Abstract
The purpose of this study is to investigate the effects of berbamine (BER), a naturally occurring small-molecule compound from Traditional Chinese Medicine (TCM) Berberis amurensis, on the growth and migration of human lung cancer A549 cell line. This cell line is the non–small cell lung cancer (NSCLC) which constitutes 80% of lung cancer cases and remains an aggressive lung cancer associated with a poor patient survival. Our present results have shown that BER significantly suppressed the in vitro and ex vivo growth of A549 cells in dose- and time-dependent manners. Furthermore, Western blot analysis confirmed that BER dose-dependently down-regulated the expression of anti-apoptotic protein Bcl-2 and up-regulated the level of pro-apoptotic protein Bax, eventually leading the reduction of Bcl-2/Bax protein ratio in A549 cells. In addition, BER significantly inhibited the A549 cell migration at the low concentrations without restraining the cell growth. More importantly, BER significantly enhanced the anticancer activity of anticancer agents such as trichostatin A (the histone deacetylase inhibitor) and celecoxib (the inhibitor of cyclooxygenase-2) by strongly reducing the viability and/or the Bcl-2/Bax protein ratio in A549 cells. Our findings suggest that BER may have the wide therapeutic and/or adjuvant therapeutic application in the treatment of human NSCLC.
Keywords: Berbamine, Trichostatin A, Celecoxib, Human lung cancer, Growth, Migration, Bcl-2/Bax
Introduction
Lung cancer is the leading cause of cancer-related mortality in the world (Parkin et al. 2005; Jemal et al. 2008). Despite the availability of assorted standard treatments, such as surgery, radiotherapy, chemotherapy, or combined regiments, non–small cell lung cancer (NSCLC), which constitutes 80% of lung cancer cases, remains an aggressive lung cancer associated with a poor patient survival. The patients with advanced disease have a median survival of approximately 10 months when treated with standard platinum-based therapy. Hence, there is an imminent need for better therapies and/or anticancer agents for NSCLC. To date, chemotherapy has been the most frequently used treatment for lung cancer and other cancers. However, some normal cells are destroyed as well by this method of treatment. To find novel natural compounds with low toxicity and high selectivity of killing cancer cells is an important area in cancer research. Due to their wide range of biological activities and low toxicity in animal models, some natural products have been used as alternative treatments for cancer. Berbamine (BER) is a naturally occurring small-molecule compound from Traditional Chinese Medicine (TCM) Berberis amurensis. In China, BER has been used to treat the clinical patients with inflammation and various cancers including leukemia, hepatoma, breast cancer and other cancers for many years. BER is also a clinical drug to treat the patients with low levels of white blood cells, which are caused by chemotherapy and/or radiotherapy. BER induced apoptosis and growth inhibition of human leukemia HL-60 and K562 cell lines without cytotoxicity to normal hematopoietic cells (Dong et al. 1997; Sun et al. 2006; Xu et al. 2006). It induced caspase-3-dependent apoptosis of leukemia NB4 cells via survivin-mediated pathway (Zhao et al. 2007). BER also caused apoptosis and cell cycle arrest, and led to loss of mitochondrial membrane potential and activate caspase-3 and caspase-9 in human hepatoma cells (Wang et al. 2007). However, whether or not BER has inhibitory activities against human lung cancer cells is unclear. In this study, we investigated the in vitro and ex vivo effects of BER on the growth and migration of the human NSCLC A549 cell line as well as its mechanisms of action. We show that BER significantly reduced the in vitro and ex vivo viability of A549 cells and regulated the expressions of Bcl-2 and Bax proteins in the cancer cells in dose-dependent manners. BER also significantly inhibited A549 cell migration and enhanced the anticancer activity of anticancer agents by strongly reducing the viability and Bcl-2/Bax protein ratio in A549 cells.
Materials and methods
Materials
Fibronectin and Boyden chambers were purchased from BD Inc. (Franklin Lakes, NJ, USA) and Costar, Corning, Inc. (Corning, NY, USA), respectively. Berbamine (BER), trichostatin A (TSA), celecoxib (S), Bay 11-7082 (Bay), RPMI 1640, penicillin, streptomycin, fetal bovine serum (FBS), 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT), trypsin/EDTA, propidium iodide (PI), and all other chemicals employed in this study were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Animal experimentation and preparation of sera from BER-treated rabbits
These were done according to our published methods with slight modifications (Zhang et al. 2001). In brief, New Zealand White rabbits (3.5–4 kg; from Luye Pharmaceutical Company, Yantai, China) were treated in accordance with guidelines established by the Animal Care and Use Committee at Yantai University. BER was orally intubated to the rabbits once daily at a dose of 100 mg/ml/kg body weight for 3 days. On the third day, the blood was then collected at 0, 1, 2 and 3 h from the rabbits (fasted for 16 h) after oral intubation of BER. The collected blood was left to clot for 2 h at room temperature and centrifuged twice at 3000×g at 4 °C for 20 min. The sera were sterilized by filtration and then heated at 56 °C for 30 min. The prepared sera were aliquoted, and stored at −80 °C until ex vivo growth assay.
Cell culture and in vitro and ex vivo growth assays
The A549 lung adenocarcinoma cell line was obtained from the American Type Culture Collection. The human cancer cell line was incubated in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS), glutamine (2 mM), penicillin (100 U/ml) and streptomycin (100 μg/ml) at 37 °C in a humidified incubator with 95% air/5% CO2 atmosphere. The in vitro and ex vivo assays were done according to our published methods (Zhang et al. 1999; Zhang et al. 2001). The cells in control group were treated with DMSO (0.1%, final concentration) in in vitro assay. The cells were cultured in RPMI 1640 medium supplemented with 10% FBS (in the case of in vitro assay) containing different concentrations of BER or in the absence or presence of existing anticancer agents (TSA, celecoxib, Bay), or 10% rabbit serum (in the case of ex vivo assay) obtained at different time points after BER was orally intubated to the rabbits for 3 days. The cell viability was measured 24, 48, and 72 h after the treatments using MTT growth assay kit. Each experiment was repeated three times.
Morphological evaluation of apoptotic cells
This was done according to our published methods (Zhang et al. 2000). In brief, A549 cells at 70% confluence were respectively treated for 48 h with BER at concentrations of 0 (0.1% DMSO, vehicle as control), 10 μM, and 20 μM. The treated cells were fixed with 1% glutaraldehyde in PBS for 30 min at room temperature, washed in PBS, and stained with 1 mM Hoechst 33258 for 30 min at room temperature. The morphological changes in the nuclear chromatin were observed under a fluorescent microscope (Nikon, TE2000-U, Tokyo, Japan), using 40× lens.
Western blot analysis
This was performed according to the method published by Chen et al. (2001), Zhang et al. (2009) and Yu et al. (2009). In brief, A549 cells were treated with BER at different concentrations (0–60 μM) in the absence or presence of trichostatin A (TSA, 20 μg/L), Bay (5 μM) and celecoxib (S, 20 μM). The treated cells were collected at 48 h. Equal amounts of cell extracts were resolved by SDS–PAGE, transferred to nitrocellulose membranes, and probed with primary antibodies to human Bcl-2, Bax, and β-Actin and then horseradish-conjugated secondary antibodies, respectively. Anti-β-Actin antibody was used as a loading control. Detection was done using an enhanced chemiluminescence system (GE Healthcare Life Sciences).
In vitro migration assay
This was performed according to the method published by Zhang et al. (2009) and Liu et al. (2009). In brief, cancer cell migration was measured by examining cell migration through fibronectin-coated polycarbonate filters, using modified transwell chambers. In brief, A549 cells (5 × 104) were seeded into the upper chamber in 200 μL of serum-free medium containing BER at the concentrations of 0–5 μM, respectively. The cells in control group were treated with DMSO (0.1%, final concentration); the lower compartment was filled with 0.66 mL of RPMI 1640 medium supplemented with 10% of FBS (as a chemoattractant). After incubation for 6 h at 37 °C, the cells that migrated to the lower surface of the filter were fixed and stained using propidium iodide. The cells on the upper side of the filter were removed using a cotton swab. The migrated cells on the underside of the filter were counted and recorded for images under a fluorescent microscope (Nikon, TE2000-U, Tokyo, Japan). Each experiment was repeated three times.
Statistical analysis
The data were expressed as mean ± standard deviation (S.D.) and analyzed by the SPSS 13.0 software to evaluate the statistical difference. One-way or two-way ANOVA followed by the appropriate post hoc test (Bonferroni) was used to establish whether significant differences existed between groups. For confirming the synergistic effect between BER and TSA or S, comparison was made by two-way ANOVA followed by Bonferroni post hoc test. Statistical analysis was done using the ANOVA and Bonferroni test. Values between different treatment groups at different times were compared. Mean concentrations and cell viability or migration (%) are shown for each group; Asterisk p < 0.05, double asterisk p < 0.01. For all tests, P values less than 0.05 were considered statistically significant. All statistical tests were two-sided.
Results and discussion
In vitro and ex vivo effects of BER on the growth in A549 cells and the anticancer activity of anticancer agents against A549 growth
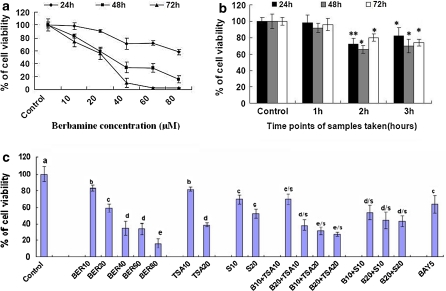
The in vitro growth assay showed that BER suppressed the growth of human lung cancer cell line A549 in dose- and time-dependent manners after the cells were treated with BER at concentrations of 10–80 μM for 24, 48 and 72 h, respectively (Fig. 1a). The ex vivo assay indicated that the rabbit sera obtained 2 h and 3 h after oral intubation of BER significantly suppressed the growth of A549 cells after the cells were treated with these sera for 24, 48 and 72 h, respectively, while the 1 h rabbit serum did not show the significant inhibitory effect (Fig. 1b). This result confirms that BER has a certain bioavailability by oral administration and the peak inhibition of the A549 cell growth is at 2 h after oral administration of BER in rabbits. There have been reports showing that the blood concentration of BER can be detected in rats (Li et al. 1995); BER appeared in blood and lung tissue of mice and rats after oral administration of BER (Lang et al. 1984; Yang et al. 1988). In the present study, we have also demonstrated that BER at the concentrations of 10 and 20 μM enhanced the anticancer activity of the anticancer agents trichostatin A at 10 and 20 μM (the histone deacetylase inhibitor) and celecoxib at 10 and 20 μM (the inhibitor of cyclooxygenase-2) by strongly reducing the viability of A549 cells after the cells were treated for 48 h with these agents (Fig. 1c). These results partially explain why BER has its therapeutic and/or adjuvant therapeutic effects on treatment for some cancer patients.
Fig. 1.
In vitro (a & c) and ex vivo (b) effects of berbamine (BER) and sera from BER-fed rabbits on the growth of human lung cancer A549 cells and the anticancer activity of anticancer agents against A549 growth. The cells were treated for 24, 48, and 72 h with BER at the concentrations indicated (a) or the sera taken from rabbits (n = 6 for each group at different time points) at 0 (as the control group), 1, 2 and 3 h after oral administration of BER in rabbits (b), respectively. (c) In vitro effects of BER on the anticancer activity of anticancer agents against A549 cell growth. The cells were treated for 48 h with BER at 10–80 μM (BER10, BER20, BER40, BER80) in the absence or presence of the anticancer agents trichostatin A (TSA) at 10 and 20 μg/L (TSA10, TSA20, the histone deacetylase inhibitor) and celecoxib at 10 and 20 μM (S10, S20, the inhibitor of cyclooxygenase-2). The effects on the A549 cell growth were examined by the MTT assay in vitro and ex vivo as described in the section of “Materials and methods”. The data are presented as the mean ± SD (Bar) for each group (n = 6). The figures (a, b and c) are the representative of 3 similar experiments performed. Statistical analysis was carried out using the ANOVA and Bonferroni test. * p < 0.05, ** p < 0.01. Values with different letters (a–e) differ significantly (p < 0.05). c/s, d/s and e/s represent the significant synergistic effects (p < 0.05) compared with the treatment with its individual compound alone. Statistically significant synergistic effects on the growth were observed in A549 cells treated with B10+TSA10, B20+TSA10, B10+TSA20, B20+TSA20, B10+S10, B20+S10, or B20+S20 compared with the individual B10, B20, TSA10, TSA20, S10, and/or S20 treatment alone (c/s: B10+TSA10, p < 0.001, two-way ANOVA; d/s: B20+TSA10, p < 0.001, two-way ANOVA; d/s: B10+S10, p < 0.001, two-way ANOVA; d/s: B20+S10, p < 0.001, two-way ANOVA; d/s: B20+S20, p < 0.001, two-way ANOVA; e/s: B10+TSA20 p < 0.001, two-way ANOVA; e/s: B20+TSA20, p < 0.0001, two-way ANOVA)
BER induced apoptosis by reducing the Bcl-2/Bax protein ratio in A549 cells
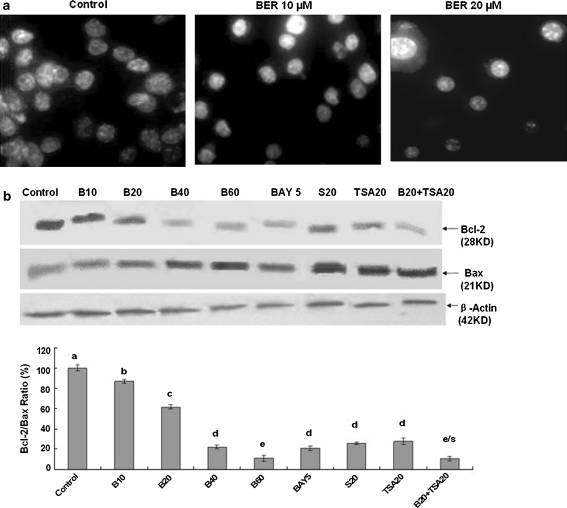
To understand the mechanisms of action of BER against the growth of human lung cancer cells, we investigated the effects of BER on apoptosis and expressions of Bcl-2/Bax proteins in A549 cells. Hoechst 33258 staining showed that the typical morphological changes, such as formation of apoptotic bodies appeared in A549 cells after the cells were treated for 48 h with BER at 10 and 20 μM, whereas the control cells without BER treatment did not show the evident apoptotic morphological changes (Fig. 2a). Furthermore, Western blot analysis confirmed that BER dose-dependently down-regulated the expression of anti-apoptotic protein Bcl-2 and up-regulated the level of pro-apoptotic protein Bax, eventually leading the reduction of the Bcl-2/Bax protein ratio in A549 cells (Fig. 2b). In addition, BER at 20 μM enhanced the activity of anticancer agent TSA at 20 μM by reducing Bcl-2 level and increasing Bax expression, causing the reduction of the Bcl-2/Bax protein ratio in A549 cells. As the positive control, Bay (the inhibitor of NF-κB) and celecoxib also strongly reduced the Bcl-2/Bax protein ratio in A549 cells (Fig. 2b). There have been studies showing that Bcl-2 and its dominant inhibitor Bax are key regulators of cell proliferation and apoptosis. Overexpression of Bcl-2 enhances cell survival by suppressing apoptosis, but overexpression of Bax accelerates cell death (Oltvai et al. 1993). Induction of apoptosis and decrease in the Bcl-2/Bax protein ratio by BER may be one of the important mechanisms of action of BER against the lung cancer cell growth by reducing the cancer cell viability.
Fig. 2.
In vitro effects of BER on induction of apoptosis (a) and reduction of ratios of Bcl-2/Bax proteins (b) in A549 cells by BER with its synergistic anticancer agents. a Induction of apoptosis in A549 cells by treatment for 48 h with BER at concentrations of 0 (0.1% DMSO vehicle as the control), 10, and 20 μM. The treated cells were stained with Hoechst 33258 and the apoptotic morphological changes in the nuclear chromatin were observed under a fluorescent microscope as described in the “Materials and methods” section. b Reduction of Bcl-2/Bax proteins in A549 cells by treatment for 48 h with BER at the concentrations of 10–60 μM (B10, B20, B40, B60) in the absence or presence of trichostatin A (TSA20) at 20 μg/L. Bay at 5 μM (BAY5, the inhibitor of NF-κB) and celecoxib at 20 μM (S20, the inhibitor of cyclooxygenase-2) were used as the positive control. The protein expressions of Bcl-2 and Bax were analyzed by Western blotting as described in the “Materials and methods” section. Anti-ß-Actin antibody was used as a sample loading control. The ratio of Bcl-2 and Bax, (the ratio of relative density of each band normalized to β-actin), shown as mean ± SD (Bar) is relative to that of 0 (0.1% DMSO vehicle) as the control (100%). For one experiment, 3 assays were carried out and only one set of gels is shown. Values with different letters (a–e) differ significantly (p < 0.05); e/s represents the significant synergistic effect (p < 0.05) compared with the treatment with its individual compound alone (e/s: B20+TSA20, p < 0.001, two-way ANOVA)
In vitro effects of BER on the A549 cell migration
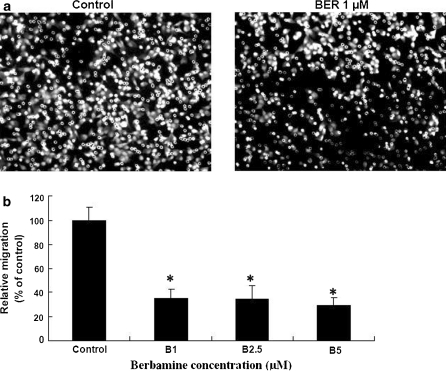
Abnormal growth and metastasis of cancer cells are regarded as the important biological characteristics of cancers. The presence of metastasis is the main cause of morbidity and mortality in millions of patients with cancer. During the complicated process of metastasis, the invasion of cancer cells is the most important and characteristic step. The migration of cancer cells is one of the important steps during the invasion. Therefore, we examined the effects of BER on the migration of A549 cells. The migration assay indicated that BER at the concentrations of 1–5 μM significantly reduced the A549 cell migration rate more than 65–72% after the cells were treated for 6 h with BER (Fig. 3). The treatment of A549 cells with different concentrations of BER (1–5 μM) for 24 h did not significantly reduce the viability of these cells (data not shown). This suggests that the inhibition of A549 cell migration by BER is not the result from the reduction of A549 cell viability. There have been studies showing that activation of PI3K/Akt and NF-κB increases the migration of cancer cell lines such as A549 (Huang et al. 2009) and MDA-MB-231 (Wei et al. 2008) cells, which can be suppressed by Ly294002, the inhibitor of PI3K/Akt. Our recent results have confirmed that BER at a low concentration without inhibiting the cell growth showed significant suppression of the phosphorylation of Akt and NF-κB expression as well as the cell migration in MDA-MB-231 cells (Wang et al. 2009). In the present study, suppression of A549 cell migration in the low concentrations of BER without restraining the cell growth may be involved in inhibiting the phosphorylation of Akt and NF-κB in the cancer cells. However, the mechanisms of action of BER against the A549 cell migration require further investigation.
Fig. 3.
In vitro effects of BER on the migration of human lung cancer A549 cells. The A549 cell migration was examined by the migration assay as described in the section of “Materials and methods”. a The photos show the propidium iodide-stained A549 cells that migrated through fibronectin-coated transwell chamber. The cells were treated for 6 h with either the vehicle (0.1% DMSO as the control) or BER at 1 μM. b Suppression of A549 cell migration by BER. The cells were treated for 6 h with BER at the concentrations indicated in the figure. Relative migration (%) ± SD (n = 6) are shown for the indicated BER concentrations and the cells in control group were treated with DMSO (0.1%, final concentration). The figure is the representative of 3 similar experiments performed. Statistical analysis was carried out using the ANOVA and Bonferroni test. * p < 0.05
BER has been used as an anticancer drug for many years in China. BER was reported to induce apoptosis in human hepatoma, colorectal adenocarcinoma, and leukemia cells (Dong et al. 1997; Wang et al. 2007; Xu et al. 2006). Our present results have confirmed that BER significantly suppressed the growth of human lung cancer A549 cells in vitro and ex vivo. Furthermore, we have also demonstrated that the induction of apoptosis by reducing the Bcl-2/Bax protein ratio in A549 cells is one of the important mechanisms of action of BER against the cancer cell growth. In addition, our results also indicate that BER reduced the A549 cell migration rate by more than 65–72% at the low concentrations without affecting the cell viability. More importantly, we show that BER enhanced the anticancer activity of the anticancer agents TSA and celecoxib by reducing the viability and/or the Bcl-2/Bax protein ratio in A549 cells. All these findings suggest that BER may have the wide therapeutic and/or adjuvant therapeutic application in the treatment of human NSCLC.
Acknowledgments
Grant support This work is supported in part by grants from the Ministry of Education of the People’s Republic of China to G.Z, the Ministry of Human Resources and Social Security of the People’s Republic of China to G.Z, Projects of Yantai University to GZ, Project from the National Natural Science Foundation of China to GZ (No. 30973553), and grants from the Department of Science and Technology of Shandong Province to GZ (Y2008C71; 2009GG10002087).
The authors declare no conflict of interest.
Abbreviations
- BER
Berbamine
- T
trichostatin A
- Bay
Bay 11-7082
- S
Celecoxib
- NSCLC
Non–small cell lung cancer
Footnotes
Huiying Duan and Jinling Luan contributed equally to this work.
References
- Chen Q, Gong B, Mahmoud-Ahmed AS, Zhou A, Hsi ED, Hussein M, Almasan A. Apo2L/TRAIL and Bcl-2-related proteins regulate type I interferon-induced apoptosis in multiple myeloma. Blood. 2001;98:2183–2192. doi: 10.1182/blood.V98.7.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Yang MM, Kwan CY. In vitro inhibition of proliferation of HL-60 cells by tetrandrine and coriolus versicolor peptide derived from Chinese medicinal herbs. Life Sci. 1997;60:135–140. doi: 10.1016/S0024-3205(96)00695-9. [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu Q, Liu K, Yagasaki K, Zhang G. Suppression of growth of highly-metastatic human breast cancer cells by norcantharidin and its mechanisms of action. Cytotechnology. 2009;59:201–208. doi: 10.1007/s10616-009-9210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Lang WF, Liu ZM, Wang WB, Ding SF, Yi MG. Studies on berbamine metabolism and its distribution with some Chinese herb in the pathologic lung tissue. J Hygiene Res. 1984;13:33–37. [Google Scholar]
- Li B, Gao Y, Li H. Determination of berbamine in rat plasma by reversed phase high performance liquid chromatography. J Harbin Med Univ. 1995;29:201–202. [Google Scholar]
- Liu Q, Duan H, Luan JL, Yagasaki K, Zhang G. Effects of theanine on growth of human lung cancer and leukemia cells as well as migration and invasion of human lung cancer cells. Cytotechnology. 2009;59:211–217. doi: 10.1007/s10616-009-9223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman C, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-O. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Sun JR, Zhang XH, He ZW, Gu Y, Yu YZ, Fang YM, Lu QH, Dong QH, Xu RZ. The mechanism of apoptosis of chronic myeloid leukemia cells induced by the novel p210 bcr/abl inhibitor berbamine. Zhonghua Yi Xue Za Zhi. 2006;86:2246–2251. [PubMed] [Google Scholar]
- Wang GY, Zhang JW, Lu QH, Xu RZ, Dong QH. Berbamine induces apoptosis in human hepatoma cell line SMMC7721 by loss in mitochondrial transmembrane potential and caspase activation. J Zhejiang Univ Sci B. 2007;8:248–255. doi: 10.1631/jzus.2007.B0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Liu Q, Zhang Y, Liu K, Yu PF, Liu K, Luan JL, Duan H, Lu ZQ, Wang FF, Wu E, Yagasaki K, Zhang G. Suppression of growth, migration and invasion of highly-metastatic human breast cancer cells by berbamine and its molecular mechanisms of action. Mol Cancer. 2009;8:81. doi: 10.1186/1476-4598-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YY, Chen YJ, Hsiao YC, Huang YC, Lai TH, Tang CH. Osteoblasts-derived TGF-beta1 enhance motility and integrin upregulation through Akt, ERK, and NF-kappaB-dependent pathway in human breast cancer cells. Mol Carcinog. 2008;47:526–537. doi: 10.1002/mc.20411. [DOI] [PubMed] [Google Scholar]
- Xu R, Dong Q, Yu Y, Zhao X, Gan X, Wu D, Lu Q, Xu X, Yu XF. Berbamine: a novel inhibitor of bcr/abl fusion gene with potent anti-leukemia activity. Leuk Res. 2006;30:17–23. doi: 10.1016/j.leukres.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Yang F, Jin SY, Li YX, Li WH, Yi MG. Absorption, distribution and elimination of tritium labeled berbamine. J Harbin Med Univ. 1988;22:103–104. [Google Scholar]
- Yu PF, Liu Q, Liu K, Yagasaki K, Wu E, Zhang G. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-κB signaling. Cytotechnology. 2009;59:219–229. doi: 10.1007/s10616-009-9225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Miura Y, Yagasaki K. Effects of green, oolong and black teas and related components on the proliferation and invasion of hepatoma cells in culture. Cytotechnology. 1999;31:37–44. doi: 10.1023/A:1008076306672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Miura Y, Yagasaki K. Induction of apoptosis and cell cycle arrest in cancer cells by in vivo metabolites of teas. Nutr Cancer. 2000;38:265–273. doi: 10.1207/S15327914NC382_16. [DOI] [PubMed] [Google Scholar]
- Zhang G, Miura Y, Yagasaki K. Inhibitory effects of theanine and sera from theanine-fed rats on receptor-mediated cancer cell beneath mesothelial-cell monolayers. Cytotechnology. 2001;36:195–200. doi: 10.1023/A:1014005423181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang H, Yu PF, Liu Q, Duan H, Luan GL, Yagasaki K, Zhang G. Effects of matrine against the growth of human lung cancer and hepatoma cells as well as lung cancer cell migration. Cytotechnology. 2009;59:191–200. doi: 10.1007/s10616-009-9211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XY, He ZW, Wu D, Xu RZ. Berbamine selectively induces apoptosis of human acute promyelocytic leukemia cells via survivin-mediated pathway. Chin Med J (Engl) 2007;120:802–806. [PubMed] [Google Scholar]