Abstract
Some of extracellular serine proteases with trypsin-like specificity of cleavage have been known to increase the release of inflammatory mediators from various cell types. For instance, two well-known trypsin-like serine proteases circulating in blood, granzyme A (GrA) and thrombin, have been found to promote interleukin (IL)-8 release from an alveolar epithelial A549 cell line. However, the mechanisms by which the proteases promote IL-8 release from the cells are not fully understood. In the present study, using A549 cells we found that (1) thrombin promoted IL-8 release from the cells via a mechanism partially involving activation of protease-activated receptor-1, a G-protein coupled receptor, whereas a recombinant form of GrA (rGrA) did it via a mechanism that does not involve the receptor activation; that (2) unlike rGrA, thrombin did not cause detachment and microtubule disruption of the cells; and that (3) the release of IL-8 induced by rGrA was inhibited in the presence of taxol, a microtubule-stabilizing reagent, whereas that induced by thrombin was not. These findings suggest that rGrA and thrombin promote the release of IL-8 from A549 cells through distinct mechanisms.
Keywords: A549 cells, Granzyme A, Interleukin-8 release, Microtubule disruption, Proteinase-activated receptor-1, Thrombin
Introduction
Extracellular serine proteases with trypsin-like specificity of cleavage (cleaving after Arg or Lys residues) have been found to play important roles in diverse aspects of cellular function. Some of them are known to be involved in the regulation of innate immune inflammatory responses. For instance, thrombin (a blood coagulation protease) and trypsin (a pancreatic digestive protease) stimulate the production and release of cytokines and chemokines in diverse cell types (Ramachandran and Hollenberg 2008; Steinhoff et al. 2005). To date, it has been recognized that the effects of thrombin and trypsin on the production of inflammatory mediators are largely attributed to their ability to activate a unique class of G-protein-coupled receptors, named protease-activated receptors (PARs). Each of PARs (PAR-1, PAR-2, PAR-3, and PAR-4) is cleaved by certain proteases at a specific site on the N-terminal extension protruding into the extracellular space. In each of the four PARs, the newly exposed N-terminus itself acts as a tethered ligand to activate the receptor through an intramolecular interaction (Ramachandran and Hollenberg 2008; Steinhoff et al. 2005). Indeed, thrombin and trypsin cleave PAR-1/PAR-4 and PAR-2, respectively, thereby triggering intracellular signaling pathways involved in the production of inflammatory mediators (Ramachandran and Hollenberg 2008; Steinhoff et al. 2005).
Granzyme A (GrA) is a serine protease with trypsin-like specificity of cleavage that is expressed in cytotoxic T-lymphocytes and natural killer cells (Kam et al. 2000). This protease is believed to function by entering virus-infected cells and growing tumors via pores formed by perforin, which is also expressed in cytotoxic cells, and participating in the apoptosis induction of abnormal cells (Chowdhury and Lieberman 2008; Kam et al. 2000). It has been found that GrA is also found in body fluids such as blood (Spaeny-Dekking et al. 1998; Tremblay et al. 2000) and that in the lung, GrA mRNA is expressed not only in cytotoxic lymphocytes infiltrating this tissue but also in alveolar type II epithelial cells and alveolar macrophages (Vernooy et al. 2007). Importantly, GrA was found to promote release of inflammatory mediators such as interleukin (IL)-6 and IL-8 from cultured cell lines (Sower et al. 1996). We also reported that a recombinant form of rat GrA (rGrA) promotes the release of IL-8 from a human alveolar type II epithelial cell line A549 (Yoshikawa et al. 2008a, b). These observations suggest that GrA, besides its roles in the killing of abnormal cells, is involved in the progression of inflammation in the extracellular environment.
The mechanisms by which GrA promotes the release of inflammatory mediators are not fully understood. We reported previously that rGrA caused detachment of A549 cells, possibly due to its ability to digest extracellular matrix components such as collagen IV and fibronectin (Yoshikawa et al. 2008a). Importantly, rGrA-induced detachment was accompanied by microtubule disruption, and IL-8 release promoted by the protease was partially but significantly inhibited in the presence of taxol, a microtubule-stabilizing reagent. These findings suggest that rGrA-promoted IL-8 release is partly due to microtubule disruption of cells. However, there may be other mechanisms by which GrA promotes IL-8 release in A549 cells.
GrA has been considered as a low-affinity ligand of PAR-1 (Parry et al. 1996; Steinhoff et al. 2005; Suidan et al. 1994). For instance, this protease induced neurite retraction, which was inhibited in the presence of an anti-PAR-1 antibody (Suidan et al. 1994). This consideration lead us to assess whether GrA promotes IL-8 release via a mechanism involving activation of the G-protein-coupled receptor. In the present study, we assessed the mechanisms by which rGrA and thrombin promote IL-8 release using A549 cells. This cell line is known to express functional PAR-1 and to promote the release of IL-8 in response to thrombin (Asokananthan et al. 2002). Consistent with the previous observation, thrombin-promoted IL-8 release was found to occur through a mechanism involving the activation of PAR-1 in the cells. However, we obtained no evidence that rGrA did it through a mechanism involving activation of the G-protein-coupled receptor. Thrombin-promoted IL-8 release was unaffected in the presence of taxol. These findings led us to suggest that these two serine proteases differentially mediate IL-8 release in A549 cells.
Materials and methods
Materials
An anti-α-tubulin antibody conjugated with fluorescein isothiocyanate, N-α-benzyloxy-l-lysine thiobenzyl ester (BLT), bovine serum albumin (BSA) (protease free), 5,5′-dithiobis-(2-nitrobenzoic acid), (DTNB) and human plasma thrombin were purchased from Sigma (St. Louis, MO, USA). A thrombin receptor agonist peptide (H-Ser-Phe-Leu-Leu-Arg-Asn-NH2, hereinafter called TRAP-6) was purchased from Bachem AG (Bubendorf, Switzerland). A nonpeptide PAR-1 antagonist, N3-cyclopropyl-7-[[4-(1-methylethyl)phenyl]methyl]-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine (SCH79797) (Ahn et al. 2000), was purchased from TOCRIS bioscience (Bristol, UK). Taxol was purchased from LKT laboratories (St. Paul, MN, USA). Fluo-3 AM was purchased from Dojindo (Kumamoto, Japan). All other reagents used are of analytical grade.
Cell culture
A549 cells were purchased from RIKEN Bioresource Center (Ibaraki, Japan) and were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal calf serum at 37 °C in 5% CO2.
Preparation of rGrA
Production of rGrA in Pichia pastoris and the purification by means of single-step chromatography using Ni2+-charged resin (HisLink™ resin, Promega, Madison, WI, USA) were performed as described previously (Hirayasu et al. 2005, 2007, 2008; Tsuzuki et al. 2003). In order to obtain the active form, the purified rGrA was incubated with 2.0 units/mL recombinant enterokinase (Novagen, Madison, WI, USA) for 18 h at 22 °C. Active rGrA was re-purified using the Ni2+-charged resin. Finally, the activated rGrA was subjected to gel filtration in serum-free DMEM supplemented with 0.1% BSA (SFM) using a NAP-10 column (GE Healthcare Japan, Tokyo). The concentration of activated rGrA was determined semiquantitatively as follows: 5 μL of gel filtrate containing rGrA was incubated in a well of a 96-well plate (Asahi Techno Glass, Tokyo, Japan) with 200 μM BLT (substrate) and 500 μM DTNB (color developer) in a HEPES buffer (25 mM HEPES, pH 8.0, 145 mM NaCl and 0.1% v/v Triton X-100) at 22 °C in a final volume of 200 μL. The color development was measured at 405 nm at 30 s intervals for 60 min using a microplate reader (Multiskan MS; Labsystems, Helsinki, Finland). The nonenzymic rate of increase in absorbance was subtracted from the enzyme-catalyzed rate. For times up to 60 min, 0.2 pmol rGrA (the amount predetermined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by silver staining using bovine trypsin as a standard) caused a linear increase in the absorbance value (0.40 per 60 min). After determination of the concentration, the remainder of gel filtrate was diluted with SFM, sterile filtered, and used for experiments.
Treatment of A549 cells with test substances and determination of IL-8 concentration in the conditioned media
A549 cells trypsinized were seeded into 48-well microtiter plates (Asahi Techno Glass) at a density of 1 × 104 cells/well and incubated for 48 h to be grown to 90% confluence. The cells were washed with phosphate buffered saline (PBS, 8 mM Na2HPO4, 1.5 mM KH2PO4, 136 mM NaCl, 2.7 mM KCl, pH 7.4), exposed to 100 μL of SFM and SFM containing test substances, and incubated for 24 h. Concentration of IL-8 in the conditioned media was determined using Human IL-8 chemiluminescent ELISA kit (Pierce, Rockford, IL, USA).
Measurement of intracellular free calcium concentration [Ca2+]i
[Ca2+]i were measured using a fluorescent Ca2+ indicator, fluo-3 AM. A549 cells were grown on 96 well microtiter plates to 80% confluence, rinsed once with a Ca2+ buffer (137 mM NaCl, 5.4 mM KCl, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 1.3 mM CaCl2, 1.0 mM MgSO4, 4.2 mM NaHCO3 containing 0.1% BSA and 1.0 mM probenecid), and loaded with 4 μM fluo-3 AM in Ca2+ buffer containing 0.04% Pluronic F270 for 30 min at 22 °C. After incubation, cells were washed once with Ca2+ buffer to remove excess dye and then exposed to Ca2+ buffer alone and the buffer containing test substances, after establishing baseline. The ratio of fluorescence emission at 485 and 528 nm was measured using PowerScan (Dainippon Sumitomo Pharma, Osaka, Japan).
Immunostaining of A549 cells with anti-α-tubulin antibody
A549 cells were cultured and incubated for 24 h with SFM containing rGrA and thrombin, as described above. After incubation, the plates were fixed with PBS containing 4% paraformaldehyde, and permealized with PBS containing 0.1% Triton X-100. After blocking with PBS containing 5% BSA, the cells were incubated for 1 h with an anti-α-tubulin antibody conjugated with fluorescein isothiocyanate (1:50 dilution in PBS). The plates were analyzed by confocal laser scanning microscopy (Fluoview FV300, Olympus, Tokyo, Japan).
Statistical analysis
Differences in values were determined by the two-tailed unpaired t-test using Instat statistical software (GraphPad, San Diego, CA, USA). A P-value of less than 0.05 was considered to be statistically significant.
Results and discussion
Promotion of IL-8 release by rGrA and thrombin in A549 cells
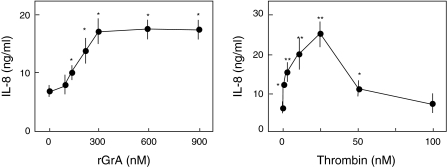
We found previously that the concentration profile for IL-8 release by rGrA in A549 cells was sigmoidal, with a threshold of 100–150 nM (Yoshikawa et al. 2008a). In the present study, we found that the ability of rGrA to promote IL-8 release reaches plateau levels at 300 nM (2.4-fold increase compared to cells incubated with SFM alone) (Fig. 1). We also assessed the concentration dependence of IL-8 release from the cells caused by thrombin. Maximal IL-8 release was observed in cells incubated with SFM containing 25 nM thrombin (3.6-fold increase compared to cells incubated with SFM alone) (Fig. 1). However, IL-8 release was not significantly higher in cells incubated with SFM containing 100 nM thrombin than in those incubated with SFM alone (Fig. 1). Higher concentrations of thrombin may degrade cell-surface components involved in the IL-8 release. Regardless, these findings suggest that thrombin is more effective than rGrA for promotion of IL-8 release in A549 cells. We have found that BLT-hydrolyzing activity of our rGrA is comparable to that of native GrA (Kam et al. 2000; Hirayasu et al. 2005). Therefore, the lower efficiency of rGrA compared with thrombin in the context of IL-8 release may not be attributed to the catalytic capacity of the recombinant enzyme.
Fig. 1.
The concentration dependence of IL-8 release by rGrA and thrombin in A549 cells. A549 cells were incubated for 24 h with SFM, SFM containing rGrA, and SFM containing thrombin. After incubation, concentration of IL-8 in the conditioned medium was determined using an ELISA kit. The concentrations for rGrA (from left to right) are 0, 100, 150, 200, 300, 600, and 900 nM. The concentrations for thrombin (from left to right) are 0, 1, 5, 15, 25, 50, and 100 nM. Data are expressed as means ± SE from three independent experiments performed in duplicate. ** and * P < 0.01 and P < 0.05, respectively, versus A549 cells incubated with SFM alone (two-tailed, unpaired t-test)
Analysis of the involvement of PAR-1 activation in rGrA-promoted IL-8 release in A549 cells
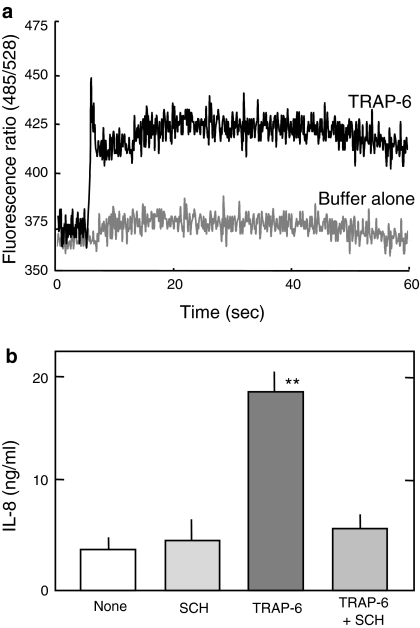
One purpose of the present study is to address whether rGrA promotes IL-8 release from A549 cells via a mechanism involving activation of PAR-1. A549 cells were found previously to express functional PAR-1 (Asokananthan et al. 2002). We first analyzed the expression of functional PAR-1 in A549 cells we used. Besides proteases, a synthetic six-amino-acid peptide (TRAP-6) can stimulate PAR-1 (Ramachandran and Hollenberg 2008; Steinhoff et al. 2005). A rise in [Ca2+]i is an indicative of PAR-1 stimulation (Asokananthan et al. 2002; Steinhoff et al. 2005). TRAP-6 was found to elicit a rise in [Ca2+]i (Fig. 2a). In addition, TRAP-6-promoted IL-8 release from A549 cells, which was significantly inhibited in the presence of a synthetic PAR-1 antagonist SCH79797 (Fig. 2b). Results shown in Fig. 2 indicate the expression of functional PAR-1 in A549 cells we used.
Fig. 2.
Expression of functional PAR-1 in A549 cells. a A rise in [Ca2+]i by TRAP-6. A549 cells were loaded with fluo-3 AM and exposed to Ca2+ buffer (Buffer alone) and Ca2+ buffer containing 100 μM TRAP-6. Responses were measured over 60 s and expressed as a fluorescence ratio (485/528 nm). Each response is the representative of five challenging. b Inhibition of TRAP-6-promoted IL-8 release by a PAR-1 antagonist. Cells were incubated for 24 h with SFM (None), SFM containing 10 μM SCH79797 (SCH), SFM containing 100 μM TRAP-6 (TRAP-6), and SFM containing 100 μM TRAP-6 and 10 μM SCH79797 (TRAP-6 + SCH). Data are expressed as means ± SE from three independent experiments performed in duplicate. ** P < 0.01 versus A549 cells treated with SFM alone (two-tailed, unpaired t-test)
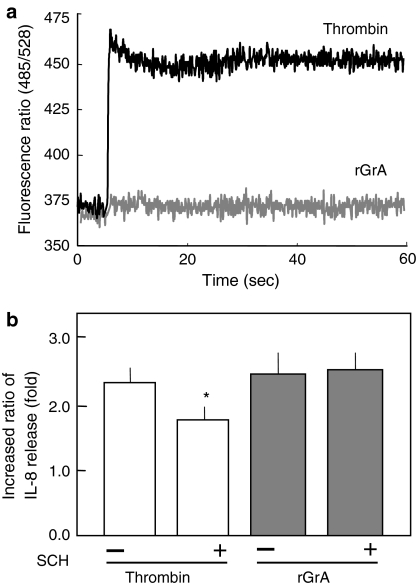
Thrombin elicited a [Ca2+]i response in A549 cells (Fig. 3a): this being consistent with the previous observation (Asokananthan et al. 2002). In the absence of SCH79797, 5 nM thrombin led to a 2.3-fold increase in IL-8 concentration (Fig. 3b). In the presence of the antagonist, 5 nM thrombin caused a 1.8-fold increase in IL-8 concentration (Fig. 3b). These findings suggest that thrombin promotes IL-8 release from A549 cells via a mechanism involving PAR-1 activation. Note that the inhibition by SCH79797 of TRAP-6-induced IL-8 release was nearly complete (Fig. 2b), whereas that of thrombin-induced IL-8 release was only partial (Fig. 3b). This implies that other mechanisms than PAR-1 activation are involved in the IL-8-inducing activity of thrombin. Activation of PAR-2 in A549 cells is known to result in elevation in [Ca2+]i and IL-8 release (Asokananthan et al. 2002). To date, however, there has been no evidence that thrombin activates PAR-2 (Ramachandran and Hollenberg 2008; Steinhoff et al. 2005). Although PAR-3 is a thrombin receptor, the stimulation by the agonist peptide (Thr-Phe-Arg-Gly-Ala-Pro-NH2) in A549 cells resulted in neither elevation in [Ca2+]i nor promotion of IL-8 release (Asokananthan et al. 2002). Stimulation of PAR-4 by the agonist peptide (Gly-Tyr-Pro-Gly-Gln-Val-NH2) in A549 cells causes a rise in [Ca2+]i and an increase in IL-8 release (Asokananthan et al. 2002). Therefore, a possible explanation for incomplete inhibition of thrombin-induced IL-8 release by SCH79797 is that PAR-4 activation-mediated mechanism still works. Also, we cannot exclude the possibility that SCH79797 efficiently inhibits the binding of TRAP-6 to PAR-1, whereas the antagonist does not efficiently inhibit the binding to the receptor of tethered ligand occurred by cleavage with thrombin.
Fig. 3.
Analyses of the involvement of PAR-1 activation in the IL-8 release by rGrA and thrombin in A549 cells. a No [Ca2+]i responses in cells challenged by rGrA. A549 cells were loaded with fluo-3 AM and exposed to 300 nM rGrA and 5 nM thrombin. Responses were measured over 60 s and expressed as a fluorescence ratio (485/528 nm). Each response is the representative of ten challengings. b Effects of a PAR-1 antagonist on the IL-8 release by rGrA and thrombin. Cells were incubated for 24 h with SFM and SFM containing proteases (5 nM thrombin or 300 nM rGrA). y-axis shows the ratio of IL-8 release in the presence of proteases to that in the absence of proteases: (−), the ratio in the absence of SCH79797 (SCH); (+), the ratio in the presence of the antagonist. Data are expressed as means ± SE from three independent experiments performed in duplicate. * P < 0.05 versus increase in IL-8 release by thrombin in the absence of SCH79797 (two-tailed, unpaired t-test)
In the concentration range of 1–300 nM, rGrA elicit no [Ca2+]i responses in A549 cells. For instance, 300 nM rGrA did not evoke significant rises in [Ca2+]i (Fig. 3a). In addition, rGrA-promoted IL-8 release was unaffected in the presence of SCH79797 (Fig. 3b). These findings suggest that rGrA-promoted IL-8 release from A549 cells does not involve activation of PAR-1. In other words, rGrA is not thought to activate PAR-1 expressed in this cell line. GrA was found previously to stimulate PAR-1 expressed in neuronal cells (Suidan et al. 1994). One possible explanation for this discrepancy is that neuronal cells may express unknown PAR-1-associated molecules that assist in interaction of GrA with PAR-1, whereas A549 cells may not.
Analysis of the involvement of microtubule disruption in thrombin-promoted IL-8 release in A549 cells
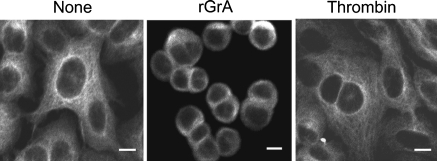
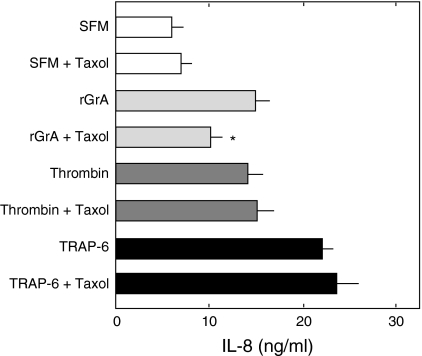
We have found that rGrA causes the detachment and microtubule disruption of A549 cells and that the promotion of IL-8 release by rGrA is significantly inhibited by taxol, a microtubule-stabilizing reagent (Yoshikawa et al. 2008a). These findings suggested that the promotion of IL-8 release by rGrA occurs via a mechanism involving microtubule disruption accompanying reduction of cell adhesion. In the present study, we assessed whether thrombin-promoted IL-8 release occurs via a mechanism involving microtubule disruption. Cells were incubated with an anti-α-tubulin antibody. We reported previously that meshworks of microtubule were found in cells incubated with SFM alone but not in those incubated with SFM containing rGrA (Yoshikawa et al. 2008a). These were confirmed in the present study (Fig. 4). Unlike rGrA, thrombin did not cause the detachment of A549 cells in the concentration range of 1–100 nM (data not shown). In addition, thrombin did not appear to disrupt microtubule structures of A549 cells. For instance, dense microtubule signals at perinucleus and microtubule meshworks were observed in cells incubated with SFM containing 100 nM thrombin (Fig. 4). The release of IL-8 induced by rGrA was inhibited by taxol, whereas that induced by thrombin was not (Fig. 5). In addition, TRAP-6-induced IL-8 release was not inhibited in the presence of taxol (Fig. 5). These findings suggest that thrombin-promoted IL-8 release does not occur through a mechanism involving microtubule disruption.
Fig. 4.
Staining of A549 cells with an anti-α-tubulin antibody. A549 cells were incubated for 24 h with SFM (None), SFM containing 300 nM rGrA, and SFM containing 100 nM thrombin. After incubation, cells were fixed and stained with an anti-α-tubulin antibody as described in “Materials and methods”. Bar = 10 μM
Fig. 5.
Effects of taxol on the release of IL-8 by rGrA thrombin, and TRAP-6 in A549 cells. A549 cells were incubated for 24 h with SFM, SFM containing 300 nM rGrA (rGrA), SFM containing 5 nM thrombin (Thrombin), and SFM containing 100 μM TRAP-6 (TRAP-6) in the absence and presence of 5 μM taxol. Data are expressed as means ± SE from three independent experiments performed in duplicate. * P < 0.05 versus A549 cells treated with SFM containing rGrA (two-tailed, unpaired t-test)
Note that inhibition of rGrA-promoted IL-8 release by taxol was only partial. This suggests that there are additional mechanisms underlying rGrA-promoted IL-8 release in A549 cells. It seems unlikely that rGrA activates PAR-2 and PAR-4 to promote it. This is supported by the observation that rGrA elicited no [Ca2+]i responses in A549 cells (Fig. 3a). GrA has been known to convert pro-IL-1β into its biologically active form via proteolytic cleavage (Irmler et al. 1995). It has also been shown that IL-1β is produced in A549 cells (Asokananthan et al. 2002) and that exogenous IL-1β stimulates IL-8 release from A549 cells (Boost et al. 2008). Therefore, it is tempting to speculate that rGrA-promoted IL-8 release from A549 cells occurs via a mechanism partially involving activation of pro-IL-1β. The primary function of extracellular GrA would be the degradation of extracellular matrix for tissue remodeling (Hirayasu et al. 2008; Kam et al. 2000; Yoshikawa et al. 2008a). It is therefore likely that the release of inflammatory mediators caused by extracellular GrA, if any, is secondary to tissue remodeling. On the other hand, thrombin besides its role in blood coagulation contributes largely to the production of inflammatory mediators through its potent ability to activate PAR-1 and PAR-4.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (18580118 to S. Tsuzuki) from the Japan Society for the Promotion of Science.
Abbreviations
- BLT
N-α-benzyloxy l-lysine thiobenzyl ester
- DTNB
5,5′-Dithiobis-(2-nitrobenzoic acid)
- GrA
Granzyme A
- IL
Interleukin
- PARs
Protease-activated receptors
- rGrA
Recombinant form of rat GrA
- SFM
Serum-free Dulbecco’s modified Eagle medium supplemented with 0.1% w/v BSA
References
- Ahn HS, Foster C, Boykow G, et al. Inhibition of cellular action of thrombin by N3-cyclopropyl- 7-[[4-(1-methylethyl)phenyl]methyl]-7H-pyrrolo[3, 2-f]quinazoline-1, 3-diamine (SCH 79797), a nonpeptide thrombin receptor antagonist. Biochem Pharmacol. 2000;60:1424–1434. doi: 10.1016/S0006-2952(00)00460-3. [DOI] [PubMed] [Google Scholar]
- Asokananthan N, Graham PT, Fink J, et al. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J Immunol. 2002;168:3577–3585. doi: 10.4049/jimmunol.168.7.3577. [DOI] [PubMed] [Google Scholar]
- Boost KA, Sadik CD, Bachmann M, et al. IFN-gamma impairs release of IL-6 by IL-1beta-stimulated A549 lung carcinoma cells. BMC Cancer. 2008;8:265. doi: 10.1186/1471-2407-8-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury D, Lieberman J. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu Rev Immunol. 2008;26:389–420. doi: 10.1146/annurev.immunol.26.021607.090404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayasu H, Yoshikawa Y, Tsuzuki S, et al. Sulfated polysaccharides derived from dietary seaweeds increase the esterase activity of a lymphocyte tryptase, granzyme A. J Nutr Sci Vitaminol (Tokyo) 2005;51:475–477. doi: 10.3177/jnsv.51.475. [DOI] [PubMed] [Google Scholar]
- Hirayasu H, Yoshikawa Y, Tsuzuki S, et al. A role of a lymphocyte tryptase, granzyme A, in experimental ulcerative colitis. Biosci Biotechnol Biochem. 2007;71:234–237. doi: 10.1271/bbb.60354. [DOI] [PubMed] [Google Scholar]
- Hirayasu H, Yoshikawa Y, Tsuzuki S, et al. A lymphocyte serine protease granzyme A causes detachment of a small-intestinal epithelial cell line (IEC-6) Biosci Biotechnol Biochem. 2008;72:2294–2302. doi: 10.1271/bbb.80140. [DOI] [PubMed] [Google Scholar]
- Irmler M, Hertig S, MacDonald HR, et al. Granzyme A is an interleukin 1 beta-converting enzyme. J Exp Med. 1995;181:1917–1922. doi: 10.1084/jem.181.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam CM, Hudig D, Powers JC. Granzymes (lymphocyte serine proteases): characterization with natural and synthetic substrates and inhibitors. Biochim Biophys Acta. 2000;1477:307–323. doi: 10.1016/s0167-4838(99)00282-4. [DOI] [PubMed] [Google Scholar]
- Parry MA, Myles T, Tschopp J, et al. Cleavage of the thrombin receptor: identification of potential activators and inactivators. Biochem J. 1996;320:335–341. doi: 10.1042/bj3200335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Hollenberg MD. Proteinases and signaling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol. 2008;153:5263–5282. doi: 10.1038/sj.bjp.0707507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sower LE, Klimpel GR, Hanna WD, et al. Extracellular activities of human granzymes. I. Granzyme A induces IL-6 and IL8 production in fibroblast and epithelial cell lines. Cell Immunol. 1996;171:159–163. doi: 10.1006/cimm.1996.0187. [DOI] [PubMed] [Google Scholar]
- Spaeny-Dekking EH, Hanna WL, Wolbink AM, et al. Extracellular granzymes A and B in humans: detection of native species during CTL responses in vitro and in vivo. J Immunol. 1998;160:3610–3616. [PubMed] [Google Scholar]
- Steinhoff M, Buddenkotte J, Shpacovitch V, et al. Proteinase-activated receptors: Transduces of proteinase-mediated signaling in inflammation and immune response. Endocrine Rev. 2005;26:1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- Suidan HS, Bouvier J, Schaerer E, et al. Granzyme A released upon stimulation of cytotoxic T lymphocytes activates the thrombin receptor on neuronal cells and astrocytes. Proc Natl Acad Sci USA. 1994;91:8112–8116. doi: 10.1073/pnas.91.17.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay GM, Wolbink AM, Cormier Y, et al. Granzyme activity in the inflamed lung is not controlled by endogenous serine protease inhibitors. J Immunol. 2000;174:5332–5340. doi: 10.4049/jimmunol.165.7.3966. [DOI] [PubMed] [Google Scholar]
- Tsuzuki S, Kokado Y, Satomi S, et al. Purification and identification of a binding protein for pancreatic secretory trypsin inhibitor: A novel role of the inhibitor as an anti-granzyme A. Biochem J. 2003;372:227–233. doi: 10.1042/BJ20021891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooy JH, Moller GM, Suylen RJ, et al. Increased granzyme A expression in type II pneumocytes of patients with severe chronic obstructive pulmonary disease. Am J Crit Care Med. 2007;175:464–472. doi: 10.1164/rccm.200602-169OC. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Hirayasu H, Tsuzuki S, et al. Granzyme A causes detachment of alveolar epithelial A549 cells accompanied by promotion of interleukin-8 release. Biosci Biotechnol Biochem. 2008;72:2481–2484. doi: 10.1271/bbb.80362. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Hirayasu H, Tsuzuki S, et al. Carrageenan inhibits granzyme A-induced detachment of and interleukin-8 release from alveolar epithelial A549 cells. Cytotechnology. 2008;58:63–67. doi: 10.1007/s10616-008-9175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]