Pulmonary Arterial Hypertension (PAH) is a serious disease and a major public health problem with approximately 1000 new patients diagnosed every year in the United States.1, 2 PAH is a progressive disease involving impaired pulmonary vascular structure and function and is ultimately lethal due to right ventricular failure. Recent insights into the pathogenesis of PAH have lead to more promising therapeutic approaches and improved outcomes; however, the mortality rates associated with PAH remain unacceptably high.1, 3, 4
PAH affects all age groups and both genders; however, there is a striking preponderance of female PAH patients which remains unexplained.5 PAH can be idiopathic, familial, or associated with other cardiovascular disorders, but the underlying pathology and pathophysiology are shared by all forms of the disease. PAH is characterized by progressive fibro-proliferative remodeling of the pulmonary arterioles, various degrees of pulmonary vasoconstriction and inflammation, thrombosis, and right ventricular hypertrophy and failure. The mainstay of current pharmacologic therapies is pulmonary vasodilation, although only a small percentage of patients have demonstrable pulmonary vasoconstriction when they undergo cardiac catheterization.6 Despite the lack of a demonstrable vasoconstrictor component, many PAH patients improve with long-term vasodilator therapy, and it is believed that pulmonary vascular remodeling could also be ameliorated as a result of vasodilator therapies. Vasoconstrictors such as angiotensin II (AngII) and endothelin-1 (ET-1) are potent stimulators of vascular smooth muscle (VSM) growth and proliferation. While many vasodilator mediators also have anti-proliferative effects on VSM, there is no definitive evidence that pulmonary vascular remodeling in humans with PAH is reversible. In addition, current vasodilator therapies are not universally successful in altering PAH progression and increasing survival. Therefore, novel approaches that directly target pulmonary vessel wall pathology are needed in order to reverse the established pulmonary vascular pathology in PAH patients.
Pathophysiological Mechanisms of PAH
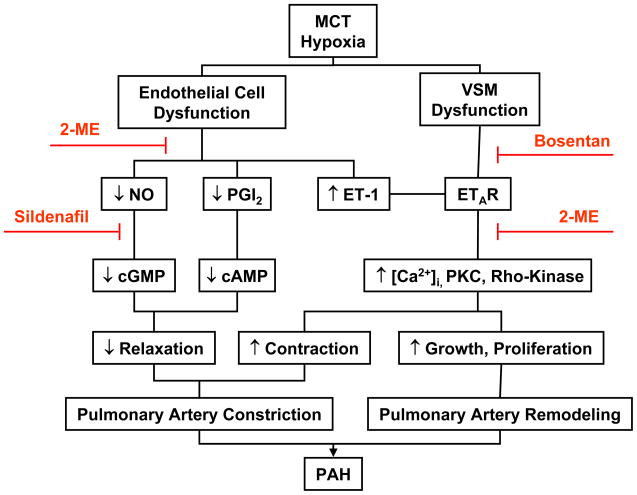
Studies in human PAH and experimental models of the disease have suggested the involvement of several molecular and signaling pathways in the development and progression of PAH.7–20 Mutations in bone morphogenetic protein receptor type II (BMPR2) and transforming growth factor beta (TGF-β) receptor have been associated with familial primary PAH.8 Decreased release or activity of endothelium-derived nitric oxide (NO) in the pulmonary circulation and loss of NO-cGMP relaxation through degradation of cGMP via phosphodiesterase 1 (PDE1) are major factors in the pathogenesis of PAH (Figure).7, 12 Several other pathways have been implicated in PAH pathogenesis as demonstrated by loss of function and interventional studies: these include cyclooxygenase-2 (COX-2) and prostacyclin (PGI2),17, 19 ET-1 15 and platelet-derived growth factor (PDGF) signaling,13 the Rho-kinase 14 and Notch3 signaling pathways 20 as well as heme oxygenase-1 (HO-1)/carbon monoxide (CO).18, 21 Nevertheless, PAH appears to be a multifactorial disorder, and more than one gene or signaling pathway is likely to be involved.
Figure.
Pathophysiological mechanisms and molecular targets in MCT-treated and hypoxia-induced model of PAH. The PAH-associated endothelial cell dysfunction and decreased pulmonary artery relaxation, and the VSM dysfunction and increased pulmonary artery constriction and remodeling are reduced by treatment with the PDE inhibitor sildenafil, or the ET-1 receptor antagonist bosentan. 2-ME enhances the stimulatory effects of sildenafil on pulmonary artery relaxation, and the inhibitory effects of bosentan on vasoconstriction and remodeling.
As demonstrated in human studies as well as studies in experimental animals, PAH is a pan-vasculopathy characterized by endothelial dysfunction, medial hypertrophy and VSM hyperplasia, and adventitial fibrosis. Using the monochrotaline (MCT) and hypoxic rat models of PAH, we and others have shown reduced pulmonary artery contraction to vasoconstrictors, decreased endothelium-dependent NO-cGMP-mediated pulmonary artery relaxation, and decreased pulmonary artery responsiveness to endogenous and exogenous nitrovasodilator.7, 22–25 The changes in pulmonary artery function are associated with extensive pulmonary artery thickening and remodeling, and increased pulmonary VSM cell growth and proliferation (Figure).26–29 These observations have made the MCT and hypoxic rat models the most commonly used animal models to investigate the pathophysiology of PAH and to test the effects of potential therapies of the disease. In addition to the significant pulmonary artery remodeling, perivascular inflammation and transdifferentiation of circulating and resident progenitor cells are thought to contribute to the pathogenesis of PAH via mechanisms that are incompletely understood.30–33
Common Therapies of PAH
The characterization of the molecular mechanisms underlying PAH has been critical to identifying novel targets for therapeutic intervention. However, as novel targets are identified, multiple obstacles to clinical application have to be overcome since bioavailability, selectivity, and potential toxicity of novel therapies need to be carefully evaluated in clinical trials. As a result, despite the identification of multiple potential new targets for intervention, only three classes of therapies are currently in use for PAH. These include PDE-5 inhibitors, PGI2 analogs, and endothelin receptor A (ETAR) antagonists. These therapies target molecular pathways that are known to be dysregulated in the setting of PAH. PDE inhibitors such as sildenafil prevent the breakdown of cGMP and consequently enhance the NO-cGMP pulmonary arterial relaxation pathway. PGI2 analogs such as iloprost stimulate the PGI2 receptors and enhance the PGI2-cAMP relaxation pathway. Non-specific ET-1 receptor antagonists such as bosentan and specific ETAR antagonists like sitaxentan and ambrisentan block ET-1 induced signaling and its effects on both VSM contraction and cell growth. To enhance their effectiveness, these therapeutic approaches are often used in combination, although the optimal combination therapy is still under investigation.34, 35 Whether used separately or combined, the goal of these approaches is to restore the balance between the NO-cGMP and PGI2-cAMP vasodilator and VSM anti-proliferative pathways, and the ET-1 induced vasoconstrictor and VSM proliferative pathway in the pulmonary vasculature. Ca2+ channel blockers may have some benefits in certain PAH patients.36, 37 Also, ample experimental evidence supports that more specific anti-proliferative, pro-apoptotic, immuno-modulatory and cell based therapies could be effective in PAH,13, 38–44 however, translation to clinical application is lagging behind these new discoveries.
Effects of sex hormones in PAH
The preponderance of PAH in females is unexplained, yet very intriguing particularly because it is opposite of the known preponderance of systemic cardiovascular disease in males. It has become increasingly appreciated that the cardiovascular effects of sex steroids and their metabolites are far more complex than initially thought. Despite attempts to define the role of sex steroid hormones in pulmonary vascular disease, significant knowledge gaps exist in this area.44 Most studies on the role of sex steroids in pulmonary vascular homeostasis focus on the vasodilator properties of estrogens, and possibly androgens, especially in the settings of experimental hypoxia.45 Specifically, 17-hydroxy-estradiol has been reported to have multiple effects on the endothelial production of NO and PGI2 which in turn lead to endothelium-dependent vasodilation. In addition, 17-hydroxy-estradiol has endothelium-independent vasodilatory effects by activating voltage-activated potassium channels in VSM cells. Similarly, progesterone and testosterone have been reported to reduce vascular tone by blocking both voltage-gated and receptor-operated Ca2+ channels in VSM cells.45 Recently, 2-methoxy-estradiol (2-ME) has been recognized as a biologically active metabolite of estradiol with estrogen receptor-independent anti-proliferative properties 46 and its therapeutic potential in cardiovascular and pulmonary vascular diseases warrant further investigation.
In this issue of the Journal of Cardiovascular Pharmacology, Tofovic and co-workers present experimental evidence supporting that 2-ME treatment ameliorates MCT-induced PAH not only in adult females, but also in adult male rats. 2-ME was as efficacious as the PDE inhibitor sildenafil and the ET-1 receptor antagonist bosentan in ameliorating MCT-induced PAH in both male and female rats. The authors also demonstrate that the combination of 2-ME with either sildenafil or bosentan confers additional protection and improves survival in MCT-treated rats (Figure). Importantly, these pharmacological interventions were initiated twelve days after administration of MCT and the establishment of PAH and pulmonary vascular remodeling thus supporting effective reversal of established disease. The authors propose that the mechanism of protection involves anti-proliferative and anti-inflammatory effects of 2-ME in the pulmonary vasculature and the lung, respectively.
Future Directions
The study by Tofovic and colleagues opens an important area for investigation with regard to the sex differences in the incidence of PAH and the role of sex hormones in the pathogenesis and management of PAH. An important question is whether the increase in the incidence of PAH in females is related to estrogen. This will be difficult to reconcile with the apparent protective effects of 2-ME in the MCT-treated model of PAH. One possibility is that the effects of estradiol on the pulmonary vasculature are different from those of estrogen metabolites. In this respect, it is important to compare the effects of 2-ME on pulmonary vessels with those of estrogen, specific estrogen receptor modulators, and other estrogen metabolites. It will also be important to study the differential effects of estrogens as compared to androgens on the pulmonary vasculature.
The specific mechanisms underlying the effects of estrogen and its metabolites on pulmonary vascular structure and function also need to be further examined. Estrogen-induced endothelium-dependent vasodilator effects on NO, PGI2 and endothelium-derived hyperpolarizing factor (EDHF) have been described in blood vessels of the systemic circulation.47 Additional inhibitory effects of estrogen on VSM cell contraction and growth have been suggested.47 Studies have suggested inhibitory effects of estrogen on the Ca2+-dependent mechanisms of VSM contraction.48 Other studies have suggested an inhibitory effect of estrogen on the expression/activity of protein kinase C and Ca2+-sensitization pathways of VSM contraction (Figure).49 In this respect, studies have suggested a role for Rho-kinase as Ca2+-sensitization pathway of VSM contraction as well as in VSM cell growth and proliferation.50 Given the beneficial effects of Rho-kinase inhibitors in experimental PAH,14 it would be important to test whether estrogen or its metabolites function by inhibiting Rho-kinase. Estrogen and its metabolites may also affect the expression/activity of matrix metalloproteinases and the composition of the extracellular matrix,51 and such effects could also improve pulmonary arterial remodeling in PAH.
Finally, while studying the effects of 2-ME in MCT-treated rat model highlights potential pulmonary vascular protective effects in PAH, it would be important to study the effects of estrogen and its metabolites in other animal models such as the hypoxia-induced model of PAH. Careful evaluation of the findings in experimental animal models could spearhead studies in human and clinical trials to determine the effects of sex hormones on the course of PAH.
Acknowledgments
Dr. R.A. Khalil was partly supported by grants from National Heart, Lung, and Blood Institute (HL-65998, HL-70659, and HL-98724) and The Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD-60702).
List of abbreviations
- cGMP
cyclic guanosine monophosphate
- COX-2
cyclooxygenase-2
- ET-1
endothelin-1
- ETAR
endothelin receptor A
- MCT
monochrotaline
- 2-ME
2-methoxy-estradiol
- NO
nitric oxide
- PAH
pulmonary arterial hypertension
- PGI2
prostacyclin
- PDE
phosphodiesterase
- VSM
vascular smooth muscle
References
- 1.Farber HW. The status of pulmonary arterial hypertension in 2008. Circulation. 2008 Jun 10;117(23):2966–2968. doi: 10.1161/CIRCULATIONAHA.108.782979. [DOI] [PubMed] [Google Scholar]
- 2.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. Feb;137(2):376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 3.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. Jul 13;122(2):164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin VV, Presberg KW, Doyle RL, Abman SH, McCrory DC, Fortin T, Ahearn G. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004 Jul;126(1 Suppl):78S–92S. doi: 10.1378/chest.126.1_suppl.78S. [DOI] [PubMed] [Google Scholar]
- 5.Frost AE, Badesch DB, Barst RJ, Benza RL, Elliott CG, Farber HW, Krichman A, Liou TG, Raskob GE, Wason P, Feldkircher K, Turner M, McGoon MD. The Changing Picture of Pulmonary Arterial Hypertension (PAH) Patients in the United States: How the REVEAL Registry Differs From Historic and Non-US Contemporary Registries. Chest. Jun 17; doi: 10.1378/chest.10-0075. [DOI] [PubMed] [Google Scholar]
- 6.Barst RJ, Langleben D, Badesch D, Frost A, Lawrence EC, Shapiro S, Naeije R, Galie N. Treatment of pulmonary arterial hypertension with the selective endothelin-A receptor antagonist sitaxsentan. J Am Coll Cardiol. 2006 May 16;47(10):2049–2056. doi: 10.1016/j.jacc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 7.Adnot S, Raffestin B, Eddahibi S, Braquet P, Chabrier PE. Loss of endothelium-dependent relaxant activity in the pulmonary circulation of rats exposed to chronic hypoxia. J Clin Invest. 1991 Jan;87(1):155–162. doi: 10.1172/JCI114965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000 Sep;26(1):81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 9.Cowan KN, Heilbut A, Humpl T, Lam C, Ito S, Rabinovitch M. Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med. 2000 Jun;6(6):698–702. doi: 10.1038/76282. [DOI] [PubMed] [Google Scholar]
- 10.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002 Jan 15;105(2):244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 11.Sweeney M, Yuan JX. Hypoxic pulmonary vasoconstriction: role of voltage-gated potassium channels. Respir Res. 2000;1(1):40–48. doi: 10.1186/rr11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schermuly RT, Pullamsetti SS, Kwapiszewska G, Dumitrascu R, Tian X, Weissmann N, Ghofrani HA, Kaulen C, Dunkern T, Schudt C, Voswinckel R, Zhou J, Samidurai A, Klepetko W, Paddenberg R, Kummer W, Seeger W, Grimminger F. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation. 2007 May 1;115(17):2331–2339. doi: 10.1161/CIRCULATIONAHA.106.676809. [DOI] [PubMed] [Google Scholar]
- 13.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005 Oct;115(10):2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, McMurtry IF. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004 Oct;287(4):L656–664. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- 15.Ivy D, McMurtry IF, Yanagisawa M, Gariepy CE, Le Cras TD, Gebb SA, Morris KG, Wiseman RC, Abman SH. Endothelin B receptor deficiency potentiates ET-1 and hypoxic pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol. 2001 May;280(5):L1040–1048. doi: 10.1152/ajplung.2001.280.5.L1040. [DOI] [PubMed] [Google Scholar]
- 16.Laudi S, Trump S, Schmitz V, West J, McMurtry IF, Mutlak H, Christians U, Weimann J, Kaisers U, Steudel W. Serotonin transporter protein in pulmonary hypertensive rats treated with atorvastatin. Am J Physiol Lung Cell Mol Physiol. 2007 Sep;293(3):L630–638. doi: 10.1152/ajplung.00110.2006. [DOI] [PubMed] [Google Scholar]
- 17.Geraci MW, Gao B, Shepherd DC, Moore MD, Westcott JY, Fagan KA, Alger LA, Tuder RM, Voelkel NF. Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension. J Clin Invest. 1999 Jun;103(11):1509–1515. doi: 10.1172/JCI5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christou H, Morita T, Hsieh CM, Koike H, Arkonac B, Perrella MA, Kourembanas S. Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ Res. 2000 Jun 23;86(12):1224–1229. doi: 10.1161/01.res.86.12.1224. [DOI] [PubMed] [Google Scholar]
- 19.Fredenburgh LE, Liang OD, Macias AA, Polte TR, Liu X, Riascos DF, Chung SW, Schissel SL, Ingber DE, Mitsialis SA, Kourembanas S, Perrella MA. Absence of cyclooxygenase-2 exacerbates hypoxia-induced pulmonary hypertension and enhances contractility of vascular smooth muscle cells. Circulation. 2008 Apr 22;117(16):2114–2122. doi: 10.1161/CIRCULATIONAHA.107.716241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Zhang X, Leathers R, Makino A, Huang C, Parsa P, Macias J, Yuan JX, Jamieson SW, Thistlethwaite PA. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med. 2009 Nov;15(11):1289–1297. doi: 10.1038/nm.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yet SF, Perrella MA, Layne MD, Hsieh CM, Maemura K, Kobzik L, Wiesel P, Christou H, Kourembanas S, Lee ME. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest. 1999 Apr;103(8):R23–29. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillespie MN, Olson JW, Reinsel CN, O’Connor WN, Altiere RJ. Vascular hyperresponsiveness in perfused lungs from monocrotaline-treated rats. Am J Physiol. 1986 Jul;251(1 Pt 2):H109–114. doi: 10.1152/ajpheart.1986.251.1.H109. [DOI] [PubMed] [Google Scholar]
- 23.Fullerton DA, Hahn AR, McIntyre RC., Jr Mechanistic imbalance of pulmonary vasomotor control in progressive lung injury. Surgery. 1996 Jan;119(1):98–103. doi: 10.1016/s0039-6060(96)80220-0. [DOI] [PubMed] [Google Scholar]
- 24.Shimoda LA, Sham JS, Sylvester JT. Altered pulmonary vasoreactivity in the chronically hypoxic lung. Physiol Res. 2000;49(5):549–560. [PubMed] [Google Scholar]
- 25.Mam V, Tanbe AF, Vitali SH, Arons E, Christou HA, Khalil RA. Impaired vasoconstriction and nitric oxide-mediated relaxation in pulmonary arteries of hypoxia- and monocrotaline-induced pulmonary hypertensive rats. J Pharmacol Exp Ther. Feb;332(2):455–462. doi: 10.1124/jpet.109.160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004 Jan 20;109(2):159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 27.Klings ES, Farber HW. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004 Jun 10;350(24):2521–2522. doi: 10.1056/NEJM200406103502418. author reply 2521–2522. [DOI] [PubMed] [Google Scholar]
- 28.Mitani Y, Ueda M, Komatsu R, Maruyama K, Nagai R, Matsumura M, Sakurai M. Vascular smooth muscle cell phenotypes in primary pulmonary hypertension. Eur Respir J. 2001 Feb;17(2):316–320. doi: 10.1183/09031936.01.17203160. [DOI] [PubMed] [Google Scholar]
- 29.Pietra GG, Edwards WD, Kay JM, Rich S, Kernis J, Schloo B, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation. 1989 Nov;80(5):1198–1206. doi: 10.1161/01.cir.80.5.1198. [DOI] [PubMed] [Google Scholar]
- 30.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009 Jun 30;54(1 Suppl):S10–19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8798–8803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol. 2006 Feb;168(2):659–669. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frid MG, Li M, Gnanasekharan M, Burke DL, Fragoso M, Strassheim D, Sylman JL, Stenmark KR. Sustained hypoxia leads to the emergence of cells with enhanced growth, migratory, and promitogenic potentials within the distal pulmonary artery wall. Am J Physiol Lung Cell Mol Physiol. 2009 Dec;297(6):L1059–1072. doi: 10.1152/ajplung.90611.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoeper MM, Markevych I, Spiekerkoetter E, Welte T, Niedermeyer J. Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J. 2005 Nov;26(5):858–863. doi: 10.1183/09031936.05.00075305. [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF, Robbins IM, Olschewski H, Rubenfire M, Seeger W. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. May 4;55(18):1915–1922. doi: 10.1016/j.jacc.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 36.Sitbon O, Humbert M, Jais X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Herve P, Simonneau G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005 Jun 14;111(23):3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 37.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992 Jul 9;327(2):76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 38.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005 Jun;115(6):1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito T, Okada T, Miyashita H, Nomoto T, Nonaka-Sarukawa M, Uchibori R, Maeda Y, Urabe M, Mizukami H, Kume A, Takahashi M, Ikeda U, Shimada K, Ozawa K. Interleukin-10 expression mediated by an adeno-associated virus vector prevents monocrotaline-induced pulmonary arterial hypertension in rats. Circ Res. 2007 Sep 28;101(7):734–741. doi: 10.1161/CIRCRESAHA.107.153023. [DOI] [PubMed] [Google Scholar]
- 40.Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, Kadowitz PJ. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007 Feb;292(2):H1120–1128. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- 41.Nishimura T, Vaszar LT, Faul JL, Zhao G, Berry GJ, Shi L, Qiu D, Benson G, Pearl RG, Kao PN. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation. 2003 Sep 30;108(13):1640–1645. doi: 10.1161/01.CIR.0000087592.47401.37. [DOI] [PubMed] [Google Scholar]
- 42.Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, Chen JZ. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. 2007 Apr 10;49(14):1566–1571. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 43.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, Grunig E, Grunig G. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med. 2008 Feb 18;205(2):361–372. doi: 10.1084/jem.20071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith AM, Jones RD, Channer KS. The influence of sex hormones on pulmonary vascular reactivity: possible vasodilator therapies for the treatment of pulmonary hypertension. Curr Vasc Pharmacol. 2006 Jan;4(1):9–15. doi: 10.2174/157016106775203090. [DOI] [PubMed] [Google Scholar]
- 45.Patel KM, Lahm T, Crisostomo PR, Herring C, Markel T, Wang M, Meldrum DR. The effects of endogenous sex hormones and acute hypoxia on vasoconstriction in isolated rat pulmonary artery rings. J Surg Res. 2008 May 1;146(1):121–126. doi: 10.1016/j.jss.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mueck AO, Seeger H. 2-Methoxyestradiol--biology and mechanism of action. Steroids. Oct;75(10):625–631. doi: 10.1016/j.steroids.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 47.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004 Feb;286(2):R233–249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 48.Murphy JG, Khalil RA. Gender-specific reduction in contractility and [Ca(2+)](i) in vascular smooth muscle cells of female rat. Am J Physiol Cell Physiol. 2000 Apr;278(4):C834–844. doi: 10.1152/ajpcell.2000.278.4.C834. [DOI] [PubMed] [Google Scholar]
- 49.Kanashiro CA, Khalil RA. Gender-related distinctions in protein kinase C activity in rat vascular smooth muscle. Am J Physiol Cell Physiol. 2001 Jan;280(1):C34–45. doi: 10.1152/ajpcell.2001.280.1.C34. [DOI] [PubMed] [Google Scholar]
- 50.Lee DL, Webb RC, Jin L. Hypertension and RhoA/Rho-kinase signaling in the vasculature: highlights from the recent literature. Hypertension. 2004 Dec;44(6):796–799. doi: 10.1161/01.HYP.0000148303.98066.ab. [DOI] [PubMed] [Google Scholar]
- 51.Wingrove CS, Garr E, Godsland IF, Stevenson JC. 17beta-oestradiol enhances release of matrix metalloproteinase-2 from human vascular smooth muscle cells. Biochim Biophys Acta. 1998 Mar 5;1406(2):169–174. doi: 10.1016/s0925-4439(97)00097-5. [DOI] [PubMed] [Google Scholar]