Abstract
Background and Objectives
An elevated concentration of lipoprotein(a) {Lp(a)} is associated with an increased prevalence and increased severity of coronary artery disease. However, the relationship between Lp(a) levels and outcomes after acute myocardial infarction (AMI) is unclear.
Subjects and Methods
Between October 2005 and June 2007, we measured serum Lp(a) levels in 832 consecutive AMI patients (age, 62.8±12.4 years, 600 men) on admission. They were divided into tertiles according to their serum Lp(a) levels {Tertile 1 (n=276), Lp(a)<13.8 mg/dL; Tertile 2 (n=279), Lp(a)=13.8-30.6 mg/dL; Tertile 3 (n=277), Lp(a)>30.6 mg/dL}.
Results
There were no differences in baseline clinical characteristics among Tertiles 1, 2, and 3, except for proportions of Killip class III-IV patients (5.8% vs. 10.0% vs. 18.8%, respectively, p<0.001). There were significant differences in left ventricular ejection fractions (57.3±11.4% vs. 55.9±12.3% vs. 53.1±13.1%, p<0.001). Among the laboratory findings, there were significant differences in total cholesterol (173.3±37.2 vs. 183.5±38.9 vs. 185.3±43.8 mg/dL, p=0.001), low density lipoprotein-cholesterol (111.3±34.3 vs. 122.9±34.7 vs. 123.3±39.4 mg/dL, p<0.001), apolipoprotein B (92.8±25.4 vs. 100.8±26.0 vs. 101.9±28.8 mg/dL, p<0.001), and amino-terminal pro-brain natriuretic peptide levels (1805.2±4343.3 vs. 2607.9±5216.3 vs. 3981.5±7689.7 pg/mL, p<0.001). After adjusting for multiple variables in the high Killip class (III-IV) subgroup, the risk estimate for major adverse cardiovascular events (MACE) at 1-year follow-up was significantly higher in Tertile 3 than in Tertiles 1 or 2 (hazard ratio 6.723, 95% confidence interval 1.037-43.593, p=0.046).
Conclusion
In patients in high Killip classes, high serum levels of Lp(a) were significantly associated with long-term adverse outcomes after AMI.
Keywords: Myocardial infarction, Lipoproteins, Prognosis
Introduction
Lipoprotein(a) {Lp(a)} consists of a low-density lipoprotein particle covalently bound to a specific glycoprotein, apolipoprotein(a), by apolipoprotein B-100.1) Since apolipoprotein(a) and plasminogen mutually compete for plasminogen receptors on endothelial cells,2) high concentrations of circulating Lp(a) may inhibit thrombolysis and fibrin clearance.3) Moreover, the binding of native Lp(a) to fibrin is significantly enhanced in the presence of homocysteine, glutathione, and cysteine.4)
A number of prospective and retrospective studies have demonstrated that increased levels of Lp(a) are associated with atherosclerosis, ischemic heart disease, and stroke.5),6) In numerous trials, patients with high Lp(a) levels had markedly increased risk of coronary heart disease.7-9) However, the relationship between Lp(a) levels and outcomes after acute myocardial infarction (AMI) is unclear. The purpose of this study was to assess the relationship between high serum Lp(a) levels and major adverse cardiac events after AMI.
Subjects and Methods
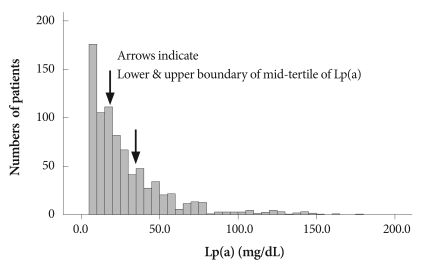
Between October 2005 and June 2007, we measured serum Lp(a) levels in 832 consecutive patients with AMI (62.8±12.4 years, 600 men) upon admission to the Heart Center of Chonnam National University Hospital, Gwangju, Korea. ST-segment elevation myocardial infarction (STEMI) was diagnosed in patients with 30 minutes of continuous chest pain, a new 2 mm ST-segment elevation on at least two contiguous electrocardiographic leads, and a creatine kinase-MB (CK-MB) level greater than three times the upper limit of normal. Non-STEMI (NSTEMI) was diagnosed by chest pain and a positive cardiac biochemical marker without new ST-segment elevation. The levels of serum Lp(a), total cholesterol, high density lipoprotein-cholesterol, triglyceride, and remnant lipoprotein cholesterol were determined on admission. Based on their serum Lp(a) levels, patients were classified into tertiles as follows: Tertile 1 (n=276), Lp(a)<13.8 mg/dL; Tertile 2 (n=279), Lp(a)=13.8-30.6 mg/dL; and Tertile 3 (n=277), Lp(a)≥30.6 mg/dL (Fig. 1). We also evaluated the following as coronary risk factors: hypertension (systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, or receiving antihypertensive drugs), diabetes mellitus (fasting glucose level ≥126 mg/dL or random blood glucose level ≥200 mg/dL), current smoking habit, hyperlipidemia (total cholesterol level ≥240 mg/dL, fasting triglyceride level ≥150 mg/dL, or receiving hyperlipidemia medication), and a family history of coronary artery disease.
Fig. 1.
Distribution of serum concentrations of Lp(a) at baseline. Arrows mark the tertile boundaries. Lp(a): lipoprotein (a).
Laboratory testing
Peripheral blood samples were obtained by direct venipuncture. The blood samples were centrifuged, and serum was removed and stored at -70℃ until the assays could be performed. Absolute CK-MB levels were determined by radioimmunoassay (Dade Behring, Inc., Miami, FL, USA). Cardiacspecific troponin I levels were measured using paramagnetic particles and a chemiluminescent immunoenzyme assay (Beckman, Coulter Inc., Fullerton, CA, USA). Serum levels of total cholesterol, triglyceride, and low- and high density lipoprotein-cholesterol, were measured by standard enzymatic methods. C-reactive protein (CRP) was analyzed by a highly-sensitive turbidimetric test using sheep antibodies against human CRP. This test has been validated against the Dade-Behring method. Serum amino-terminal pro-brain natriuretic peptide was measured using an electrochemiluminescence sandwich immunoassay and an Elecsys 2010 analyzer (Roche Diagnostics, Mannheim, Germany). Lp(a) was measured by an immunonephelometric assay using a latex Lp(a) reagent composed of polystyrene particles coated with a rabbit antihuman Lp(a) γ-globulin fraction. Apolipoprotein A1, apolipoprotein B, and Lp(a) were measured using a Behring Nephelometer II (Dade Behring Inc., Deerfield, IL, USA).
Coronary angiography
Conventional coronary angiography was performed using a digital flat-panel fluoroscopy system (Phillips) via femoral or radial approaches, applying nonionic contrast material (Ultravist 370, Schering, Berlin, Germany). At least four orthogonal views were obtained.
Study outcomes
The primary outcome for this analysis was a composite of major adverse cardiovascular events (MACE), including cardiac death, noncardiac death, nonfatal myocardial infarction, repeat percutaneous coronary intervention, and coronary artery bypass grafting, over one year. Repeat percutaneous coronary interventions included target-lesion revascularization (TLR), target-vessel revascularization (TVR), and non-TVR.
Statistical analysis
Continuous variables with normal distributions were presented as means±standard deviation and were compared by 1-way analysis of variance. To test for differences between Lp(a) tertiles, categorical variables were compared using the χ2 test. Cox proportional hazards models were used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) comparing MACE-free survival rates between Lp(a) tertiles. We used models that adjusted for age, sex, smoking, total cholesterol, low-density lipoprotein cholesterol, high-sensitivity C-reactive protein (hsCRP), apolipoprotein B, Killip class, left ventricular ejection fraction, amino-terminal pro-brain natriuretic peptide, multivessel disease, and number of stents. These variables were selected on the basis of previous studies and multivariable linear regression analyses. We determined that the proportional hazard assumption was satisfied by examining plots of the log-negative-log of the within-group survivorship functions versus log-time. Total cholesterol, low density lipoprotein-cholesterol, and apolipoprotein B levels were adjusted for Lp(a) contribution,10) according to compositional data in which cholesterol accounts for ≒ 30% and apolipoprotein B for ≒ 16% of total Lp(a) mass.11),12) Thus, total Lp(a) mass was multiplied by 0.3, and this value was subtracted from total cholesterol and low density lipoprotein-cholesterol values in all individuals. Similarly, total Lp(a) mass multiplied by 0.16 was subtracted from apolipoprotein B values. A p less than 0.05 was deemed significant. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) software, version 15.0 (SPSS Inc., Chicago, IL, USA) for Windows (Microsoft Corp., Redmond, Washington, USA).
Results
Baseline characteristics
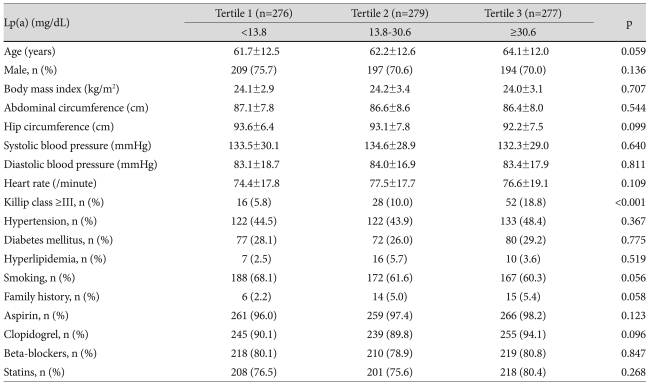
Baseline characteristics are summarized in Table 1. The mean age was higher in Tertile 3 than in Tertiles 1 and 2 (64.1±12.0 vs. 61.7±12.5 vs. 62.2±12.6 years, respectively, p=0.059) and there was a trend for females to have increased Lp(a) levels. Body mass indices, abdominal circumferences, and hip circumferences, were almost the same between tertiles. There were also no differences seen in blood pressures, heart rates, and past medical histories. However, there was a higher incidence of Killip classes III and IV as Lp(a) levels increased {5.8% (Tertile 1) vs. 10.0% (Tertile 2) vs. 18.8% (Tertile 3), p<0.001} (Table 1).
Table 1.
Baseline clinical characteristics of patients by Lp(a) tertiles
Continuous variables are expressed as means±standard deviation. Lp(a): lipoprotein(a)
Laboratory findings
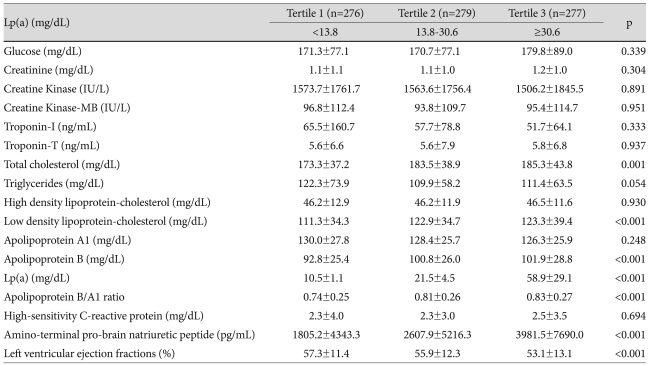
Laboratory findings are summarized in Table 2. Both serum glucose and creatinine levels were highest in Tertile 3, but the increases were not statistically significant. There were no significant differences seen in cardiac enzyme levels. Among Tertiles 1, 2, and 3, total cholesterol was highest in Tertile 3 (173.3±37.2 vs. 183.5±38.9 vs. 185.3±43.8 mg/dL, respectively, p=0.001), as was low density lipoprotein-cholesterol (111.3±34.3 vs. 122.9±34.7 vs. 123.3±39.4 mg/dL, respectively, p<0.001). There was a trend toward a decrease in mean apolipoprotein A1 levels as Lp(a) levels increased. The mean concentration of apolipoprotein B was highest in Tertile 3 (p<0.001), and therefore the apolipoprotein B/apolipoprotein A1 ratio tended to increase as Lp(a) levels increased (p<0.001). Although the hsCRP was also increased in Tertile 3, the increase was not statistically significant. Among Tertiles 1, 2, and 3, the amino-terminal pro-brain natriuretic peptide level was significantly higher in Tertile 3 (1805.2±4343.3 vs. 2607.9±5216.3 vs. 3981.5±7690.0 pg/mL, respectively, p<0.001). The left ventricular ejection fraction was significantly decreased in Tertile 3 (57.3±11.4 vs. 55.9±12.3 vs. 53.1±13.1%, p<0.001).
Table 2.
Comparisons of laboratory findings between Lp(a) tertiles
Lp(a): lipoprotein(a)
Coronary angiography findings
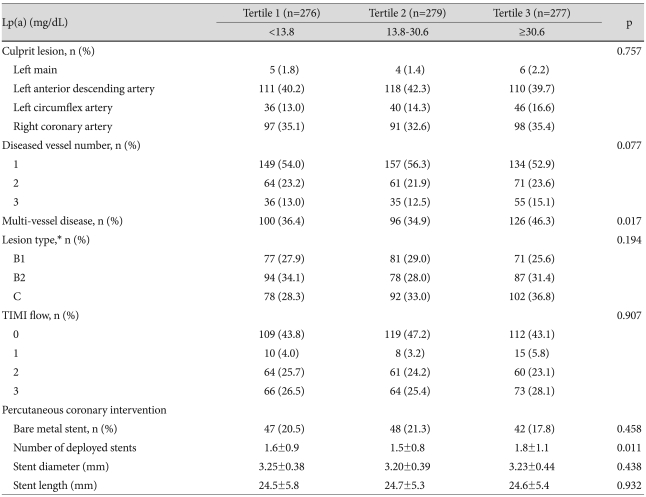
Coronary angiography findings are summarized in Table 3. There were no significant differences in occurrences of culprit lesions, American College of Cardiology/American Heart Association lesion types, Thrombolysis in Myocardial Infarction flow grades, and stent diameters and lengths. However, among Tertiles 1, 2, and 3, multivessel disease was most common (36.4% vs. 34.9% vs. 46.3%, respectively, p=0.017), and the number of deployed stents was highest in Tertile 3 (1.6±0.9 vs. 1.5±0.8 vs. 1.8±1.1, respectively, p=0.011).
Table 3.
Comparisons of coronary angiographc findings betweeg Lp(a) tertiles
*Lesion type according to American College of Cardiology/American Heart Association. Lp(a): lipoprotein(a), TIMI: thrombolysis in myocardial infarction
Clinical outcomes
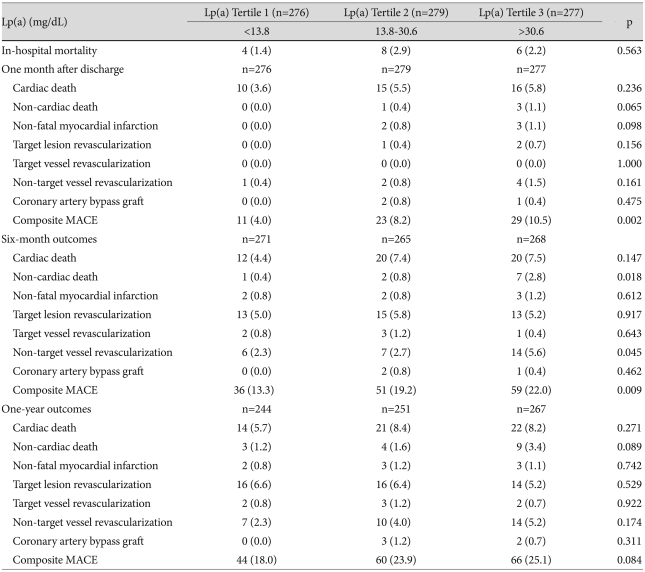
Comparisons of clinical outcomes between groups are summarized in Table 4. In-hospital mortalities were not significantly different among tertiles, but one-month and six-month outcomes were different. The percent of patients with composite MACE was highest in Tertile 3 at both one-month (4.0% vs. 8.2% vs. 10.5%, p=0.002) and six-month follow-ups (13.3% vs. 19.2% vs. 22.0%; p=0.009), but by one-year follow-up there were no significant differences between numbers of patients with composite MACE among the tertiles (Table 5).
Table 4.
Comparisons of clinical outcomes betweeg Lp(a) tertiles
Lp(a): lipoprotein(a), MACE: major adverse cardiac event
Table 5.
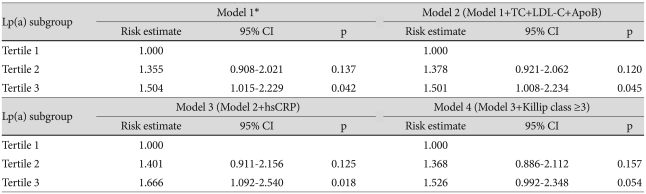
Risk estimates for a major adverse cardiac event according to Lp(a) tertile
*Model 1 adjusted for age, sex, and smoking. Lp(a): lipoprotein(a), TC: total cholesterol, LDL-C: low density lipoprotein-cholesterol, ApoB: apolipoprotein B, hsCRP: high sensitivity C-reactive protein
To analyze the association between serum Lp(a) levels and clinical outcomes, the Cox proportional hazards model was applied with adjustment of multiple variables. Higher levels of baseline Lp(a) were associated with increased risk for MACE until levels were adjusted according to hsCRP values (HR 1.666, 95% CI 1.092-2.540; p=0.018). But when adjusted for Killip class, the increased risk for MACE was not significant.
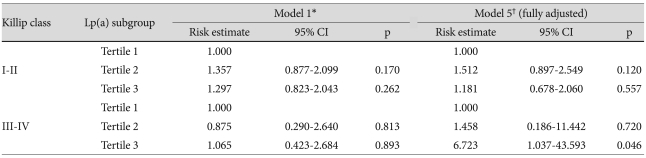
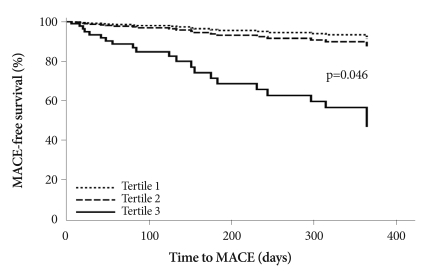
The lack of significance was also seen with adjustments according to amino-terminal pro-brain natriuretic peptide, left ventricular ejection fraction, number of stents, and multivessel disease. Therefore, we analyzed risk estimates according to Killip class, one of the confounding variables. After completely adjusting for the risk for MACE, the risk in Tertile 3 was 6.723 (95% CI 1.037-43.593; p=0.046) compared to the referent Tertile (Table 6) (Fig. 2). The other three variables did not show significant risk estimates for MACE.
Table 6.
Risk estimates for a major adverse cardiac event according to Lp(a) tertile and Killip class
*Model 1 includes age, gender, and smoking, †Model 5 includes age, gender, smoking, total cholesterol, low density lipoprotein-cholesterol, apolipoprotein B, high-sensitivity C-reactive protein, log N-terminal pro-B type natriuretic peptide, multivessel disease, number of stents, left ventricular ejection fraction. Lp(a): lipoprotein(a)
Fig. 2.
Fully-adjusted time-to-clinical outcomes by baseline Lp(a) tertiles. Hazard ratios have been adjusted for age; sex; smoking; total cholesterol; low density lipoprotein-cholesterol; high-sensitivity C-reactive protein; apolipoprotein B; Killip class; left ventricular ejection fraction; amino-terminal pro-brain natriuretic peptide; multivessel disease; and number of stents. MACE: major adverse cardiovascular events, Lp(a): lipoprotein (a).
Discussion
The results of this study indicate that high serum levels of Lp(a) are significantly associated with long-term adverse outcomes in AMI patients, especially in those in a high Killip class. Thus, preprocedural Lp(a) levels could provide data for risk stratification in patients with AMI.
Kamstrup et al.8) have observed stepwise increases in risk for myocardial infarction with increasing levels of Lp(a) in the general population, but we do not know the severities of the conditions of the patients in their study. In the present study, patients with higher Lp(a) levels also had more serious initial presentations. There were more patients in a high Killip class in the highest Lp(a)-level tertile. Left ventricular ejection fraction also decreased linearly with increasing serum Lp(a) levels. After multifactorial adjustment using the Cox proportional hazards model, it seems unlikely that the differences in left ventricular ejection fraction among the Lp(a) tertiles were actually of clinical significance; whereas high Killip classes were seen to be an independent risk factor for MACE (HR 1.997, 95% CI 1.220-3.271; p=0.006).
The reason why Lp(a) was only associated with MACE in patients in high Killip classes remains unclear. One potential mechanism may be oxidative stress on Lp(a) in acute heart failure caused by AMI. It has been reported that the biological effects of oxidized Lp(a) are more potent than those of native Lp(a). These effects include stimulation of atherosclerosis, inhibition of vessel dilatation, and mitogenic stimulation of human vascular smooth muscle cells.13) Therefore, we suggest that high serum Lp(a) is predictive of more adverse outcomes in patients in higher Killip classes than in lower Killip classes. Second, decreased oxygen delivery to the ischemic myocardium should be considered. Patients in high Killip classes with pulmonary edema or in cardiogenic shock have increased wedge pressure, and thus impaired oxygen and carbon dioxide exchange in the lungs. Since apolipoprotein(a) and plasminogen compete for plasminogen receptors on endothelial cells,2) high serum Lp(a) may inhibit thrombolysis and fibrin clearance.3) These two effects could aggravate myocardial ischemia and might result in worse outcomes.
Basically, Lp(a) is not easily modified by statin therapy.14) Although there are contradictory findings about the effect of statins on Lp(a) serum concentrations,15) it is uncertain whether statin use prevents the coronary heart disease associated with Lp(a).16) Nearly 80% of patients in our study were administered statins, and since the numbers of patients taking statins were almost equally distributed among the tertiles, statin therapy probably did not influence our results. Some reports have indicated that niacin; certain inhibitors of the cholesteryl ester transfer protein; and mipomersen, an antisense oligonucleotide directed at human apoB-100; reduce Lp(a) levels about 20%, 40%, and 70%, respectively.17-19) No patients in this study took any of these medications. Therefore, medical therapy seems unlikely to be a confounder in this study.
Some studies have demonstrated association of high serum Lp(a) levels with restenosis after percutaneous coronary angioplasty.20),21) However, it has also been reported that serum Lp(a) levels do not influence restenosis after elective coronary stenting.22),23) Hoffmann et al.24) have demonstrated by serial intravascular ultrasound studies that in-stent restenosis results from neointimal tissue proliferation, which has been attributed to Lp(a) in vitro.25) However, Morita et al.26) studied patients with high Lp(a) levels undergoing POBA as well as PCI and found that there was less difference of TLR rate in the PCI group. Although not completely understandable, it has been suggested that the metallic scaffolding in stents strongly inhibits recoil, which results in the suppression of in-stent restenosis in patients with high serum Lp(a) levels. Our results are consistent with this data. The TLR rates were very similar among the tertiles.
With respect to revascularization in newly developed lesions, it is difficult to determine a causal relationship for Lp(a). Lp(a) promotes growth of vascular smooth muscles cells and inhibits plasminogen activity, so that it contributes to the development of atherosclerosis and atherothrombosis.26) We suggest that these Lp(a) effects could explain the trend toward a high non-TVR rate in patients with high Lp(a) levels. However, the incidence of non-TVR was too small to analyze by the Cox proportional hazards model.
Regarding clinical outcomes in this study, there are some results that need to be clarified. First, one- and six-month outcomes showed significant differences among tertiles, but no significant differences were found in the one-year outcomes. An analysis of the nationwide Korea Acute Myocardial Infarction Registry, which enrolled 13,133 patients with AMI, showed that most adverse cardiac events occurred within the first six-months.27) The less frequent occurrence of adverse cardiac events between the six-month to one-year follow-ups may have influenced our results. Second, there were significant differences between the tertiles in the number of noncardiac deaths at the six-month follow-up. These deaths were caused by bleeding, sepsis, and aggravation of underlying diseases. However, the number of patients in this group was too small to interpret the results accurately.
The present study was limited in several respects. First, this was a retrospective study, and therefore subject to the limitations inherent in this type of clinical investigation. Second, the results of this study should be verified by prospective investigations, because it was a single-center study. Third, there was a problem with laboratory standardization. International reference material for Lp(a) laboratory standardization only became available in 2000.28) Even after standardization, there might be some variations in Lp(a) measurements. Standardization of serum Lp(a) measurement should be undertaken, and we need to use assay systems that are insensitive to apo(a) isoforms.16) Fourth, Lp(a) tends to increase in AMI or in surgery as an acute phase reactant.29) There was difficulty timing the measurements and assessments of Lp(a) levels. Last, hormone replacement therapy (HRT) in female patients, which can affect serum Lp(a) levels, was not considered in this study. However, the study population comprised mainly men, and it has been recently determined that far fewer Asian women take HRT compared with women in Western countries.30) Nevertheless, HRT should be considered in future studies.
In conclusion, elevated levels of serum Lp(a) are significantly associated with one-year adverse outcomes in AMI patients in high Killip classes. Since Lp(a) may promote atherosclerosis of the vascular wall by its involvement in calcification,13) in the future it may be determined to be a very important risk factor. Additional prospective studies may determine causal relationships and treatments.
Acknowledgments
This study was supported by grants from the Korea Healthcare Technology R&D project (A084869), Ministry for Health, Welfare, & Family Affairs, Republic of Korea, and the Cardiovascular Research Foundation, Asia.
References
- 1.Lee CK. Lipoprotein(a), Lp(a) Korean Circ J. 1993;23:631–633. [Google Scholar]
- 2.Hajjar KA, Gavish D, Breslow JL, Nachman RL. Lipoprotein (a) modulation of endothelial surface fibrinolysis and its potential role in atherosclerosis. Nature. 1989;339:303–305. doi: 10.1038/339303a0. [DOI] [PubMed] [Google Scholar]
- 3.Harpel PC, Gordon BR, Parker TS. Plasmin catalyzes binding of lipoprotein (a) to immobilized fibrinogen and fibrin. Proc Natl Acad Sci U S A. 1989;86:3847–3851. doi: 10.1073/pnas.86.10.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harpel PC, Chang VT, Borth W. Homocysteine and other sulfhydryl compounds enhance the binding of lipoprotein (a) to fibrin: a potential biochemical link between thrombosis, atherogenesis, and sulfhydryl compound metabolism. Proc Natl Acad Sci U S A. 1992;89:10193–10197. doi: 10.1073/pnas.89.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SH, Shin GJ. Lipoprotein (a) as a risk factor for coronary heart disease: whether related with NIDDM or not. Korean Circ J. 1996;26:507–513. [Google Scholar]
- 6.Jürgens G, Taddei-Peters WC, Költringer P, et al. Lipoprotein (a) serum concentration and apolipoprotein (a) phenotype correlate with severity and presence of ischemic cerebrovascular disease. Stroke. 1995;26:1841–1848. doi: 10.1161/01.str.26.10.1841. [DOI] [PubMed] [Google Scholar]
- 7.Danesh J, Collins R, Peto R. Lipoprotein (a) and coronary heart disease: meta-analysis of prospective studies. Circulation. 2000;102:1082–1085. doi: 10.1161/01.cir.102.10.1082. [DOI] [PubMed] [Google Scholar]
- 8.Kamstrup PR, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein (a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117:176–184. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 9.Foody JM, Milberg JA, Pearce GL, Sprecher DL. Lipoprotein (a) associated with coronary artery disease in older women: age and gender analysis. Atherosclerosis. 2000;153:445–451. doi: 10.1016/s0021-9150(00)00427-5. [DOI] [PubMed] [Google Scholar]
- 10.Jenner JL, Ordovas JM, Lamonfava S, et al. Effects of age, sex, and menopausal status on plasma lipoprotein (A) levels: the Framingham Offspring Study. Circulation. 1993;87:1135–1141. doi: 10.1161/01.cir.87.4.1135. [DOI] [PubMed] [Google Scholar]
- 11.Seman LJ, Breckenridge WC. Isolation and partial characterization of apolipoprotein (a) from human lipoprotein (a) Biochem Cell Biol. 1986;64:999–1009. doi: 10.1139/o86-133. [DOI] [PubMed] [Google Scholar]
- 12.Carlson LA, Hamsten A, Asplund A. Pronounced lowering of serum levels of lipoprotein Lp(a) in hyperlipemic subjects treated with nicotinic-acid. J Intern Med. 1989;226:271–276. doi: 10.1111/j.1365-2796.1989.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 13.Morishita R, Ishii J, Kusumi Y, et al. Association of serum oxidized lipoprotein (a) concentration with coronary artery disease: potential role of oxidized lipoprotein (a) in the vascular wall. J Atheroscler Thromb. 2009;16:410–418. doi: 10.5551/jat.no224. [DOI] [PubMed] [Google Scholar]
- 14.Kostner GM, Gavish D, Leopold B, Bolzano K, Weintraub MS, Breslow JL. HMG CoA reductase inhibitors lower LDL cholesterol without reducing Lp(a) levels. Circulation. 1989;80:1313–1319. doi: 10.1161/01.cir.80.5.1313. [DOI] [PubMed] [Google Scholar]
- 15.Marcovina SM, Koschinsky ML, Albers JJ, Skarlatos S. Report of the National Heart, Lung, and Blood Institute Workshop on Lipoprotein (a) and Cardiovascular Disease: recent advances and future directions. Clin Chem. 2003;49:1785–1796. doi: 10.1373/clinchem.2003.023689. [DOI] [PubMed] [Google Scholar]
- 16.Maher VM, Brown BG, Marcovina SM, Hillger LA, Zhao XQ, Albers JJ. Effects of lowering elevated LDL cholesterol on the cardiovascular risk of lipoprotein (a) JAMA. 1995;274:1771–1774. [PubMed] [Google Scholar]
- 17.Insull W, Jr, McGovern ME, Schrott H, et al. Efficacy of extended-release niacin with lovastatin for hypercholesterolemia: assessing all reasonable doses with innovative surface graph analysis. Arch Intern Med. 2004;164:1121–1127. doi: 10.1001/archinte.164.10.1121. [DOI] [PubMed] [Google Scholar]
- 18.Merki E, Graham MJ, Mullick AE, et al. Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein (a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein (a) transgenic mice. Circulation. 2008;118:743–753. doi: 10.1161/CIRCULATIONAHA.108.786822. [DOI] [PubMed] [Google Scholar]
- 19.Kastelein JJ, Wedel MK, Baker BF, et al. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation. 2006;114:1729–1735. doi: 10.1161/CIRCULATIONAHA.105.606442. [DOI] [PubMed] [Google Scholar]
- 20.Hearn JA, Donohue BC, Báalbaki H, et al. Usefulness of serum lipoprotein (a) as a predictor of restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1992;69:736–739. doi: 10.1016/0002-9149(92)90497-m. [DOI] [PubMed] [Google Scholar]
- 21.Desmarais RL, Sarembock IJ, Ayers CR, Velmon SM, Powers ER, Gimple LW. Elevated serum lipoprotein (a) is a risk factor for clinical recurrence after coronary balloon angioplasty. Circulation. 1995;91:1403–1409. doi: 10.1161/01.cir.91.5.1403. [DOI] [PubMed] [Google Scholar]
- 22.Ribichini F, Steffenino G, Dellavalle A, et al. Plasma lipoprotein (a) is not a predictor for restenosis after elective high-pressure coronary stenting. Circulation. 1998;98:1172–1177. doi: 10.1161/01.cir.98.12.1172. [DOI] [PubMed] [Google Scholar]
- 23.Rhew JY, Jeong MH, Hong YJ, et al. The effects of lipoprotein (a) on coronary stent restenosis. Korean Circ J. 2001;31:476–483. [Google Scholar]
- 24.Hoffmann R, Minz GS, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis: a serial intravascular ultrasound study. Circulation. 1996;94:1247–1254. doi: 10.1161/01.cir.94.6.1247. [DOI] [PubMed] [Google Scholar]
- 25.Grainger DJ, Kirschenlohr HL, Metcalfe JC, Weissberg PL, Wade DP, Lawn RM. Proliferation of human smooth muscle cells promoted by lipoprotein (a) Science. 1993;260:1655–1658. doi: 10.1126/science.8503012. [DOI] [PubMed] [Google Scholar]
- 26.Morita Y, Himeno H, Yakuwa H, Usui T. Serum lipoprotein (a) level and clinical coronary tenosis progression in patients with myocardial infarction: re-revascularization rate is high in patients with high-Lp(a) Circ J. 2006;70:156–162. doi: 10.1253/circj.70.156. [DOI] [PubMed] [Google Scholar]
- 27.Sim DS, Kim JH, Jeong MH. Differences in clinical outcomes between patients with ST-elevation versus non-ST-elevation acute myocardial infarction in Korea. Korean Circ J. 2009;39:297–303. doi: 10.4070/kcj.2009.39.8.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcovina SM, Albers JJ, Scanu AM, et al. Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein (a) Clin Chem. 2000;46:1956–1967. [PubMed] [Google Scholar]
- 29.Kim CJ, Kwak MH, Kim KM, Ryu WS, Park JT, Ryoo UH. Lipoprotein (a) as an acute phase reactant. Korean J Lipidol. 1996;6:111–115. [Google Scholar]
- 30.Heinemann K, Rübig A, Strothmann A, Nahum GG, Heinemann LA. Prevalence and opinions of hormone therapy prior to the Women's Health Initiative: a multinational survey on four continents. J Womens Health (Larchmt) 2008;17:1151–1166. doi: 10.1089/jwh.2007.0584. [DOI] [PubMed] [Google Scholar]