Abstract
Background
An important focus of tumor immunotherapy has been the identification of appropriate antigenic targets. Serum-based screening approaches have led to the discovery of hundreds of tumor-associated antigens recognized by IgG. Our efforts to identify immunologically recognized proteins in prostate cancer have yielded a multitude of antigens, however prioritizing these antigens as targets for evaluation in immunotherapies has been challenging. In this report, we set out to determine whether the evaluation of multiple antigenic targets would allow the identification of a subset of antigens that are common immunologic targets in patients with prostate cancer.
Methods
Using a phage immunoblot approach, we evaluated IgG responses in patients with prostate cancer (n=126), patients with chronic prostatitis (n=45), and men without prostate disease (n=53).
Results
We found that patients with prostate cancer or prostatitis have IgG specific for multiple common antigens. A subset of 23 proteins was identified to which IgG were detected in 38% of patients with prostate cancer and 33% patients with prostatitis versus 6% of controls (p<0.001 and p=0.003, respectively). Responses to multiple members were not higher in patients with advanced disease, suggesting antibody immune responses occur early in the natural history of cancer progression.
Conclusions
These findings suggest an association between inflammatory conditions of the prostate and prostate cancer, and suggest that IgG responses to a panel of commonly recognized prostate antigens could be potentially used in the identification of patients at risk for prostate cancer or as a tool to identify immune responses elicited to prostate tissue.
Keywords: IgG, autoantibody, prostate cancer, prostatitis, high-throughput immunoblot
INTRODUCTION
A major effort over the last two decades in the design and evaluation of anti-tumor immunotherapies has been to identify antigenic molecules that might serve as targets for an anti-tumor immune attack. The identification of tumor antigen-specific immune responses in patients with cancer has served as preclinical evidence that such responses can exist and might be augmented by means of active immunotherapy. Consequently, early efforts sought to identify the antigens recognized by tumor-infiltrating lymphocytes (1). Subsequent studies were aided by the observation that antigen-specific T-cells were frequently accompanied by antigen-specific IgG (2). The development of serum-based screening approaches, including SEREX (serological identification of antigens by recombinant expression cloning), has permitted the rapid identification of many tumor antigen-specific IgG in patients with many different tumor types (3). In fact, these methods have been so robust that literally hundreds of antigenic proteins have been identified, leading efforts to define the cancer “immunome” of immunologically recognized cancer-associated proteins as a defined set of rational targets for tumor vaccine development (4,5). However, the multitude of immunologically recognized proteins associated with cancer has also presented challenges in terms of prioritizing particular antigens for vaccine evaluation. A recent consensus panel led by the National Cancer Institute has sought to define a more limited set of antigens, and criteria for prioritizing antigens, for future evaluation in combination with other immune-active therapies (6).
Over the last several years, we and others have used IgG screening methodologies such as SEREX to identify immunologically recognized proteins of the prostate using sera from individual patients with prostate cancer to specifically prioritize antigens for consideration as vaccine targets for prostate cancer (7–9). To further identify antigens that might represent natural prostate tissue antigens, we have also used sera from patients with chronic prostatitis to screen a normal prostate tissue cDNA expression library, prioritizing those recognized by multiple individuals with prostatitis (10). Moreover, to identify other potential target antigens, we have used a similar SEREX methodology to identify antigens recognized in subjects after treatment with immune-active therapies, including flt3 ligand (11) and standard androgen deprivation therapy (12). Finally, in an effort to identify immunologically recognized prostate tumor-specific antigens not expressed in normal prostate tissue, we have also sought to identify prostate cancer-relevant cancer-testis antigens (CTA), proteins normally only expressed in MHC class I-deficient germ cells but aberrantly expressed in solid tumors of different histologic types. For these studies, sera from patients with prostate cancer were used to directly screen a testis tissue cDNA expression library or a panel of defined CTA family members (13,14).
In the current report we hypothesized that from among these multiple previously identified antigens we might identify a subset of commonly recognized prostate cancer-associated antigens. Moreover, we suspected that in a disease such as prostate cancer with a long natural history, immune responses might develop over time with progressive disease, such that multiple antigens might be recognized in patients with advanced, metastatic disease. We reasoned that the evaluation of multiple antigens might lead to the identification of a subset recognized by the majority of patients, and this might have future utility in diagnosing prostate cancer, as has been previously suggested (15). Using a phage immunoblot approach (16), 126 prostate cancer-associated antigens previously identified from other studies were compiled into a single panel and probed with sera from patients with prostate cancer, chronic prostatitis, and normal male controls. We report here the identification of a subset of prostate-associated antigens commonly recognized by IgG in patients with prostate cancer, including patients with newly diagnosed disease. These antigens were also frequently recognized in patients with clinical prostatitis. These findings suggest a possible association of prostate inflammation with prostate cancer, and suggest that evaluation of IgG to defined antigens might have utility in identifying patients at risk for prostate cancer.
MATERIALS AND METHODS
Patient Populations
Sera were obtained from patients with chronic prostatitis (n=45, median age 42, range 19–62), prostate cancer (n=151, median age 67, range 44–86), and men without a history of known prostate disease (n=78, median age 32, range 18–62). Sera were also obtained from male patients with other cancers (melanoma, n=4; renal n=17; testicular, n=5; other, n=5; median age 58, range 30–74). Among the patients with prostate cancer, 18 were collected from patients at the time of diagnosis prior to definitive radiation treatment or prostatectomy (pretreatment), 32 were collected from patients after definitive treatment without evidence of disease recurrence (limited stage), 44 were collected from patients with metastatic disease recurrence on androgen deprivation therapy (androgen-dependent), and 57 were collected from patients with castrate-resistant, metastatic disease. All subjects gave written Institutional review-board approved consent for the use of their blood for immunological studies. Blood was collected at the University of Wisconsin-Madison Hospital and Clinics (Madison, WI) or at the University of Washington Medical Center (Seattle, WA), and sera were stored in aliquots at −20°C to −80°C until used for analysis.
High Throughput Immunoblot (HTI)
Phage immunoblot analysis was performed, similar to what we have previously described (16). For this, panels of 128 lambda phage encoding unique prostate-associated antigens were assembled (Table I). Phage included those initially identified in patients with prostate cancer or chronic prostatitis by SEREX (10–13), or were constructed to express specific genes of interest based on prior studies (16,17). Included in these panels were 29 cancer-testis antigens, 41 antigens identified in patients with chronic prostatitis, 28 antigens identified in patients treated with androgen deprivation (ADT), and 30 antigens identified in patients treated with other therapies. 100,000 pfu lambda phage encoding these antigens were robotically spotted in triplicate in a 16×24 array onto lawns of E. coli (XL-1 blue strain) growing in agar-containing OmniTray plates. Replicates for individual antigens were staggered in position across the array to account for regional variations on individual filters. For initial studies, phage encoding human immunoglobulin G (IgG) were spotted as a positive control and an empty phage construct was similarly spotted as a negative control. Plates were allowed to dry at room temperature for 20 minutes and incubated at 37°C for 4 hours after which nitrocellulose membranes suffused with10-mM isopropyl β-D-thiogalactopyranoside (IPTG) (Fisher Scientific, Pittsburgh, PA) were overlain. Plates were incubated at 37°C overnight to allow recombinant protein expression. After 16–20 hours, membranes were removed, washed twice in TBST (50mM TrispH 7.2, 100mM NaCl, 0.5%Tween-20) for 10 minutes and once in TBS (50mM Tris pH 7.2, 100mM NaCl) for an additional 10 minutes. Membranes were blocked in blocking solution (TBST + 1% BSA), and incubated at 4°C with human sera (diluted 1:100 in blocking solution) overnight. Membranes were washed the following day and blocked prior to detection of human IgG with a mouse anti-human IgG antibody conjugated to alkaline phosphatase (Sigma, St. Louis, MO). Membranes were washed again and immunoreactivity detected by development with 0.3mg/mL nitro blue tetrazolium chloride (NBT) (Fisher Scientific) and 0.15mg/mL 5-bromo 4-chloro 3-indoylphosphate (BCIP) (Fisher Scientific) in 100-mM Tris 9.5, 100-mM NaCl, and 5-mM MgCl2. Membranes were washed with large volumes of deionized water and dried at room temperature prior to evaluation (Figure 1). Membranes were scanned using a color image scanner and the digital format aligned with a 16×24 grid using densitometry software (ImageQuant TL, Amersham Biosciences, GE Healthcare Life Sciences, Piscataway, NJ). For initial studies, immunoreactivity was quantified by measuring the density at each spot; values of replicates for individual antigens were averaged. Background correction was then made by subtracting the average of empty phage construct replicate densities on individual membrane and normalized by dividing by the average of IgG positive control replicate densities on each membrane. Transformation of densitometry data resulted in density values for individual antigens relative to a negative control and a positive control (set at 0.0 and 1.0 respectively). For subsequent studies, immunoreactivity was judged as “positive” or “negative” by visual inspection, as previously described (10,16). Antigens for which 0–1 of replicates determined immunoreactive with individual sera were defined as negative for immunoreactivity, and 2–3 of the replicates determined immunoreactive were defined as positive.
Table I. Prostate-associated antigen panel.
Shown are the lambda phage-encoded antigens, and GenBank accession numbers, used for the current studies and obtained from previous studies from patients with prostatitis (PRO) (10), patients with prostate cancer treated with androgen deprivation therapy (ADT) (12), patients with prostate cancer treated with various other therapies (PCA) (9,11), or specific cancer-testis antigens (CTA) (13,16).
| Designation | GenBank Accession |
Gene name |
|---|---|---|
| PRO1 | NM_006423.1 | Rab acceptor |
| PRO2 | NM_007124.1 | U-trophon |
| PRO3 | NM_000484.1 | Amyloid Beta (4) precursor protein |
| PRO4 | NM_002709.1 | Protein Phosphatase 1 |
| PRO5 | AC005822.1 | hRPK.209-J-20 DNA from chromosome 17 |
| PRO6 |
AF308301.1 / NM_030811.2 |
NY-BR-87/Ribosomal protein S26 |
| PRO7 | NM_014761.1 | KIAA0174 from chromosome 16 |
| PRO8 | AF273042.1 | Cutaneous T cell lymphoma tumor antigen sel-1 |
| PRO9 | AK096728.1 | FLJ39409 Cdna |
| PRO10 | NM_001747.1 | Macrophage capping protein gelsolin-like (CAPG) |
| PRO11 | NM_000717.2 | Carbonic anhydrase IV |
| PRO12 | AL136040.5 | BAC C-2506P8 from chromosome 14 |
| PRO13 | AC084864.4 | RP11-738B7 DNA from chromosome 7 |
| PRO14 | NM_001813.1 | Centromere protein E |
| PRO15 | M27274.1 | Prostate specific antigen (PSA) |
| PRO16 | NM_012116.2 | Cas-Br-M ecotropic retroviral transforming sequence c (CBLC) |
| PRO17 | BC006286.3 | Dual specificity phosphatase 12 |
| PRO18 |
NM_144767.1 / BC017368.1 / AF126008.1 |
Protein kinase A anchor protein 13/lymphoid blast crisis oncogene/breast cancer nuclear receptor-binding auxiliary protein (BRX) |
| PRO19 | AC073879.7 | BAC RP11-752K22 from chromosome 2 |
| PRO20 | AB017363.1 | Frizzled-1 |
| PRO21 | AL365273.25 | RP11-429G19 DNA from chromosome 10 |
| PRO22 | XM_047011.2 | o-fucosyltansferase |
| PRO23 | NM_017582.3 | NICE5 |
| PRO24 | NM_014190.1 | Adducin 1 |
| PRO25 | AL138752.5 | RP11-3J10 on chromosome 9 |
| PRO26 | BC024007.1 | Chitobiase |
| PRO27 | AK001572.1 | FLJ10710 cDNA |
| PRO28 | AL356915.19 | RP11-3J10 on chromosome 13 |
| PRO29 |
NM_006117.1 / AF257175.1 |
Peroxisomal D3,D2 enoyl CoA isomerase/Hepatocellular carcimona- associated antigen 64 |
| PRO30 | XM_033511.8 | Helicase with SNF2 domain |
| PRO31 |
AF039689.1 / XM_083939.1 / AF432221.1 |
NY-CO-7/STUB1/CLL-associated antigen KW-8 |
| PRO32 | AC021558.10 | RP11-746L20 DNA from chromosome 8 |
| PRO33 | NM_031946.2 | Centaurin gamma 3 |
| PRO34 | BC034250.1 | Pituitary tumor-transforming 1 interacting protein |
| PRO35 | AC069506.14 | BAC RP11-321G3 |
| PRO36 | AC011489.6 | CTB-179K24 DNA on chromosome 19 |
| PRO37 | NM_032415.2 | Caspase domain recruitment (CARD11) |
| PRO38 | NM_003379.3 | Cytovillin 2 |
| PRO39 | BC029529.1 | Beta tubulin |
| PRO40 | L07872.1 | Recombination signal binding protein (RBPJK) |
| PRO41 | NM_006455.1 | Nucleolar autoantigen / MAD-Pro-34 |
| CTA1 | NM_004988 | Mage A1 |
| CTA2 | BC007343 | SSX-2 |
| CTA3 | AJ003149 | Ny-ESO1 |
| CTA4 | NM_021123 | Gage 7 |
| CTA5 | U90841 | SSX-4 |
| CTA6 | BC015020 | NXF-2 |
| CTA7 | BC022011 | TPX 1 |
| CTA8 | BC009538 | Xage 1 |
| CTA9 | BC002833 | Lage 1 |
| CTA10 | BC010897 | Page 1 |
| CTA11 | BC081566 | Mage E1 |
| CTA12 | BC054023 | Span XC |
| CTA13 | BC064547 | Adam 2 |
| CTA14 | BC037775 | TSP 50 |
| CTA15 | BC034320 | NY-SAR 35 |
| CTA16 | BC022064 | Fate 1 |
| CTA17 | BC009230 | Page 5 |
| CTA18 | BC023635 | Lip1 |
| CTA19 | BC032457 | SPA17 |
| CTA20 | BE387798 | Mage A8 |
| CTA21 | BE897525 | Mage B1 |
| CTA22 | BC026071 | Mage B2 |
| CTA23 | BC017723 | Mage A4 |
| CTA24 | BC001003 | SSX-1 |
| CTA25 | BC069397 | Gage 2 |
| CTA26 | BC069470 | Gage 4 |
| CTA27 | BC016803 | Mage A3 |
| CTA28 | NM_002762 | MAD-CT-1 |
| CTA29 | AK097414 | MAD-CT-2 |
| PCA1 | NM_025161.4 | Chromosome 17 gene contig. |
| PCA2 | NM_000972.2 | Ribosomal protein L7a |
| PCA3 | NM_152636.2 | Chromosome 12 gene contig. |
| PCA4 | NM_001134194.1 | Prostatic acid phosphotase |
| PCA5 | NM_003291.2 | Tripeptidyl peptidase II |
| PCA6 | NM_006690.3 | Matrix metallopeptidase 24 |
| PCA7 | NM_000990.4 | Ribosomal protein L27a |
| PCA8 | NM_002652.2 | Prolactin-induced protein |
| PCA9 | NM_005349.2 | Immunoglobulin Kappa J region |
| PCA10 | NM_005817.2 | Mannose-6-phosphate receptor binding protein 1 |
| PCA11 | NM_013267.2 | Glutaminase 2 |
| PCA12 | NM_018979.2 | WNK lysine deficient protein kinase 1 |
| PCA13 | NM_020718.3 | Ubiquitin specific peptidase 31 |
| PCA14 | NM_003007.2 | Semenogelin I, transcript variant 1 |
| PCA15 | NM_022735.3 | Acyl-coenzyme A binding domain containing 3 |
| PCA16 | NM_001040284.1 | PAP associated domain |
| PCA17 | NM_020187.2 | Chromosome 3 gene contig. |
| PCA18 | NM_002712.1 | Protein phosphotase 3, regulatory subunit 7 |
| PCA19 | NM_014220.2 | Transmembrane 4 L6 family member 1 |
| PCA20 | NM_001135592.1 | Ribosomal protein S27a |
| PCA21 | NM_012401.2 | Plexin B2 |
| PCA22 | NM_000985.3 | Ribosomal protein L17 |
| PCA23 | NM_080608.3 | Chromosome 20 gene contig. |
| PCA24 | NM_005165.2 | Aldolase C |
| PCA25 | NM_000969.3 | Ribosomal protein L5 |
| PCA26 | NM_001958.2 | Eukaryotic translation elongation factor 1 alpha 1 |
| PCA27 | NM_025108.2 | Chromosome 16 gene contig. |
| PCA28 | NM_015358.2 | Zinc-finger protein, CW type with coiled-coil domain 3 |
| PCA29 | NM_001130410.1 | Acetyl-coenzyme A acyltransferase 1 |
| PCA30 | NM_000044.2 | Androgen receptor ligand-binding domain |
| ADT1 | NM_004750.3 | Mitogen-activated protein kinase-activated protein kinase 2 |
| ADT2 | NM_001103.2 | actinin alpha 2 |
| ADT3 | NM_002518.3 | neuronal PAS domain protein 2 (NPAS2) |
| ADT4 | NM_033138.2 | caldesmon 1 (CALD1) |
| ADT5 | NM_001459.2 | fms-related tyrosine kinase 3 ligand |
| ADT6 | NM_022474.2 | membrane protein, palmitoylated 5 (MAGUK p55 subfamily member 5) |
| ADT7 | NM_006022.2 | TSC22 domain family, member 1 (TSC22D1) |
| ADT8 | NM_001001522.1 | transgelin |
| ADT9 | NM_022758.4 | Chromosome 6 gene contig |
| ADT10 | NM_002695.3 | polymerase (RNA) II (DNA directed) polypeptide E (POLR2E) |
| ADT11 | NM_001018160.1 | NEDD8 activating enzyme subunit 1 (NAE1) |
| ADT12 | NM_015313.1 | Rho guanine nucleotide exchange factor 12 (ARHGEF12) |
| ADT13 | NM_152374.1 | Chromosome 1 gene contig |
| ADT14 | NM_001100873.1 | Chromosome 16 gene contig |
| ADT15 | NM_001136204.1 | Regulator of chromosome condensation 2 (RCC2) |
| ADT16 | NT_006713.15 | Chromosome 5 gene contig |
| ADT17 | NM_025157.3 | Paxillin (PXN) |
| ADT18 | NM_016143.2 | NSFL1 (p97) cofactor (p47) NSFL1C |
| ADT19 | NM_004913.2 | Chromosome 16 gene contig |
| ADT20 | NM_207356.2 | Chromosome 1 gene contig |
| ADT21 | NM_003104.4 | Sorbitol dehydrogenase (SORD) |
| ADT22 | NT_006316.16 | Chromosome 4 gene contig |
| ADT23 | NM_013336.3 | Sec 61 alpha 1 subunit |
| ADT24 | NM_002474.2 | Myosin heavy chain 11 (MYH11) |
| ADT25 | NR_002819.2 | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) |
| ADT26 | NC_000001.10 | Similar to lamanin receptor 1 |
| ADT27 | NT_009952.14 | Chromosome 13 gene contig |
| ADT28 | NM_021239.2 | RNA binding motif protein 25 (RBM 25) |
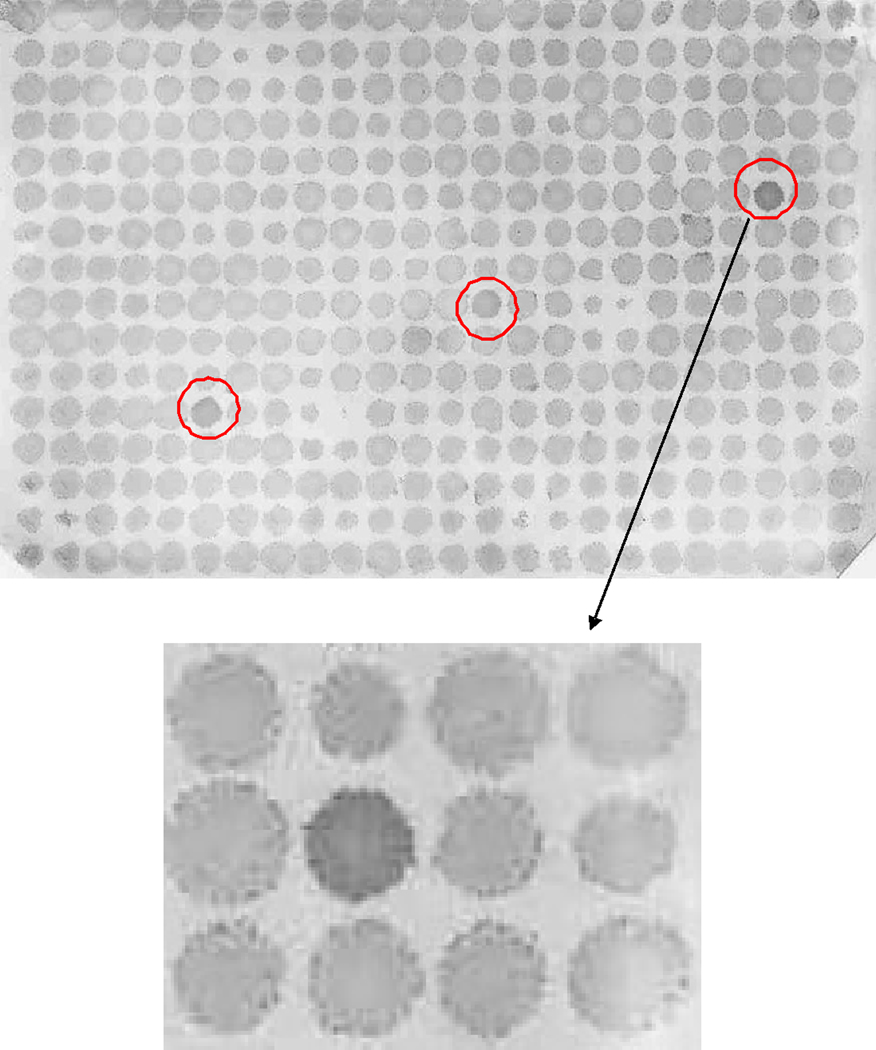
Figure 1. High-throughput immunoblot analysis.
125 unique prostate cancer-associated antigen-encoding phage were spotted in triplicate in a 384-spot array onto bacterial lawns. Expressed proteins were transferred onto a nitrocellulose membrane and probed overnight with patient sera. IgG immune responses were detected using a mouse anti-human IgG antibody and immunoreactivity was quantified by densitometry. Shown is an example of a membrane, and detail, with immunoreactive replicate phage plaques indicated by the circles.
Statistical analyses
Data collected from preliminary studies analyzing patients with castrate-resistant prostate cancer and normal male blood donors were plotted as relative density values representing antibody responses to 125 prostate cancer-associated antigens for individual patients. Median relative density values for individual antigens were calculated in both castrate-resistant prostate cancer samples and normal control samples and the non-parametric Wilcoxon Rank Sum test was used to compare the medians of the two groups. The Benjamini-Hochberg False-Discovery Rate (FDR) method for multiple testing was used to control the type I error (18). The frequencies of IgG responses were compared between populations using Fisher’s exact test. Receiver Operating Curve (ROC) analysis was performed to identify a subset of prostate cancer-associated antigens with the highest predictive value for detecting prostate cancer cases when compared to normal controls. The positive likelihood ratio value was used to quantify the predictive value for individual markers. The proportions of patients with at least one positive IgG response and the proportions of patients with at least three positive IgG responses in the subset of predictive markers were compared between populations by performing logistic regression analysis where population groups were included as factors. Dunnett’s multiple testing procedure was used to compare the proportions between the patient populations and the control (normal) group. All p-values are two-sided, with p<0.05 indicating statistical significant differences. The data analysis was performed using SAS® version 9.2 software (SAS Corp., Cary, NC).
RESULTS
Patients with prostate cancer have higher frequencies of detectable IgG specific for prostate cancer-associated antigens than men without cancer
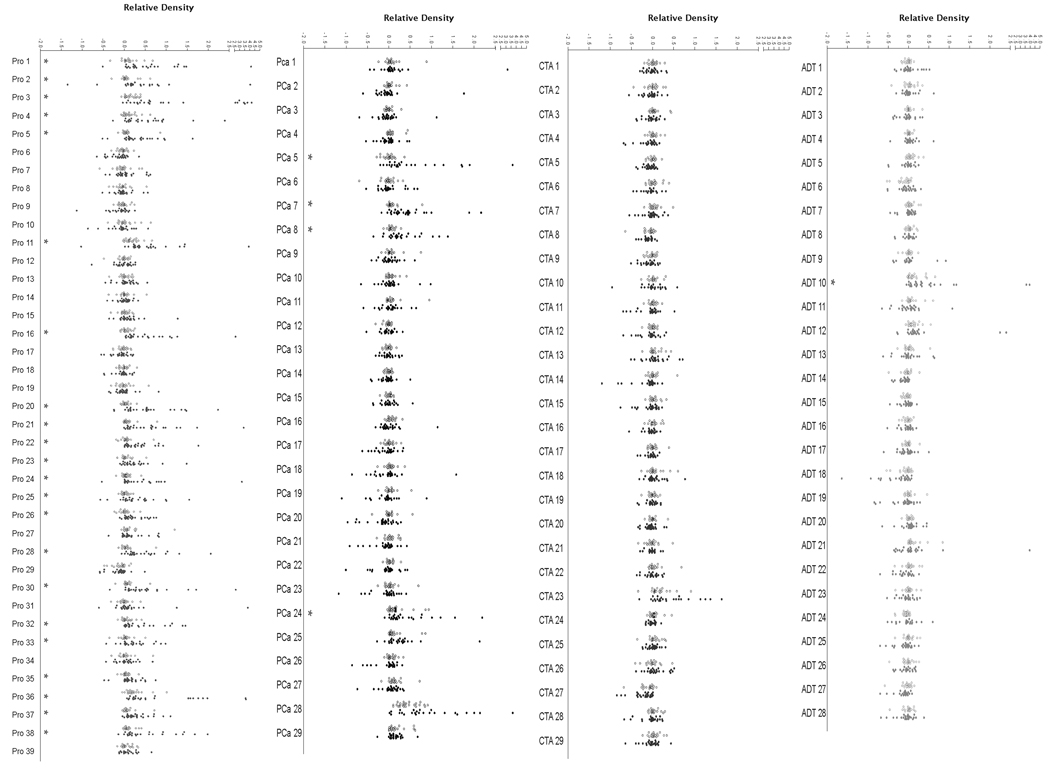
We have previously reported prostate tissue and prostate cancer-associated antigens identified by SEREX analysis using sera from patients with prostatitis (10), patients with prostate cancer (9,13), patients with prostate cancer treated with various immune modulating agents (11,12), or using phage encoding specific cancer-testis antigens (CTA) (16). To determine if specific antigens might be commonly recognized in the sera of patients with prostate cancer, and if a particular set of antigens appeared to be more specifically recognized, phage encoding 125 unique, prostate cancer-associated antigens obtained from these prior studies (Table I) were spotted in replicate onto bacterial lawns, transferred to nitrocellulose membranes, and probed with individual sera samples, as illustrated in Figure 1. IgG specific for phage-encoded plaques were detected, the immunoreactivity quantified by densitometry, and then normalized to internal controls (0 = reactivity equivalent to phage not encoding a protein; 1 = reactivity equivalent to phage encoding human IgG). Using sera from 25 patients with castrate-resistant metastatic prostate cancer or 25 healthy male blood donors, immunoreactivity could be detected to multiple antigens in the patient population (Figure 2). Of the 125 antigens evaluated, median IgG responses, as measured densitometrically, to 27 (22%) were significantly higher in the sera of patients compared with controls (p<0.05, Wilcoxon Rank Sum test, Figure 2). Moreover, of these 27 antigens, 22 were to those previously identified in patient with chronic prostatitis.
Figure 2. Antigens previously identified as prostatitis antigens are commonly recognized by IgG in patients with prostate cancer.
Prostate antigen arrays were screened for IgG immunoreactivity, and mean relative density scores were defined for each antigen using sera from healthy male control blood donors (n=25, open circles) and men with castrate-resistant prostate cancer (n=25, closed circles). Antigens are grouped by the studies from which they were originally identified. Asterisks denote significant higher median reactivity for the cancer population compared to the control population (p < 0.05, Wilcoxon Rank Sum test) and adjusted for multiple testing using the Benjamini-Hochberg FDR method.
IgG responses to prostate-associated antigens are common in patients with early and late stages of prostate cancer, and patients with prostatitis
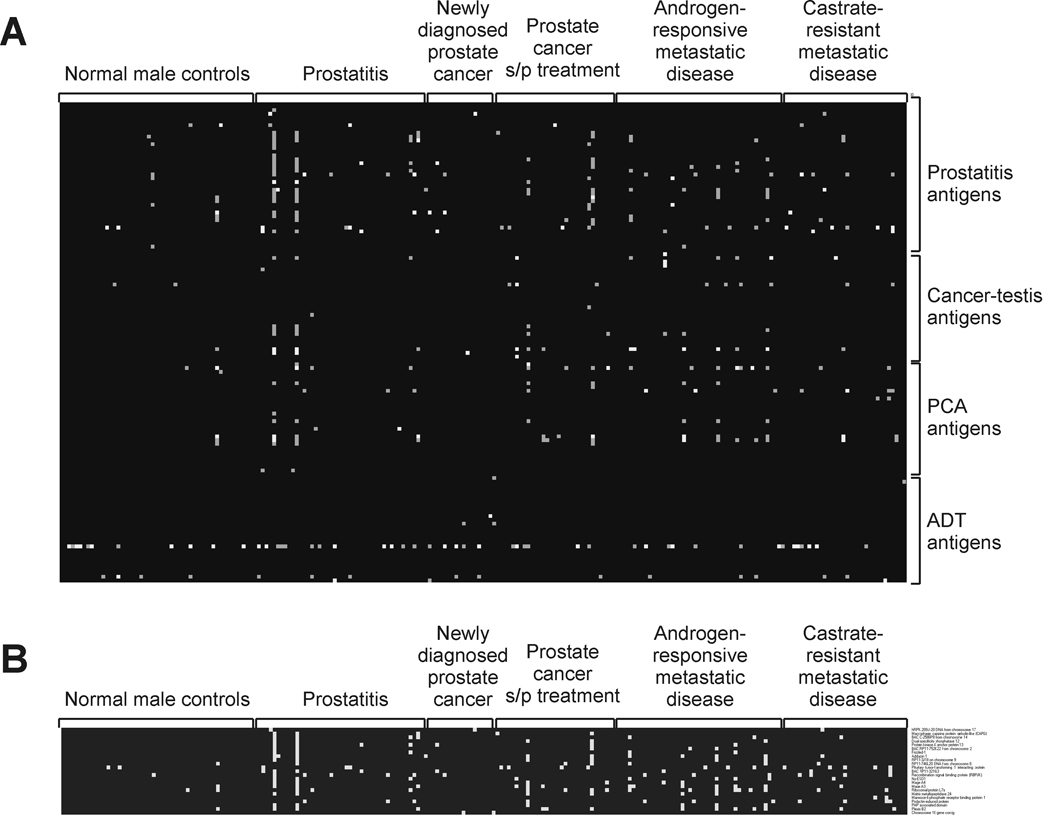
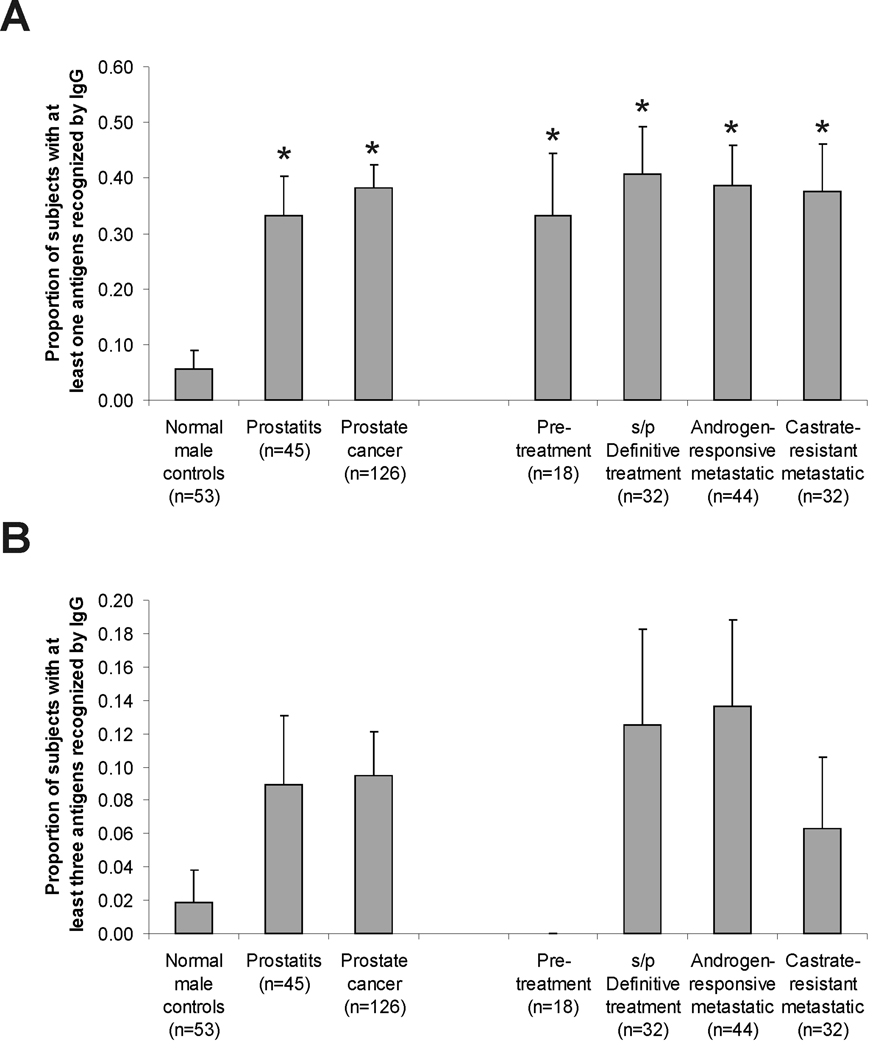
The observation that multiple IgG responses were detectable in patients with advanced prostate cancer, and to prostatitis antigens in particular, suggested that prostate cancer and clinical prostatitis might share common antigenic targets, and further that antibody responses might occur with the development and/or progression of disease. Moreover, the finding that IgG responses were significantly higher in patients with prostate cancer than in men without prostate cancer suggested that a subset of antigens might be identified with predictive value for prostate cancer. In order to test these possibilities, and determine if recognition of particular antigens could distinguish patients with prostate cancer from patients with prostatitis or men without known prostate disease, we conducted a similar analysis using sera from a larger population of healthy male control blood donors (n=53), patients with chronic prostatitis (n=45), and patients with different stages of prostate cancer (n=126). Sera from patients included men with newly diagnosed prostate cancer obtained pre-treatment (n=18), patients with treated disease and no evidence of recurrence (n=32), patients with metastatic disease responsive to androgen deprivation therapy (n=44), and additional patients with castrate-resistant metastatic disease (n=32). In addition, the panel of antigens was modified slightly to exclude two antigens (Pro39 and ADT28) not immunologically recognized in the initial screen, and to include phage encoding additional antigens (Mad-Pro-30, Mad-Pro-34 and the androgen receptor ligand-binding domain) that we had previously identified as immunologically recognized antigens from other studies (10,17). To account for background reactivity of individual membranes and variability in plaque immunoreactivity, visual inspection was used to score each of the replicate individual plaques as immunoreactive or not. As demonstrated in Figure 3A, immunoreactivity could be readily identified to multiple antigens. Overall, however, responses to at least one antigen were not more frequently observed in the patient population (72/126, 57%) or prostatitis population (28/45, 62%) compared with the control population (22/53, 42%; p=0.11 and p=0.08, respectively). No single antigen was identified to which immunoreactivity was significantly more frequent in the patient population than the control population. Instead, a subset of 23 predictive antigens with high (>4) positive likelihood ratio values was identified based on a ROC analysis by comparing IgG responses among prostate cancer cases to normal controls. The list of the 23 antigens is shown in Table II. A subset analysis showed that the proportion of patients with a detectable IgG response to at least one of the 23 antigens was significantly higher in the prostate cancer patient population when compared to the normal control population (Figure 4A), that is, 48/126 (38%) versus 3/53 (6%) (p<0.001). IgG responses were also detectable in the sera from patients with prostatitis (15/45, 33%), significantly higher than the control population (p=0.003), however not significantly different from the patient population (Figure 4A). As shown in Figure 4B, the presence of IgG to three or more of these antigens was more common in patients with prostate cancer (12/126, 10%) and patients with prostatitis (4/45, 9%) relative to male control blood donors (1/53, 2%), however responses to multiple members was not significantly higher in patients with more advanced disease.
Figure 3. IgG responses to prostate-associated antigens are common in patients with early and late stages of prostate cancer.
Immunoblot analysis was conducted as described using sera from additional healthy male control blood donors (n=53), patients with chronic prostatitis (n=45), patients with newly diagnosed prostate cancer pre-treatment (n=18), patients with previously diagnosed prostate cancer without evidence of disease recurrence (n=32), patients with metastatic disease on androgen-deprivation therapy (n=44), and additional patients with castrate-resistant metastatic disease (n=32). Panel A: Shown is a heatmap analysis of the relative immunoreactivity for each antigen, grouped by the study from which they were originally identified, and for each patient or control group. Spots are graded in color intensity from 3 of 3 replicates visually immunoreactive (white), 2 of 3 (grey), to not immunoreactive. Panel B: Subset analysis for the 23 antigens with the highest positive likelihood ratio values when comparing prostate cancer cases to normal control cases.
Table 2. Subset antigen panels.
Shown are the subsets of lambda phage-encoded antigens with highest specificity for prostate cancer based on receiver operating curve analysis (see also Figure 3B). Specifically, antigens with a positive likelihood ratio > 4 (sensitivity / (1-specificity)) are included, and the positive likelihood ratio is shown.
| Antigen subset with highest individual positive likelihood ratio for prostate cancer vs. normal controls | ||
|---|---|---|
| Designation | Positive Likelihood Ratio |
Gene name |
| ADT14 | ∞ | Chromosome 16 gene contig |
| CTA3 | ∞ | NY-ESO-1 |
| CTA23 | ∞ | Mage A4 |
| PCa6 | ∞ | Matrix metallopeptidase 24 |
| PCa8 | ∞ | Prolactin-induced protein |
| PCa10 | ∞ | Mannose-6-phosphate receptor binding protein 1 |
| PCa16 | ∞ | PAP-associated domain |
| Pro5 | ∞ | hRPK.209-J-20 DNA from chromosome 17 |
| Pro10 | ∞ | Macrophage capping protein gelsolin-like (CAPG) |
| Pro12 | ∞ | BAC C-2506P8 from chromosome 14 |
| Pro17 | ∞ | Dual specificity phosphatase 12 |
| Pro18 | ∞ | Protein kinase A anchor protein 13/lymphoid blast crisis oncogene/breast cancer nuclear receptor-binding auxiliary protein (BRX) |
| Pro19 | ∞ | BAC RP11-752K22 from chromosome 2 |
| Pro20 | ∞ | Frizzled-1 |
| Pro24 | ∞ | Adducin 1 |
| Pro25 | ∞ | RP11-3J10 on chromosome 9 |
| Pro35 | ∞ | BAC RP11-321G3 |
| CTA27 | 8.4 | Mage A3 |
| PCa21 | 8.4 | Plexin B2 |
| Pro40 | 6.7 | Recombination signal binding protein (RBPJK) |
| PCa2 | 5.5 | Ribosomal protein L7a |
| Pro34 | 5.4 | Pituitary tumor-transforming 1 interacting protein |
| Pro32 | 4.2 | RP11-746L20 DNA from chromosome 8 |
Figure 4. IgG responses to prostate tissue-associated antigens occurs irrespective of stage of prostate cancer.
Shown is the proportion and standard error of subjects in each population recognizing at least one (panel A) or three (panel B) of the antigens identified in Table II. Asterisks denote higher frequency compared to the normal control population (p < 0.05, computed by logistic regression analysis and Dunnett’s method for multiple comparisons).
IgG responses to prostate-associated antigens were uncommon in sera from male patients with other malignancies
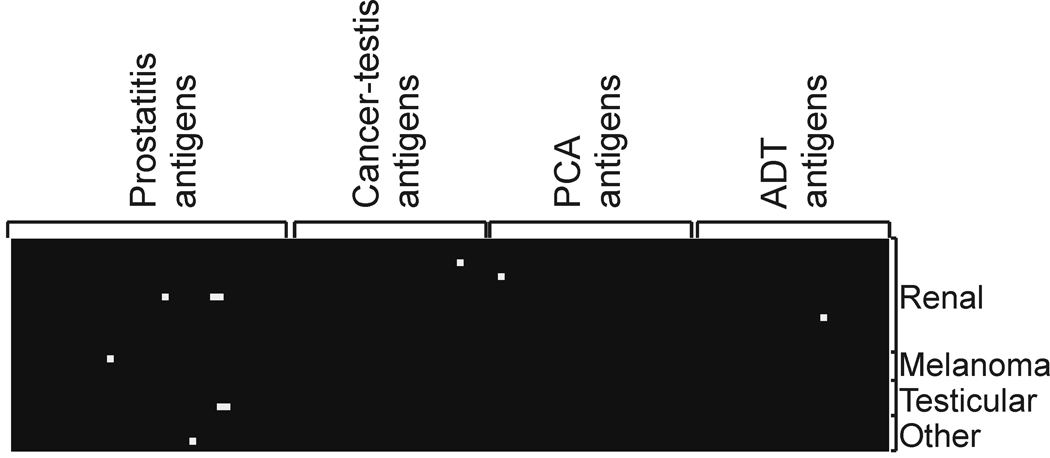
Given that the majority of antigens recognized were not prostate-specific in terms of expression, we reasoned that it was possible that the responses detected were signatures for non-prostate-specific inflammatory conditions. We consequently evaluated IgG responses to this same panel of antigens using sera obtained from male patients (n=31) with other, non-prostate, cancers (renal cell cancer, n=17; testicular cancer, n=5; melanoma, n=4; head and neck cancer, n=1; GI stromal tumor, n=1; bladder cancer, n=1; non-small cell lung cancer, n=1). As shown in Figure 5, while IgG responses were detectable to at least one of the 126 antigens in 7/31 sera samples (23%), only one response in 1/31 sera samples (3%) was detected to one of the 23 prioritized antigens. These were not statistically different in frequency from responses identified in the non-cancer control population. Moreover, the one response identified was to the Pro32 antigen with the lowest positive likelihood ratio (Table II).
Figure 5. IgG responses to prostate-associated antigens are uncommon in sera from male patients with other malignancies.
Immunoblot analysis was conducted as described using sera from an additional 31 male patients with non-prostate cancers (renal cell cancer, n=17; testicular cancer, n=5; melanoma, n=4; head and neck cancer, n=1; GI stromal tumor, n=1; bladder cancer, n=1; non-small cell lung cancer, n=1). Shown is a heatmap analysis of the immunoreactivity for each antigen, grouped by the study from which they were originally identified, and for each patient tumor type. Spots were scored positive (white) if visually immunoreactive in at least 2 of 3 replicates.
DISCUSSION
In the current report, we set out to evaluate serum antibody responses to a panel of prostate tissue- and prostate cancer-associated antigens in order to determine if a subset of these antigens were commonly recognized in patients with prostate cancer, and whether higher numbers of antigens were recognized in patients with more advanced disease. In an initial small screen we identified that immune responses to proteins originally identified as immunological targets in patients with chronic prostatitis were commonly recognized in patients with advanced prostate cancer relative to volunteer male blood donors. This suggested to us that many of these prostatitis antigens represent true immunologically recognized antigens of the prostate, many of which are recognized with the development of prostate cancer. Our subsequent studies specifically evaluated whether patients with prostate cancer of earlier stage, and patients with prostatitis, had IgG responses to these antigens, and whether responses to multiple antigens was more frequent with later stages of disease. Overall we found that immune responses to at least one member of a subset of 23 of these antigens were detectable as frequently in patients with early stage disease as in patients with advanced, metastatic disease. In addition, immune responses were as frequently detected in patients with clinical prostatitis, however were uncommon in sera from male subjects with other types of cancer. Responses to multiple members of this panel were not more frequent in patients with more advanced prostate cancer than patients with prostatitis. This latter observation may be related to the group of antigens prioritized, associated primarily with prostatitis, and not to gene products that might only be expressed in advanced tumors. These findings suggest an association between prostate tissue inflammation and cancer, and at least with this group of antigens, suggest that “antigen spread” does not necessarily occur with increased tumor burden.
Chronic inflammation is highly associated with the development of several solid tumors, notably lung and colorectal cancers. At present, much experimental and epidemiological evidence also suggests that chronic inflammation may similarly drive the development of prostate cancers (19–22). Tumor-infiltrating lymphocytes are commonly seen in prostate tumor specimens, and chronic inflammatory cells are commonly seen adjacent to the earliest premalignant lesions of the prostate (23). Moreover, in rodent models chronic prostatitis appears to drive the development of prostate tumors (24). Viral and bacterial pathogens have been cited as possible etiologic agents for human prostate inflammation and cancer, however a definitive causal link has not been established (25). Human prostate-infiltrating lymphocytes, obtained at the time of surgery, have been demonstrated to be oligoclonal, suggesting these lymphocytes may recognize tissue-specific antigens (26,27). Our current findings, while not suggesting causality, suggest an association between chronic inflammatory conditions such as prostatitis and prostate cancer, or at least that common targets of a prostate-associated immune response can be recognized in these disorders. Our findings further suggest that there may be a subset of common antigens expressed by the prostate, recognition of which can be shared by multiple individuals. While the detection of these IgG responses itself does not appear to be highly sensitive or specific as a test to distinguish prostate cancer from patients with prostatitis, the evaluation of IgG responses to prostate-associated antigens could potentially identify patients in a premalignant inflammatory state at risk for developing prostate cancer. We plan to test this hypothesis using sera obtained from patients without a history of prostatitis but who developed prostate cancer within several years, suggesting this could be developed as a test to identify subjects in a premalignant stage at risk for developing prostate cancer.
Other groups have sought to identify antibody responses to prostate tissue antigens as a means of prostate cancer detection. In reports by Wang and Bradford, the investigators identified autoantibodies specific for peptides expressed by prostate cancer tissue using phage-encoded epitope screening (15,28). They reported that the specificity and sensitivity for prostate cancer diagnosis exceeded that obtained with standard serum PSA screening using phage-encoded peptides derived from these screens. In separate reports, this same investigator group has evaluated antibody responses to individual proteins, including huntingtin-interacting protein 1 (HIP1) (29) and alpha-methylacyl-CoA racemase (AMACR) (30), and demonstrated that antibody responses detectable to these proteins are highly specific for prostate cancer. Our findings support the findings of these groups, and add further feasibility to the general field of antibody-based diagnostics. However, given that our current panel of antigens was chosen from prior studies, the prioritized antigens identified here are not entirely prostate-specific in terms of expression. Notwithstanding, their recognition appears to be biased towards patients with prostate disease, given that they were not similarly recognized in sera from patients with other malignancies. This is not entirely unexpected, as antibodies to ubiquitously expressed antigens are common in patients with autoimmune disorders such as systemic lupus erythematosis (31). If this panel of antigens were to be developed as a diagnostic test for prostate cancer, future studies would explore the frequency of recognition of these antigens using larger numbers of sera from patients with other malignancies.
Our demonstration of antibody responses to antigens identified as prostatitis antigens, and other antigens similarly recognized by patients with prostatitis, suggests that IgG responses to many antigens might develop very early in the transformation process. In addition, most groups assessing immune responses to prostate-associated antigens have used control samples from normal male control blood donors or patients with benign prostatic hypertrophy. Our findings suggest that sera from patients with prostatitis should be considered in these studies as well, since antibody responses in this nonmalignant population are detectable, including responses to cancer-testis antigens that would be predicted to be tumor specific. In any case, the association of immune responses to defined antigens with a premalignant inflammatory state might be useful for identifying patients at risk for developing cancer, potentially well before an elevation of a serum protein such as PSA could be detected. If this is true, this concept of identifying antigens recognized in inflammatory tissue states could have application to the other malignancies associated with chronic inflammation for which serum-based tests are not available.
Advantages of the phage immunoblot approach we describe here are the ease of transfection and protein expression in bacteria, particularly for novel proteins for which there are no available reagents. In addition, the ability to simultaneously evaluate IgG responses to multiple proteins at once from a particular serum sample is an advantage over traditional ELISA. However, the sensitivity of this approach is lower than ELISA, and in fact we did not detect IgG responses to antigens such as PSA or AR LBD in individual sera samples in which we could identify low level IgG by ELISA (data not shown). In addition, background reactivity to E. coli produced regional variations on some membranes that made densitometry evaluation difficult. For defining individual antigen reactivity as strictly “positive” or “negative” we relied on visual interpretation of replicates which significantly adds to the time and potential subjectivity of the interpretation. Notwithstanding, we believe this method provides a robust means to prioritize antigens for which more sensitive methods could be developed.
Finally, the identification of antibody responses to multiple prostate-associated proteins provides us a potential tool for the evaluation of immune responses to the prostate. For example, in addition to antigen-specific immunotherapies, many new immune-based therapies have entered clinical testing for which there is not a defined target antigen. In the case of prostate cancer, agents such as cell-based vaccines and T-cell checkpoint inhibitors have shown evidence of anti-tumor effect in clinical trials (32–34). A challenge in the development of these therapies, however, has been the absence of biomarkers for tumor-specific immunological effect that are associated with clinical responses. Studies with anti-CTLA-4 monoclonal antibodies, in particular, have sought to identify whether amplification of other T-cell co-stimulatory molecules (35), or antibodies to defined antigenic tumor-associated proteins (33,36), might be useful as biomarkers. For whole cell tumor vaccines where there is not a specific defined antigen being targeted, surrogate antigens known to be expressed by the tumor vaccine have been used as a means of monitoring immune responses from the vaccine (37). The use of immunologically-recognized surrogate antigens, including HER-2/neu, MUC1 and p53, has been possible in the case of breast cancer where IgG responses to these antigens have been identified. In the case of prostate cancer, however, there has not been a defined panel of commonly recognized antigenic proteins. Most of the antigens identified in this report are not specifically expressed in prostate tissue, and the cancer-testis antigens identified here are recognized in sera from patients with different malignancies. Nonetheless, the antigens identified might still serve as a panel of gene products commonly associated with prostate inflammation. Future studies will explore whether IgG responses to other members of this panel are similarly recognized in other malignancies or other inflammatory conditions. As new immune-based and immune-modulating agents are developed, it will be important to establish tools able to define whether immune responses to specific tumors are elicited. The ability to rapidly evaluate antibody responses to a panel of commonly recognized antigens, such as we report here, before or after treatment could be useful in these determinations.
Acknowledgements
This work was supported by NIH (K23 RR16489), and by the US Army Medical Research and Materiel Command Prostate Cancer Research Program (W81XWH-06-1-0184). We thank Eli Caldwell and Danielle Willborn-Johnson for technical assistance.
References
- 1.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 2.Jäger E, Chen YT, Drijfhout JW, Karbach J, Ringhoffer M, Jager D, Arand M, Wada H, Noguchi Y, Stockert E, Old LJ, Knuth A. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998;187(2):265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci U S A. 1995;92(25):11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jongeneel V. Towards a cancer immunome database. Cancer Immun. 2001;1:3. [PubMed] [Google Scholar]
- 5.De Groot AS. Immunome-derived vaccines. Expert opinion on biological therapy. 2004;4(6):767–772. doi: 10.1517/14712598.4.6.767. [DOI] [PubMed] [Google Scholar]
- 6.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunphy EJ, Johnson LE, Olson BM, Frye TP, McNeel DG. New approaches to identification of antigenic candidates for future prostate cancer immunotherapy. Update Canc Ther. 2006;22:273–284. [Google Scholar]
- 8.Fossa A, Siebert R, Aasheim HC, Maelandsmo GM, Berner A, Fossa SD, Paus E, Smeland EB, Gaudernack G. Identification of nucleolar protein No55 as a tumour-associated autoantigen in patients with prostate cancer. Br J Cancer. 2000;83(6):743–749. doi: 10.1054/bjoc.2000.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mooney CJ, Dunphy EJ, Stone B, McNeel DG. Identification of autoantibodies elicited in a patient with prostate cancer presenting as dermatomyositis. Int J Urol. 2006;13(3):211–217. doi: 10.1111/j.1442-2042.2006.01263.x. [DOI] [PubMed] [Google Scholar]
- 10.Dunphy EJ, Eickhoff JC, Muller CH, Berger RE, McNeel DG. Identification of antigen-specific IgG in sera from patients with chronic prostatitis. J Clin Immunol. 2004;24(5):492–501. doi: 10.1023/B:JOCI.0000040920.96065.5a. [DOI] [PubMed] [Google Scholar]
- 11.Dunphy EJ, McNeel DG. Antigen-specific IgG elicited in subjects with prostate cancer treated with flt3 ligand. J Immunother. 2005;28(3):268–275. doi: 10.1097/01.cji.0000158853.15664.0c. [DOI] [PubMed] [Google Scholar]
- 12.Morse MD, McNeel DG. Prostate Cancer Patients Treated with Androgen Deprivation Therapy Develop Persistent Changes in Adaptive Immune Responses. Human immunology. 2010 doi: 10.1016/j.humimm.2010.02.007. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoeppner LH, Dubovsky JA, Dunphy EJ, McNeel DG. Humoral immune responses to testis antigens in sera from patients with prostate cancer. Cancer Immun. 2006;6:1–7. [PubMed] [Google Scholar]
- 14.Dubovsky JA, McNeel DG. Inducible expression of a prostate cancer-testis antigen, SSX-2, following treatment with a DNA methylation inhibitor. Prostate. 2007;67(16):1781–1790. doi: 10.1002/pros.20665. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Yu J, Sreekumar A, Varambally S, Shen R, Giacherio D, Mehra R, Montie JE, Pienta KJ, Sanda MG, Kantoff PW, Rubin MA, Wei JT, Ghosh D, Chinnaiyan AM. Autoantibody signatures in prostate cancer. N Engl J Med. 2005;353(12):1224–1235. doi: 10.1056/NEJMoa051931. [DOI] [PubMed] [Google Scholar]
- 16.Dubovsky JA, Albertini MR, McNeel DG. MAD-CT-2 identified as a novel melanoma cancer-testis antigen using phage immunoblot analysis. J Immunother. 2007;30(7):675–683. doi: 10.1097/CJI.0b013e3180de4d19. [DOI] [PubMed] [Google Scholar]
- 17.Olson BM, McNeel DG. Antibody and T-cell responses specific for the androgen receptor in patients with prostate cancer. Prostate. 2007;67(16):1729–1739. doi: 10.1002/pros.20652. [DOI] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Stat Soc, Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 19.Palapattu GS, Sutcliffe S, Bastian PJ, Platz EA, De Marzo AM, Isaacs WB, Nelson WG. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2005;26(7):1170–1181. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- 20.Narayanan NK, Nargi D, Horton L, Reddy BS, Bosland MC, Narayanan BA. Inflammatory processes of prostate tissue microenvironment drive rat prostate carcinogenesis: preventive effects of celecoxib. Prostate. 2009;69(2):133–141. doi: 10.1002/pros.20862. [DOI] [PubMed] [Google Scholar]
- 21.McDowell KL, Begley LA, Mor-Vaknin N, Markovitz DM, Macoska JA. Leukocytic promotion of prostate cellular proliferation. Prostate. 2009 doi: 10.1002/pros.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60(1):78–83. doi: 10.1016/s0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 23.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155(6):1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilardoni MB, Rabinovich GA, Oviedo M, Depiante-Depaoli M. Prostate cancer induction in autoimmune rats and modulation of T cell apoptosis. J Exp Clin Cancer Res. 1999;18(4):493–504. [PubMed] [Google Scholar]
- 25.Schlaberg R, Choe DJ, Brown KR, Thaker HM, Singh IR. XMRV is present in malignant prostatic epithelium and is associated with prostate cancer, especially high-grade tumors. Proc Natl Acad Sci U S A. 2009;106(38):16351–16356. doi: 10.1073/pnas.0906922106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, Ellis TM, Wojcik EM, Yang D, Flanigan RC, Waters WB, Kast WM, Kwon ED. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98(25):14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ Prostate. 2009;69(15):1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradford TJ, Wang X, Chinnaiyan AM. Cancer immunomics: using autoantibody signatures in the early detection of prostate cancer. Urol Oncol. 2006;24(3):237–242. doi: 10.1016/j.urolonc.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 29.Bradley SV, Oravecz-Wilson KI, Bougeard G, Mizukami I, Li L, Munaco AJ, Sreekumar A, Corradetti MN, Chinnaiyan AM, Sanda MG, Ross TS. Serum antibodies to huntingtin interacting protein-1: a new blood test for prostate cancer. Cancer Res. 2005;65(10):4126–4133. doi: 10.1158/0008-5472.CAN-04-4658. [DOI] [PubMed] [Google Scholar]
- 30.Sreekumar A, Laxman B, Rhodes DR, Bhagavathula S, Harwood J, Giacherio D, Ghosh D, Sanda MG, Rubin MA, Chinnaiyan AM. Humoral immune response to alpha-methylacyl-CoA racemase and prostate cancer. J Natl Cancer Inst. 2004;96(11):834–843. doi: 10.1093/jnci/djh145. [DOI] [PubMed] [Google Scholar]
- 31.Szalat R, Ghillani-Dalbin P, Jallouli M, Amoura Z, Musset L, Cacoub P, Sene D. Anti-NuMA1 and anti-NuMA2 (anti-HsEg5) antibodies: Clinical and immunological features: A propos of 40 new cases and review of the literature. Autoimmunity reviews. 2010 doi: 10.1016/j.autrev.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Small EJ, Sacks N, Nemunaitis J, Urba WJ, Dula E, Centeno AS, Nelson WG, Ando D, Howard C, Borellini F, Nguyen M, Hege K, Simons JW. Granulocyte macrophage colony-stimulating factor--secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res. 2007;13(13):3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 33.Fong L, Kwek SS, O'Brien S, Kavanagh B, McNeel DG, Weinberg V, Lin AM, Rosenberg J, Ryan CJ, Rini BI, Small EJ. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69(2):609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PubMed] [Google Scholar]
- 34.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13(6):1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Liakou CI, Kamat A, Pettaway C, Ward JF, Tang DN, Sun J, Jungbluth AA, Troncoso P, Logothetis C, Sharma P. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc Natl Acad Sci U S A. 2009;106(8):2729–2734. doi: 10.1073/pnas.0813175106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, Ku GY, Jungbluth AA, Segal NH, Rasalan TS, Manukian G, Xu Y, Roman RA, Terzulli SL, Heywood M, Pogoriler E, Ritter G, Old LJ, Allison JP, Wolchok JD. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105(51):20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dols A, Meijer SL, Hu HM, Goodell V, Disis ML, Von Mensdorff-Pouilly S, Verheijen R, Alvord WG, Smith JW, 2nd, Urba WJ, Fox BA. Identification of tumor-specific antibodies in patients with breast cancer vaccinated with gene-modified allogeneic tumor cells. J Immunother. 2003;26(2):163–170. doi: 10.1097/00002371-200303000-00009. [DOI] [PubMed] [Google Scholar]