite robust CD4 counts.
The occurrence of KS at higher than expected CD4 counts has been previously reported [9–13]. However, our study is unique in that we describe the changing trends of CD4 counts at KS diagnosis over the entire HIV epidemic and demonstrate a rising proportion of cases at higher CD4 counts. To our knowledge, only one other study examined CD4 trends at KS diagnosis over time, but found no change in CD4 counts between the pre- and post-HAART eras; however, their population had high rates of drug use and poor antiretroviral adherence [12], whereas our population had free medical care, excellent reported medication adherence, and low rates of drug use (<1%) [14].
Similar to other studies in the HAART era [9, 10, 15], 35% of participants were on HAART and 9% had an HIV RNA level <400 copies/ml at KS diagnosis. Such cases are somewhat surprising since HAART has reduced the number of KS cases by its effects on HIV suppression and potential anti-angiogenic effects [10, 16]. Some KS cases in the setting of HAART may be related to the immune reconstitution inflammatory syndrome (IRIS) [17, 18]; however, most of our cases were not associated with HAART introduction.
Given these trends, determining if HAART use at higher CD4 counts will reduce the impact of KS is of clinical importance. We found a suggestion of increased risk of KS among those not on HAART compared to those on HAART with CD4 counts ≥350 cells/mm3. Prior studies have shown that KS in the setting of HAART results in less aggressive and more localized disease [19].
In summary, KS remains an important disease among HIV-infected persons, despite achievement of higher CD4 counts. Among patients with access to HAART, the proportion of KS cases occurring at high CD4 counts appears to be rising. Future studies are needed to determine whether earlier HAART initiation will further decrease the burden of KS among HIV-infected persons.
Acknowledgments
Support for this work (IDCRP-000-04) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed through the Uniformed Services University of the Health Sciences. This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072.
The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, the DoD or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
Footnotes
This work is original and has not been published elsewhere. Part of these data will be presented at the 48th Annual Meeting of the Infectious Disease Society of America, 21–24 October 2010, Vancouver, Canada.
References
- 1.Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Adult and Adolescent Spectrum of Disease Project and HIV Outpatient Study Investigators. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 2.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. for the HIV/AIDS Cancer Match Study. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 3.Crum-Cianflone N, Hullsiek KH, Marconi V, Weintrob A, Ganesan A, Barthel RV, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23:41–50. doi: 10.1097/QAD.0b013e328317cc2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farizo KM, Buehler JW, Chamberland ME, Whyte BM, Froelicher ES, Hopkins SG, et al. Spectrum of disease in persons with human immunodeficiency virus infection in the United States. JAMA. 1992;267:1798–1805. [PubMed] [Google Scholar]
- 6.Centers for Disease Control (CDC) 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep. 1992;41:961–962. [PubMed] [Google Scholar]
- 7.Mocroft A, Kirk O, Clumeck N, Gargalianos-Kakolyris P, Trocha H, Chentsova N, et al. The changing pattern of Kaposi sarcoma in patients with HIV, 1994–2003: the EuroSIDA Study. Cancer. 2004;100:2644–2654. doi: 10.1002/cncr.20309. [DOI] [PubMed] [Google Scholar]
- 8.Weintrob AC, Fieberg AM, Agan BK, Ganesan A, Crum-Cianflone NF, Marconi VC, et al. Increasing age at HIV seroconversion from 18 to 40 years is associated with favorable virologic and immunologic responses to HAART. J Acquir Immune Defic Syndr. 2008;49:40–47. doi: 10.1097/QAI.0b013e31817bec05. [DOI] [PubMed] [Google Scholar]
- 9.Maurer T, Ponte M, Leslie K. HIV-associated Kaposi's sarcoma with a high CD4 count and a low viral load. N Engl J Med. 2007;357:1352–1353. doi: 10.1056/NEJMc070508. [DOI] [PubMed] [Google Scholar]
- 10.Mani D, Neil N, Israel R, Aboulafia DM. A retrospective analysis of AIDS-associated Kaposi's sarcoma in patients with undetectable HIV viral loads and CD4 counts greater than 300 cells/mm(3) J Int Assoc Physicians AIDS Care (Chic Ill) 2009;8:279–285. doi: 10.1177/1545109709341852. [DOI] [PubMed] [Google Scholar]
- 11.Krown SE, Lee JY, Dittmer DP. AIDS Malignancy Consortium. More on HIV-associated Kaposi's sarcoma. N Engl J Med. 2008;358:535–536. doi: 10.1056/NEJMc072994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallafent JH, Buskin SE, De Turk PB, Aboulafia DM. Profile of patients with Kaposi's sarcoma in the era of highly active antiretroviral therapy. J Clin Oncol. 2005;23:1253–1260. doi: 10.1200/JCO.2005.04.156. [DOI] [PubMed] [Google Scholar]
- 13.Stebbing J, Sanitt A, Teague A, Powles T, Nelson M, Gazzard B, et al. Prognostic significance of immune subset measurement in individuals with AIDS-associated Kaposi's sarcoma. J Clin Oncol. 2007;25:2230–2235. doi: 10.1200/JCO.2007.10.7219. [DOI] [PubMed] [Google Scholar]
- 14.Brodine SK, Shaffer RA, Starkey MJ, Tasker SA, Gilcrest JL, Louder MK, et al. Drug resistance patterns, genetic subtypes, clinical features, and risk factors in military personnel with HIV-1 seroconversion. Ann Intern Med. 1999;131:502–506. doi: 10.7326/0003-4819-131-7-199910050-00004. [DOI] [PubMed] [Google Scholar]
- 15.Chan J, Kravcik S, Angel JB. Development of Kaposi's sarcoma despite sustained suppression of HIV plasma viremia. J Acquir Immune Defic Syndr. 1999;22:209–210. doi: 10.1097/00126334-199910010-00017. [DOI] [PubMed] [Google Scholar]
- 16.Lebbe C, Blum L, Pellet C, Blanchard G, Verola O, Morel P, et al. Clinical and biological impact of antiretroviral therapy with protease inhibitors on HIV-related Kaposi's sarcoma. AIDS. 1998;12:F45–F49. doi: 10.1097/00002030-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Connick E, Kane MA, White IE, Ryder J, Campbell TB. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma during potent antiretroviral therapy. Clin Infect Dis. 2004;39:1852–1855. doi: 10.1086/426078. [DOI] [PubMed] [Google Scholar]
- 18.Nathan RV. Suspected immune reconstitution inflammatory syndrome associated with the proliferation of Kaposi's sarcoma during HAART. AIDS. 2007;21:775. doi: 10.1097/QAD.0b013e3280ad3da6. [DOI] [PubMed] [Google Scholar]
- 19.Nasti G, Martellotta F, Berretta M, Mena M, Fasan M, Di Perri G, et al. Impact of highly active antiretroviral therapy on the presenting features and outcome of patients with acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer. 2003;98:2440–2446. doi: 10.1002/cncr.11816. [DOI] [PubMed] [Google Scholar]