Abstract
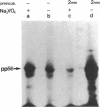
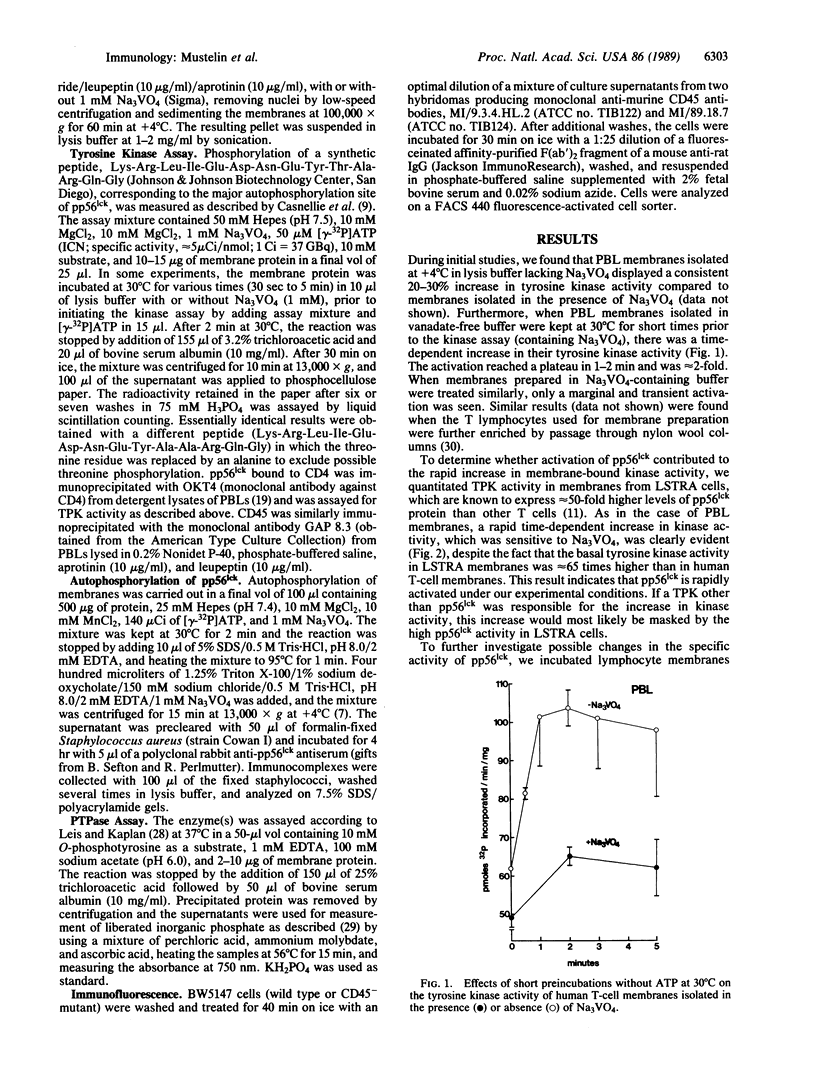
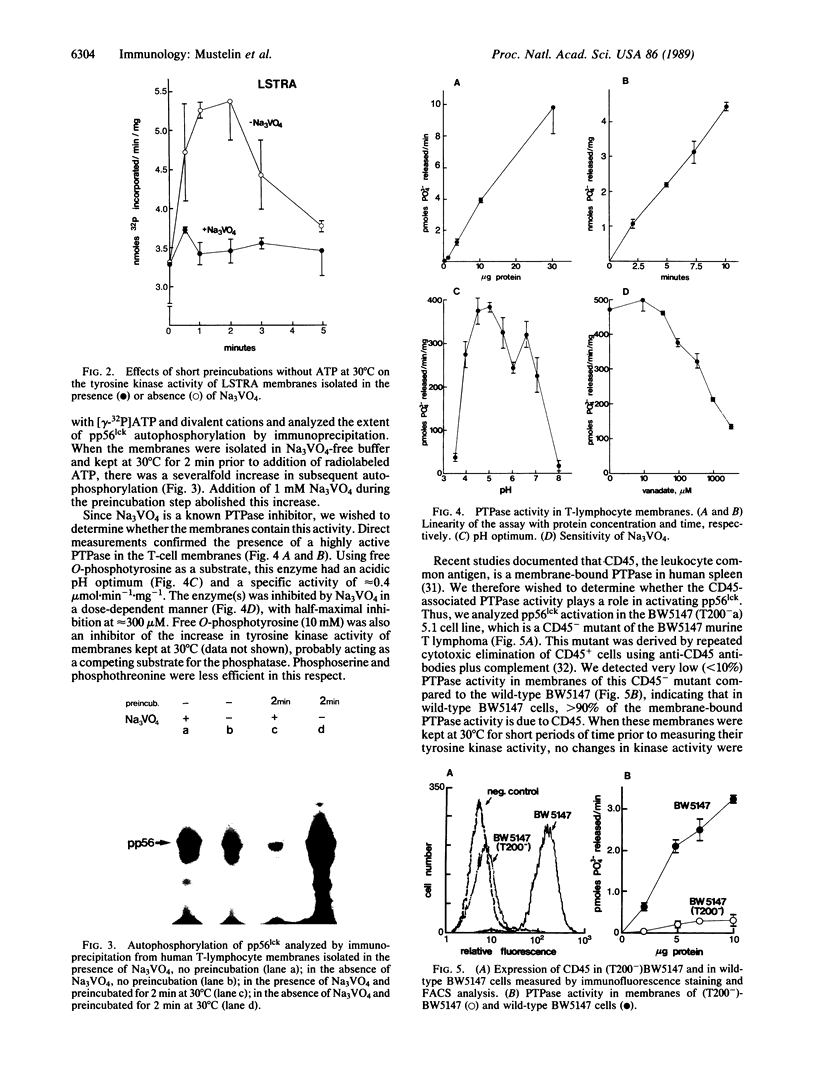
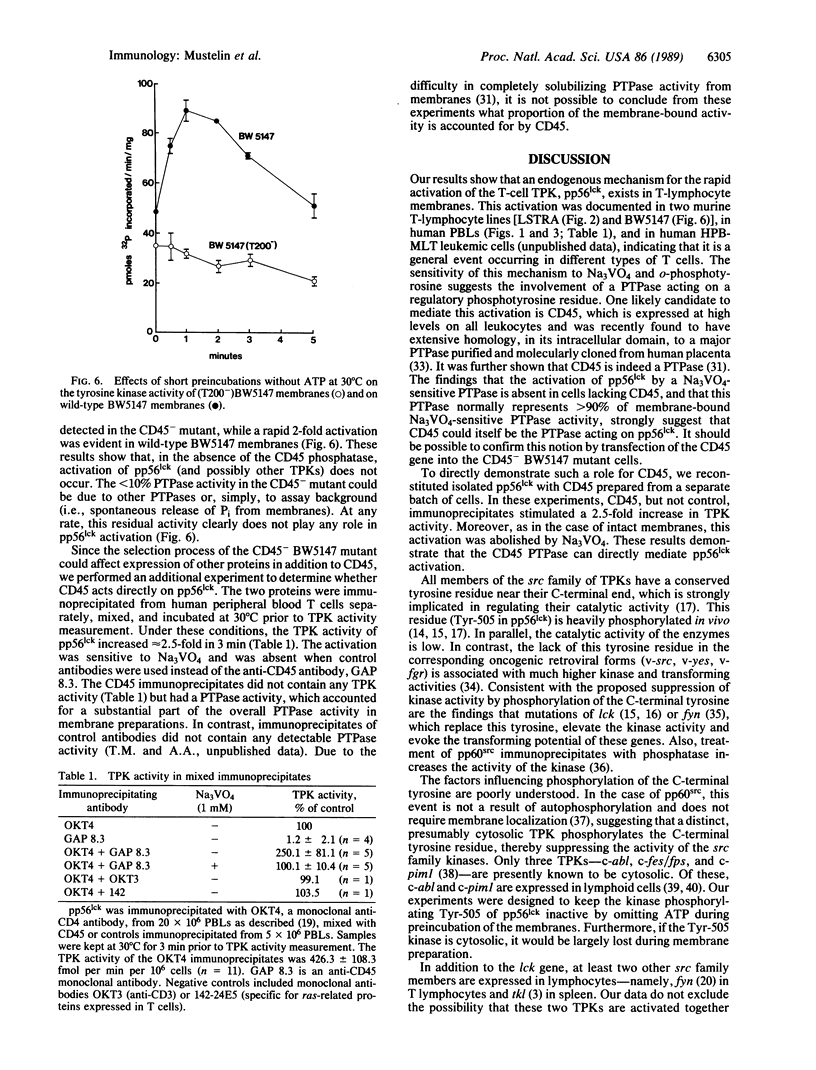
T lymphocytes express a tyrosine protein kinase (TPK; protein-tyrosine kinase; ATP:protein-tyrosine O-phosphotransferase, EC 2.7.1.112), pp56lck that is encoded by the lck protooncogene. This TPK was recently found to be associated with the intracellular domain of the T-cell surface glycoproteins, CD4 and CD8, suggesting that it plays an important role in T-cell development and activation. We have studied the regulation of pp56lck and found that this kinase can be rapidly activated by an endogenous mechanism present in T-lymphocyte membranes. This activation was sensitive to sodium orthovanadate and O-phosphotyrosine, consistent with the involvement of a phosphotyrosine phosphatase (PTPase; protein-tyrosine-phosphatase; protein-tyrosine-phosphate phosphohydrolase, EC 3.1.3.48) in pp56lck activation. Based on a recent report demonstrating that CD45, the leukocyte common antigen, is a membrane-bound PTPase, we analyzed its role in pp56lck activation. CD45 was found to be the major (greater than 90%) PTPase in membranes of the murine T-lymphoma line BW5147. Moreover, activation of pp56lck was undetectable in a mutant BW5147 line lacking CD45 expression (and the associated PTPase activity). In contrast, activation of pp56lck was readily detected in the wild-type lymphoma line. More important, when immunoprecipitated CD45 was added to pp56lck, the TPK activity of the latter increased greater than 2-fold within minutes. This effect of CD45 was completely blocked by sodium orthovanadate. These findings indicate an important role for the CD45 PTPase in pp56lck activation. This role could be mediated by direct dephosphorylation of a regulatory tyrosine residue in pp56lck.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrein K. E., Sefton B. M. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4247–4251. doi: 10.1073/pnas.85.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Neriah Y., Bauskin A. R. Leukocytes express a novel gene encoding a putative transmembrane protein-kinase devoid of an extracellular domain. Nature. 1988 Jun 16;333(6174):672–676. doi: 10.1038/333672a0. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Suchard S. J., Nagpal M. L., Glenney J. R., Jr A T-lymphoma transmembrane glycoprotein (gp180) is linked to the cytoskeletal protein, fodrin. J Cell Biol. 1985 Aug;101(2):477–487. doi: 10.1083/jcb.101.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Sefton B. M. Myristic acid is attached to the transforming protein of Rous sarcoma virus during or immediately after synthesis and is present in both soluble and membrane-bound forms of the protein. Mol Cell Biol. 1984 Dec;4(12):2697–2704. doi: 10.1128/mcb.4.12.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casnellie J. E., Harrison M. L., Pike L. J., Hellström K. E., Krebs E. G. Phosphorylation of synthetic peptides by a tyrosine protein kinase from the particulate fraction of a lymphoma cell line. Proc Natl Acad Sci U S A. 1982 Jan;79(2):282–286. doi: 10.1073/pnas.79.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casnellie J. E. Sites of in vivo phosphorylation of the T cell tyrosine protein kinase in LSTRA cells and their alteration by tumor-promoting phorbol esters. J Biol Chem. 1987 Jul 15;262(20):9859–9864. [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K., Walsh K. A., Fischer E. H. The leukocyte common antigen (CD45): a putative receptor-linked protein tyrosine phosphatase. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7182–7186. doi: 10.1073/pnas.85.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., King C. S. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol Cell Biol. 1986 Dec;6(12):4467–4477. doi: 10.1128/mcb.6.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmrich F. Cross-linking of CD4 and CD8 with the T-cell receptor complex: quaternary complex formation and T-cell repertoire selection. Immunol Today. 1988 Oct;9(10):296–300. doi: 10.1016/0167-5699(88)91320-5. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H. Do CD4 or CD8 molecules provide a regulatory signal in T-cell activation? Immunol Today. 1988 May;9(5):132–134. doi: 10.1016/0167-5699(88)91198-X. [DOI] [PubMed] [Google Scholar]
- Gacon G., Gisselbrecht S., Piau J. P., Boissel J. P., Tolle J., Fischer S. High level of tyrosine protein kinase in a murine lymphoma cell line induced by Moloney leukemia virus. EMBO J. 1982;1(12):1579–1582. doi: 10.1002/j.1460-2075.1982.tb01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D., Maddon P., Sekaly R., Talle M. A., Godfrey M., Long E., Goldstein G., Chess L., Axel R., Kappler J. Functional interaction between human T-cell protein CD4 and the major histocompatibility complex HLA-DR antigen. Nature. 1987 Aug 13;328(6131):626–629. doi: 10.1038/328626a0. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hyman R., Trowbridge I. Two complementation classes of T200 (Ly-5) glycoprotein-negative mutants. Immunogenetics. 1981 Mar 1;12(5-6):511–523. doi: 10.1007/BF01561692. [DOI] [PubMed] [Google Scholar]
- Iba H., Cross F. R., Garber E. A., Hanafusa H. Low level of cellular protein phosphorylation by nontransforming overproduced p60c-src. Mol Cell Biol. 1985 May;5(5):1058–1066. doi: 10.1128/mcb.5.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr T-cell development. Accessories or coreceptors? Nature. 1988 Sep 15;335(6187):208–210. doi: 10.1038/335208a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kawakami T., Kawakami Y., Aaronson S. A., Robbins K. C. Acquisition of transforming properties by FYN, a normal SRC-related human gene. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3870–3874. doi: 10.1073/pnas.85.11.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga Y., Caccia N., Toyonaga B., Spolski R., Yanagi Y., Yoshikai Y., Mak T. W. A human T cell-specific cDNA clone (YT16) encodes a protein with extensive homology to a family of protein-tyrosine kinases. Eur J Immunol. 1986 Dec;16(12):1643–1646. doi: 10.1002/eji.1830161229. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Tonks N. K., Fischer E. H., Clark E. A. CD45 regulates signal transduction and lymphocyte activation by specific association with receptor molecules on T or B cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8628–8632. doi: 10.1073/pnas.85.22.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. F., Kaplan N. O. An acid phosphatase in the plasma membranes of human astrocytoma showing marked specificity toward phosphotyrosine protein. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6507–6511. doi: 10.1073/pnas.79.21.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mally M. I., Vogt M., Swift S. E., Haas M. Oncogene expression in murine splenic T cells and in murine T-cell neoplasms. Virology. 1985 Jul 15;144(1):115–126. doi: 10.1016/0042-6822(85)90310-1. [DOI] [PubMed] [Google Scholar]
- Marchildon G. A., Casnellie J. E., Walsh K. A., Krebs E. G. Covalently bound myristate in a lymphoma tyrosine protein kinase. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7679–7682. doi: 10.1073/pnas.81.24.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Cooper J. A., King C. S., Ziegler S. F., Tinker D. A., Overell R. W., Krebs E. G., Perlmutter R. M. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck). Mol Cell Biol. 1988 Feb;8(2):540–550. doi: 10.1128/mcb.8.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Overell R. W., Meier K. E., Krebs E. G., Perlmutter R. M. Translational activation of the lck proto-oncogene. Nature. 1988 Mar 10;332(6160):171–173. doi: 10.1038/332171a0. [DOI] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- McCarthy S. A., Kruisbeek A. M., Uppenkamp I. K., Sharrow S. O., Singer A. Engagement of the CD4 molecule influences cell surface expression of the T-cell receptor on thymocytes. Nature. 1988 Nov 3;336(6194):76–79. doi: 10.1038/336076a0. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Altman A. Do CD4 and CD8 control T-cell activation via a specific tyrosine protein kinase? Immunol Today. 1989 Jun;10(6):189–192. doi: 10.1016/0167-5699(89)90322-8. [DOI] [PubMed] [Google Scholar]
- Renshaw M. W., Capozza M. A., Wang J. Y. Differential expression of type-specific c-abl mRNAs in mouse tissues and cell lines. Mol Cell Biol. 1988 Oct;8(10):4547–4551. doi: 10.1128/mcb.8.10.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh S. M., Brugge J. S. Investigation of factors that influence phosphorylation of pp60c-src on tyrosine 527. Mol Cell Biol. 1988 Jun;8(6):2465–2471. doi: 10.1128/mcb.8.6.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988 Nov 3;336(6194):73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- Strebhardt K., Mullins J. I., Bruck C., Rübsamen-Waigmann H. Additional member of the protein-tyrosine kinase family: the src- and lck-related protooncogene c-tkl. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8778–8782. doi: 10.1073/pnas.84.24.8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telerman A., Amson R., Zakut-Houri R., Givol D. Identification of the human pim-1 gene product as a 33-kilodalton cytoplasmic protein with tyrosine kinase activity. Mol Cell Biol. 1988 Apr;8(4):1498–1503. doi: 10.1128/mcb.8.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks N. K., Charbonneau H., Diltz C. D., Fischer E. H., Walsh K. A. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry. 1988 Nov 29;27(24):8695–8701. doi: 10.1021/bi00424a001. [DOI] [PubMed] [Google Scholar]
- Trevillyan J. M., Lin Y., Chen S. J., Phillips C. A., Canna C., Linna T. J. Human T lymphocytes express a protein-tyrosine kinase homologous to p56LSTRA. Biochim Biophys Acta. 1986 Oct 10;888(3):286–295. doi: 10.1016/0167-4889(86)90228-4. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Horak I. D., Horak E. M., Bookman M. A., Bolen J. B. Alterations of the lymphocyte-specific protein tyrosine kinase (p56lck) during T-cell activation. Mol Cell Biol. 1988 Oct;8(10):4353–4361. doi: 10.1128/mcb.8.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronova A. F., Buss J. E., Patschinsky T., Hunter T., Sefton B. M. Characterization of the protein apparently responsible for the elevated tyrosine protein kinase activity in LSTRA cells. Mol Cell Biol. 1984 Dec;4(12):2705–2713. doi: 10.1128/mcb.4.12.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]