Amyotrophic lateral sclerosis (ALS), the most common adult-onset motor neuron disease (MND), is characterized clinically by progressive motor weakness leading to death within one to five years and neuropathologically by loss of upper and lower motor neurons, corticospinal tract degeneration, gliosis, and ubiquitinated neuronal inclusions in the spinal cord and cerebrum [1,2,3,4]. ALS is largely a sporadic disease but about 10% of cases show an autosomal dominant pattern of inheritance and of these about 20% have mutations in the Cu/Zn superoxide dismutase-1 (SOD1) gene [5,6]. Recently, mutations in two DNA/RNA-binding proteins called TDP-43 (TAR DNA-binding protein of 43 kDa encoded by the TARDBP gene) [7,8,9,10] and FUS (encoded by the fused in sarcoma (FUS) gene) were reported as causes of (~10%) familial and (~2%) sporadic forms of ALS [11,12]. Together, frontotemporal lobar degeneration (FTLD) and ALS form a spectrum of disorders linked by a common molecular pathology called TDP-43 proteinopathy [13,14]. In cases with combined MND and FTLD, TDP-43-immunoreactive inclusions are found in the spinal cord, brainstem, limbic areas and frontal and temporal lobes. The selective vulnerability of different regions of the neuraxis helps to explain the variability in clinical phenotype associated with TDP-43 proteinopathy and other proteinopathies. Although most TARDBP mutations have been reported in autosomal dominant ALS, a few cases have included both FTLD-TDP and ALS [15,16]. In only a few cases of ALS/FTLD with a pathogenic TARDBP mutation has neuropathological confirmation of TDP-43 proteinopathy been demonstrated [17,18,19,20,21]; thus, the contribution of any one mutation to a particular phenotype remains uncertain.
We report the neuropathology of one patient with a family history of MND and a TARDBP A315T mutation. The clinical history and molecular genetics of this family have been reported previously [7]. The procedures employed were approved by the Washington University School of Medicine Human Studies Committee. Family members older than 18 years were included after informed consent was obtained. All individuals from the ALS kindred were interviewed by an experienced research clinician or nurse, using a modified version of the Family History Interview developed by the Consortium to Establish a Registry for Alzheimer’s Disease. For individuals with neurological symptoms, the visit included a clinical and neurological examination and a consensus history derived from the individual and/or nearest relatives. When possible, available medical records were also analyzed.
A full autopsy was performed. The left hemi-brain and spinal cord were fixed in formalin for two weeks, sliced, examined macroscopically, and prepared for histology. Paraffin wax sections were cut at 6 μm, as previously described [22]; the right cerebrum and portions of the spinal cord were sliced unfixed (1 cm thickness), snap frozen, and stored at −80°C. Histological stains included: haematoxylin and eosin, Luxol fast blue-haematoxylin with periodic acid-Schiff, and a modified Bielschowsky silver impregnation. Immunohistochemistry (IHC) was performed using the following antibodies: anti-ubiquitin (1:1,000, rabbit polyclonal antibody; DAKO, Glostrup, Denmark), anti-TDP-43 (1:4,000, rabbit polyclonal antibody; ProteinTech Inc, Chicago, Illinois), anti-phosphorylated TDP-43 (1:40,000, rabbit polyclonal antibody; pTDP-43; Cosmo Bio Inc., Carlsbad, CA), anti-phosphorylated tau (1:2,000 PHF-1, mouse monoclonal antibody, kindly supplied by Dr Peter Davies, Albert Einstein Medical School, Bronx, NY), anti-α-synuclein (1:500, mouse monoclonal antibody LB-509; Zymed, San Francisco, CA), anti-phosphorylated neurofilament H (1:10,000, mouse monoclonal antibody SMI31; Sternberger Monoclonals Inc., Maryland, USA) and anti-Aβ (10D5, 1:2,000 mouse monoclonal antibody, Elan Pharmaceuticals, San Francisco, CA).
For fine structural studies, frontal lobe neocortex and anterior horn of spinal cord were fixed overnight at 4 °C in modified Karnovsky’s fixative containing 3% glutaraldehyde and 1% paraformaldehyde in sodium cacodylate buffer pH 7.4, postfixed in phosphate cacodylate-buffered 21% OsO4 for 1 h, dehydrated in graded ethanols with a final dehydration in propylene oxide and embedded in EMbed-812 (EMS, Hatfield, PA). One μm thick plastic sections were examined by light microscopy after staining with toluidine blue. Ultrathin sections (90 nm thick) were cut onto formvar coated grids. Sections were post-stained with uranyl acetate and Venable’s lead citrate and viewed with a JEOL model 1200EX electron microscope (JEOL, Tokyo, Japan).
Exon 6 of TARDBP was sequenced from high molecular weight DNA that had been extracted from brain tissue and amplified using gene specific intronic primers. Direct sequencing was performed using Big Dye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Wellesley, MA) and standard protocols (primer sequence available on request). Reactions were run on an ABI3100 and mutation analysis was performed using Sequencher software v4.6 (Gene Codes Corporation, Ann Arbor, MI, USA). The mutation was confirmed only if it was observed in both forward and reverse sequence reads. In addition, the missense mutation (G>A) inserts a unique restriction site, Rsa I, that allows a rapid screen independent of sequencing.
The patient (subject II-4; [7]) developed right leg weakness at age 83, which progressed to involve both legs requiring wheelchair dependence within two years. Mental status, cranial nerves, sensory examination, reflexes, co-ordination and gait were normal at initial examination; upper motor neuron findings were absent. Motor and sensory nerve conduction were normal, but electromyography (EMG) showed denervation in the arms both proximally and distally, with fasciculation potentials in the legs, and occasional large motor unit potentials. Magnetic resonance imaging (MRI) of brain and spinal cord were normal, as was blood work including absent anti-GM1 antibodies; SOD1 gene testing was normal. Asymmetric arm weakness and respiratory weakness developed at age 85. At age 86 there was severe atrophy and weakness in the lower and upper extremities with widespread fasciculations, but mental status, cranial nerve function and sensation remained normal.
The cause of death was bronchopneumonia consequent to amyotrophic lateral sclerosis. Direct sequencing of brain-derived DNA confirmed the presence of the heterozygous missense mutation Ala-315-Thr (A315T) in the TARDBP gene. The fresh brain weighed 1,160 g. Examination of the cerebral arteries and the circle of Willis revealed mild patchy atherosclerosis. The cranial nerves were unremarkable. There was mild cortical atrophy, but the precentral gyrus was of normal size and proportion. Coronal slicing revealed mild dilatation of the lateral ventricles. The hippocampi appeared unremarkable. The brainstem and cerebellum were of normal size and proportion. There substantia nigra and locus coeruleus were a little depigmented. The anterior roots of the spinal cord appeared thinned.
Microscopy of the brain and spinal cord revealed diagnostic features of MND (Figure 1), accompanied by modest Alzheimer’s disease changes and mild to moderate small vessel disease (Table 1). AD changes were most pronounced in limbic areas. The cerebral cortex showed moderate neuronal loss and gliosis only in the entorhinal cortex; elsewhere, including the motor neurons of the precentral gyrus, losses were minimal. Diffuse plaques were frequent and widespread in the cortex, but neurofibrillary tangles and neuritic plaques occurred in significant numbers only in the basal forebrain, hippocampal formation, amygdala, entorhinal cortex, temporal lobe and insula. These features were consistent with stage III in the Braak and Braak staging of tangles and amyloid stage C [23,24]. Cerebral amyloid angiopathy (CAA) was mild to moderate.
Figure 1.
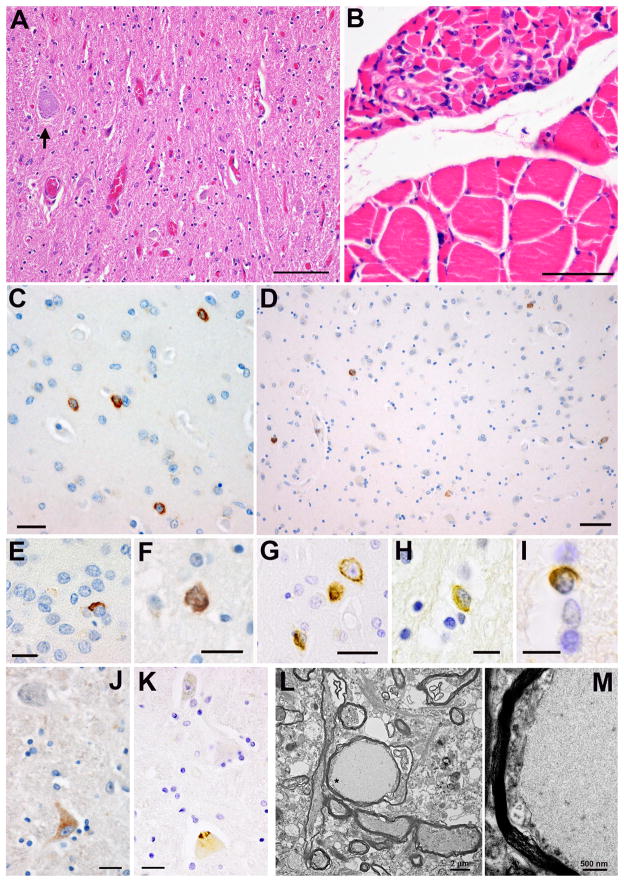
Neuropathology of familial MND with TARDBP A315T mutation. Severe neuronal loss and gliosis in the anterior horn of the spinal cord (A). Haematoxylin and eosin; bar = 100 μm. Grouped muscle fiber atrophy in the quadriceps muscle, with small caliber fibers, widespread syncytial knots, and angulated fibers (B). Fatty replacement and subtly vacuolated fibers were also noted (not shown). Haematoxylin and eosin; bar = 50 μm. Neuronal and glial TDP-43-immunoreactive cytoplasmic inclusions in the cerebrum (C–I). NCI in the putamen (C); bar = 25 μm. NCI in the amygdala (D); bar = 25 μm. NCI in the dentate gyrus (E); bar = 20 μm. NCI in the parahippocampal gyrus (F); bar = 20 μm. NCI in the subiculum (G); bar = 20 μm. TDP-43-immunoreactive inclusions in an astrocyte (H); bar = 10 μm and an oligodendrocyte in the spinal cord (I); bar = 10 μm. (C–I) Immunohistochemistry with anti-phosphorylated TDP-43 antibodies. TDP-43-immunoreactive neuronal cytoplasmic inclusions are present in motor neurons: a TDP-43-immunoreactive pre-inclusion in the cytoplasm of a motor neuron in the hypoglossal nucleus (J) and a compact and skein-like NCI in a single motor neuron of the anterior horn (K). Immunohistochemistry with anti-phosphorylated TDP-43 antibodies. Bars = 25μm. Fine structure of neuroaxonal swellings in the spinal cord (L, M). Low power electron micrograph showing a round inclusion in an axon (L); bar = 2 μm. (M) A high power image of the area marked in (L) with * shows a homogeneous fibrillary matrix with eccentric and displaced organelles; bar = 500 nm.
Table 1.
Pathological findings in familial MND with TARDBP A315T mutation
| Regions | Neuron loss/gliosis | Molecular pathology | ||||||
|---|---|---|---|---|---|---|---|---|
| pTDP | Aβ | pTau | ||||||
| NCI | DP | CP | CAA | NFT | NP | |||
| Cerebral cortex | ||||||||
| Frontal lobe | Midfrontal gyrus | + | 0 | +++ | + | ++ | + | + |
| Cingulate gyrus | + | + | +++ | ++ | + | + | + | |
| Precentral gyrus | + | 0 | + | + | ++ | 0 | 0 | |
| Insula gyrus | + | + | +++ | + | 0 | ++ | ++ | |
| Temporal lobe | Superior temporal gyrus | + | 0 | ++ | + | ++ | ++ | 0 |
| Parietal lobe | Angular gyrus | + | 0 | +++ | + | ++ | + | + |
| Occipital lobe | Calcarine sulcus | + | 0 | ++ | + | +++ | 0 | 0 |
| Limbic areas | ||||||||
| Amygdala | Amygdala | + | + | ++ | + | + | +++ | + |
| Entorhinal cortex | ++ | + | ++ | + | + | +++ | + | |
| Hippocampus | Dentate fascia | 0 | + | + | 0 | + | + | 0 |
| Cornu ammonis (CA1) | + | + | ++ | 0 | + | +++ | + | |
| Parahippocampal gyrus | ++ | + | +++ | ++ | + | +++ | ++ | |
| Subcortical areas | ||||||||
| Anterior olfactory nucleus | + | + | +++ | + | 0 | ++ | ++ | |
| Nucleus basalis of Meynert | + | + | ++ | + | + | ++ | + | |
| Striatum | + | + | ++ | + | 0 | + | 0 | |
| Globus pallidus | + | 0 | 0 | 0 | 0 | + | 0 | |
| Thalamus | + | + | 0 | 0 | 0 | + | 0 | |
| Subthalamic nucleus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Midbrain | ||||||||
| Midbrain tectum | + | 0 | 0 | 0 | 0 | + | 0 | |
| Occulomotor nucleus | + | 0 | 0 | 0 | 0 | + | 0 | |
| Red nucleus | + | 0 | 0 | 0 | 0 | 0 | 0 | |
| Substantia nigra | + | 0 | 0 | 0 | 0 | + | 0 | |
| Pons | ||||||||
| Locus coeruleus | + | 0 | 0 | 0 | 0 | + | 0 | |
| Reticular formation | + | 0 | 0 | 0 | 0 | + | 0 | |
| Pontine nuclei | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Medulla oblongata | ||||||||
| Hypoglossal nucleus | + | + | 0 | 0 | 0 | + | 0 | |
| Dorsal vagal nucleus | + | 0 | 0 | 0 | 0 | 0 | 0 | |
| Reticular formation | 0 | + | 0 | 0 | 0 | + | 0 | |
| Inferior olivary nucleus | + | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cerebellum | ||||||||
| Purkinje cells | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Granule cells | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Dentate nucleus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Spinal cord | ||||||||
| Cervical segment | +++ | + | 0 | + | 0 | 0 | 0 | |
| Thoracic segment | +++ | + | 0 | 0 | 0 | 0 | 0 | |
| Lumbar segment | +++ | + | 0 | 0 | 0 | 0 | 0 | |
| Sacral segment | +++ | + | 0 | 0 | 0 | 0 | 0 | |
pTDP, phosphorylated TDP-43; Aβ, β-amyloid parenchymal deposits; pTau, phosphorylated tau protein. Loss of neurons: 0, none; +, mild; ++, moderate; +++, severe. The numbers of TDP-43-immunoreactive neuronal cytoplasmic inclusions (NCIs), Aβ deposits (DP, diffuse plaques; CP, cored plaques; and cerebral amyloid angiopathy (CAA)), and pTau (NFT, neurofibrillary tangles and NP, neuritic plaques) were assessed using a semi-quantitative rating scale: 0, none; +, sparse; ++, moderate; +++, numerous.
TDP-43 pathology was largely restricted to limbic areas, the deep gray nuclei, the medulla, and the spinal cord (Figure 1C to K). A few TDP-43-positive inclusions were identified in the insula and the anterior cingulate gyrus; a few neurons in the latter structure showed more diffuse staining, possibly indicating a pre-inclusion stage. The amygdala and entorhinal cortex showed more pronounced TDP-43 pathology. TDP-43-immunoreactive neuronal cytoplasmic inclusions (NCIs) with pre-inclusion, compact, and skein-like morphology were identified in both structures, and occasional TDP-43-immunoreactive glial cells were noted in the amygdala (Figure 1C to K). TDP-43-immunoreactive NCIs were sparse in the dentate fascia, hippocampus and parahippocampal gyrus. Isolated TDP-43 immunoreactive NCI were present in the putamen and thalamus. In the medulla, the hypoglossal nucleus showed some neuronal loss, and rare motor neurons exhibited TDP-43-immunoreactivity in a diffuse cytoplasmic pattern, or in compact and skein-like inclusions (Figure 1J and K). A TDP-43-positive oligodendroglial inclusion was also identified (Figure 1I), but no Bunina bodies were seen. The spinal cord showed severe loss of neurons and gliosis in multiple levels (Figure 1A). Sparse ubiquitin- or TDP-43-immunoreactive inclusions were present in the few surviving motor neurons. Pallor and axonal loss in the corticospinal tracts were not readily appreciated.
Sections of the quadriceps muscle (Figure 1B) showed grouped muscle fibre atrophy, characterized by small calibre, widespread syncytial knots, angulated fibres, fatty replacement, subtly vacuolated fibres, and scattered fine intracellular yellow-brown pigment. No inflammation, regenerating fibres, or significant fibrosis were noted. Sections of the gastrocnemius muscle showed similar changes. Peripheral organs were generally unremarkable except that the lung showed evidence of acute bronchopneumonia and chronic inflammation with foreign body giant cell reaction.
Ultrastructural examination of the anterior horn revealed rounded structures (Figure 1L and M), most about 5–10 μm in cross section, composed of a fibrillary matrix. Some of these structures appeared to be extracellular; others appeared within a subset of myelinated cell processes, surrounded by densely packed organelles in the scanty, peripherally displaced axoplasm. These structures were morphologically different from spheroids commonly seen in degenerating corticospinal tracts. Analogous structures apparently visible on routine paraffin sections of the spinal cord did not show immunoreactivity with any of the antibodies applied in this case. The nature and significance of these structures, and their relevance to the patient’s disease process are unclear.
In summary, microscopy revealed the stigmata of motor neuron disease: lower motor neuron loss. TDP-43-immunoreactive inclusions, of varying forms, were seen in only a few of the surviving motor neurons. The lower motor neurons, particularly of the spinal cord, were severely involved; the upper motor neurons were relatively spared. In addition, TDP-43-immunoreactive neuronal and glial inclusions were seen in varying numbers in neocortical, archicortical, and subcortical nuclei (Table 1). We were unable to show cleavage of TDP-43 by immunoblotting, possibly as a consequence of the low number of inclusions in surviving neurons (data not shown). Microscopy of representative neocortical areas also showed Alzheimer’s disease changes consistent with stage III in the Braak and Braak staging of tangles and amyloid stage C [23,24], and small vessel disease (cerebral amyloid angiopathy and arteriolosclerosis), but these changes were modest and unlikely to have contributed to the clinical symptoms. In conclusion, the patient’s motor problems can be explained by one neurodegenerative disease: motor neuron disease with TARDBP A315T mutation.
A missense mutation, at a highly conserved residue within TARDBP gene in one member of a kindred multiply affected with MND, resulted in late-onset MND. TDP-43-immunoreactive inclusions were found in the spinal cord and at several sites in the neuraxis indicating widespread expression of the pathological protein, comparable to the widespread distribution of inclusions reported with other mutations [17,19]. The selective vulnerability of spinal cord motor neurons to TDP-43 mutations remains to be elucidated but may share mechanisms of neurodegeneration with other MND-causing genes including SOD1 and FUS. Features of MND with TARDBP A315T mutation have recently been recapitulated in a transgenic mouse model which also showed pathology in the spinal cord and cerebrum [25,26]. This and other models will facilitate the elucidation of the mechanisms of neurodegeneration and the selective vulnerability of different populations of neurons.
Acknowledgments
We thank the clinical, genetic, neuropathology and technical staff for making information and tissue samples available for this study and we thank the family whose generosity made this research possible. Support for this work was provided by grants from the National Institute on Aging of the National Institutes of Health (P50-AG05681, P01-AG003991), the Hope Center for Neurological Disorders, the Buchanan Fund, the Charles F. & Joanne Knight Alzheimer Research Initiative, the McDonnell Center for Molecular and Cellular Neurobiology, and the Barnes-Jewish Foundation.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124 (Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 2.Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59:1077–79. doi: 10.1212/wnl.59.7.1077. [DOI] [PubMed] [Google Scholar]
- 3.Okamoto K, Hirai S, Yamazaki T, Sun XY, Nakazato Y. New ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett. 1991;129:233–36. doi: 10.1016/0304-3940(91)90469-a. [DOI] [PubMed] [Google Scholar]
- 4.Wightman G, Anderson VE, Martin J, Swash M, Anderton BH, Neary D, Mann D, Luthert P, Leigh PN. Hippocampal and neocortical ubiquitin-immunoreactive inclusions in amyotrophic lateral sclerosis with dementia. Neurosci Lett. 1992;139:269–74. doi: 10.1016/0304-3940(92)90569-s. [DOI] [PubMed] [Google Scholar]
- 5.Siddique T, Lalani I. Genetic aspects of amyotrophic lateral sclerosis. Adv Neurol. 2002;88:21–32. [PubMed] [Google Scholar]
- 6.Lagier-Tourenne C, Cleveland DW. Rethinking ALS: the FUS about TDP-43. Cell. 2009;136:1001–4. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gitcho MA, Baloh RH, Chakraverty S, Mayo K, Norton JB, Levitch D, Hatanpaa KJ, White CL, III, Bigio EH, Caselli R, Baker M, Al Lozi MT, Morris JC, Pestronk A, Rademakers R, Goate AM, Cairns NJ. TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol. 2008;63:535–38. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande VC, Bouchard JP, Lacomblez L, Pochigaeva K, Salachas F, Pradat PF, Camu W, Meininger V, Dupre N, Rouleau GA. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–74. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 9.Yokoseki A, Shiga A, Tan CF, Tagawa A, Kaneko H, Koyama A, Eguchi H, Tsujino A, Ikeuchi T, Kakita A, Okamoto K, Nishizawa M, Takahashi H, Onodera O. TDP-43 mutation in familial amyotrophic lateral sclerosis. Ann Neurol. 2008;63:538–42. doi: 10.1002/ana.21392. [DOI] [PubMed] [Google Scholar]
- 10.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–72. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, McKenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH., Jr Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–8. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 12.Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al Saraj S, Al Chalabi A, Leigh PN, Blair IP, Nicholson G, de Belleroche J, Gallo JM, Miller CC, Shaw CE. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–11. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, Kwong LK, Forman MS, Ravits J, Stewart H, Eisen A, McClusky L, Kretzschmar HA, Monoranu CM, Highley JR, Kirby J, Siddique T, Shaw PJ, Lee VM, Trojanowski JQ. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007;61:427–34. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]
- 14.Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL, III, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VM. Mackenzie, IRA TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007;171:227–40. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benajiba L, Le BI, Camuzat A, Lacoste M, Thomas-Anterion C, Couratier P, Legallic S, Salachas F, Hannequin D, Decousus M, Lacomblez L, Guedj E, Golfier V, Camu W, Dubois B, Campion D, Meininger V, Brice A. TARDBP mutations in motoneuron disease with frontotemporal lobar degeneration. Ann Neurol. 2009;65:470–73. doi: 10.1002/ana.21612. [DOI] [PubMed] [Google Scholar]
- 16.Gitcho MA, Bigio EH, Mishra M, Johnson N, Weintraub S, Mesulam M, Rademakers R, Chakraverty S, Cruchaga C, Morris JC, Goate AM, Cairns NJ. TARDBP 3′-UTR variant in autopsy-confirmed frontotemporal lobar degeneration with TDP-43 proteinopathy. Acta Neuropathol. 2009;118:633–45. doi: 10.1007/s00401-009-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Deerlin VM, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB, Clay D, Wood EM, Chen-Plotkin AS, Martinez-Lage M, Steinbart E, McCluskey L, Grossman M, Neumann M, Wu IL, Yang WS, Kalb R, Galasko DR, Montine TJ, Trojanowski JQ, Lee VM, Schellenberg GD, Yu CE. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 2008;7:409–16. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Bo R, Ghezzi S, Corti S, Pandolfo M, Ranieri M, Santoro D, Ghione I, Prelle A, Orsetti V, Mancuso M, Soraru G, Briani C, Angelini C, Siciliano G, Bresolin N, Comi GP. TARDBP (TDP-43) sequence analysis in patients with familial and sporadic ALS: identification of two novel mutations. Eur J Neurol. 2009;16:727–32. doi: 10.1111/j.1468-1331.2009.02574.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuhnlein P, Sperfeld AD, Vanmassenhove B, Van DV, Lee VM, Trojanowski JQ, Kretzschmar HA, Ludolph AC, Neumann M. Two German kindreds with familial amyotrophic lateral sclerosis due to TARDBP mutations. Arch Neurol. 2008;65:1185–89. doi: 10.1001/archneur.65.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutherford NJ, Zhang YJ, Baker M, Gass JM, Finch NA, Xu YF, Stewart H, Kelley BJ, Kuntz K, Crook RJ, Sreedharan J, Vance C, Sorenson E, Lippa C, Bigio EH, Geschwind DH, Knopman DS, Mitsumoto H, Petersen RC, Cashman NR, Hutton M, Shaw CE, Boylan KB, Boeve B, Graff-Radford NR, Wszolek ZK, Caselli RJ, Dickson DW, Mackenzie IR, Petrucelli L, Rademakers R. Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis. PLoS Genet. 4(9):e1000193. doi: 10.1371/journal.pgen.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daoud H, Valdmanis PN, Kabashi E, Dion P, Dupre N, Camu W, Meininger V, Rouleau GA. Contribution of TARDBP mutations to sporadic amyotrophic lateral sclerosis. J Med Genet. 2009;46:112–4. doi: 10.1136/jmg.2008.062463. [DOI] [PubMed] [Google Scholar]
- 22.Behrens MI, Mukherjee O, Tu PH, Liscic RM, Grinberg LT, Carter D, Paulsmeyer K, Taylor-Reinwald L, Gitcho M, Norton JB, Chakraverty S, Goate AM, Morris JC, Cairns NJ. Neuropathologic heterogeneity in HDDD1: a familial frontotemporal lobar degeneration with ubiquitin-positive inclusions and progranulin mutation. Alzheimer Dis Assoc Disord. 2007;21:1–7. doi: 10.1097/WAD.0b013e31803083f2. [DOI] [PubMed] [Google Scholar]
- 23.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 24.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci U S A. 2009;106:18809–14. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wils H, Kleinberger G, Janssens J, Pereson S, Joris G, Cuijt I, Smits V, Ceuterick-de Groote C, Van Broeckhoven C, Kumar-Singh S. TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci U S A. 2010;107:3858–63. doi: 10.1073/pnas.0912417107. [DOI] [PMC free article] [PubMed] [Google Scholar]