Gold Catalysis
Gold(I)-catalyzed carbon-carbon bond forming reactions continue to fascinate the synthetic community but are less well investigated than many conventional metal catalysts.[1] In many cases a cationic gold(I)-species activates an unsaturated C-C bond and isomerizes or functionalizes it to build molecular complexity through reactive intermediates that include gold-π-complexes, gold-vinyls, and gold-carbenes. Support for these intermediates is mainly based on gold(I) organometallic chemistry, though computational studies and the isolation of proposed catalytic intermediates have been reported.[2–5]
We report mechanistic studies on a seemingly simple gold(I)-catalyzed reaction and demonstrate the importance of dinuclear organometallic intermediates. While similar dinuclear species have been postulated in DFT studies,[5b] di-gold intermediates have yet to be detected in situ and their reactivity characterized.
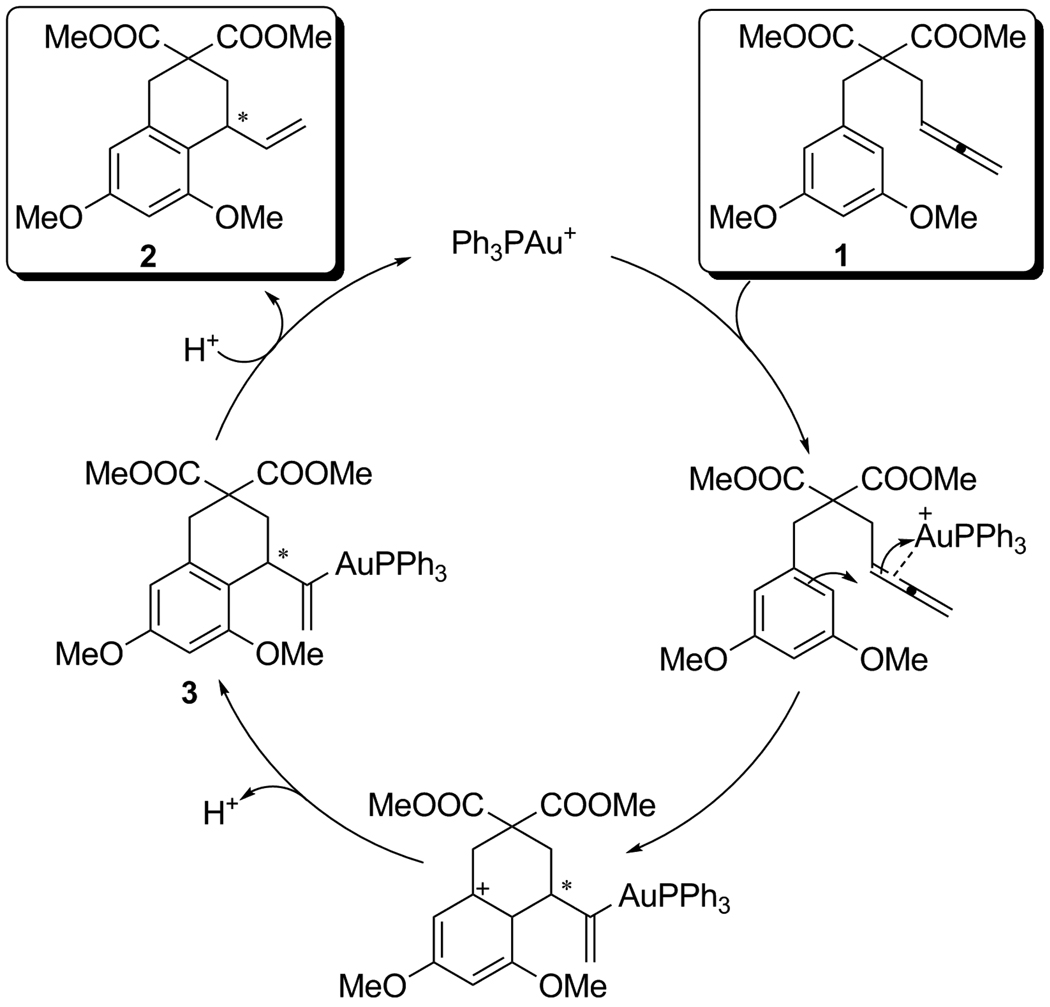
We previously showed that the transformation of 1 to 2 could be catalyzed by R3PAu+.[6] The proposed cycle is shown in Scheme 1, with R3PAu+ initiating the process by π-coordination to 1,[7] which activated it for nucleophilic attack by the aromatic ring.[1b] Subsequent rearomatization generated vinyl-gold(I) complex 3, which protodeaurated with the generated acid to give 2 and R3PAu+.[8] The seemingly well-behaved nature of this reaction led us to initiate studies to refute or validate this mechanism, and this has led to surprising results concerning the nature of the catalyst resting state.
Scheme 1.
Proposed cycle for the gold(I)-catalyzed hydroarylation of allenes.
Preliminary studies identified the Gagosz catalyst Ph3PAuNTf2 (4) as a convenient source of Ph3PAu+ that did not require in situ activation with silver salts.[9] Monitoring by 31P-NMR the reaction of 1 with 10 mol% 4 indicated that the catalyst rests as a single species 5a, with two distinct peaks in a 1:1 ratio (~36 ppm).[10] Upon completion of the reaction, these peaks diminished, and the Gagosz catalyst reappeared at 30 ppm. The stoichiometric reaction of 1 with 4 in the presence of 2,6-di-tert-butyl-pyridine (to inhibit protodeauration) resulted in incomplete consumption of 1, but gave the same peaks at 36 ppm. Addition of a second equivalent of 4 converted all starting material to 5a.
The 1H-NMR of isolated 5a showed a single carbocycle consistent with a vinyl-gold(I) connectivity, but with other features inconsistent with 3. These included the two peaks at ~36 ppm (31P-NMR) rather than the expected singlet at ~44 ppm,[3b–h] and the 2:1 ratio of the PPh3 to carbocycle resonance in the 1H-NMR. A closer examination of the multiplicity of the vinyl peaks in the 1H-NMR additionally revealed a heteronuclear coupling between the vinyl proton that was anti to Au and two phosphorous atoms, rather than the single phosphine expected for a simple vinyl-gold(I) complex like 3. These data therefore suggested that the resting state 5a was a doubly metalated variant of 3.
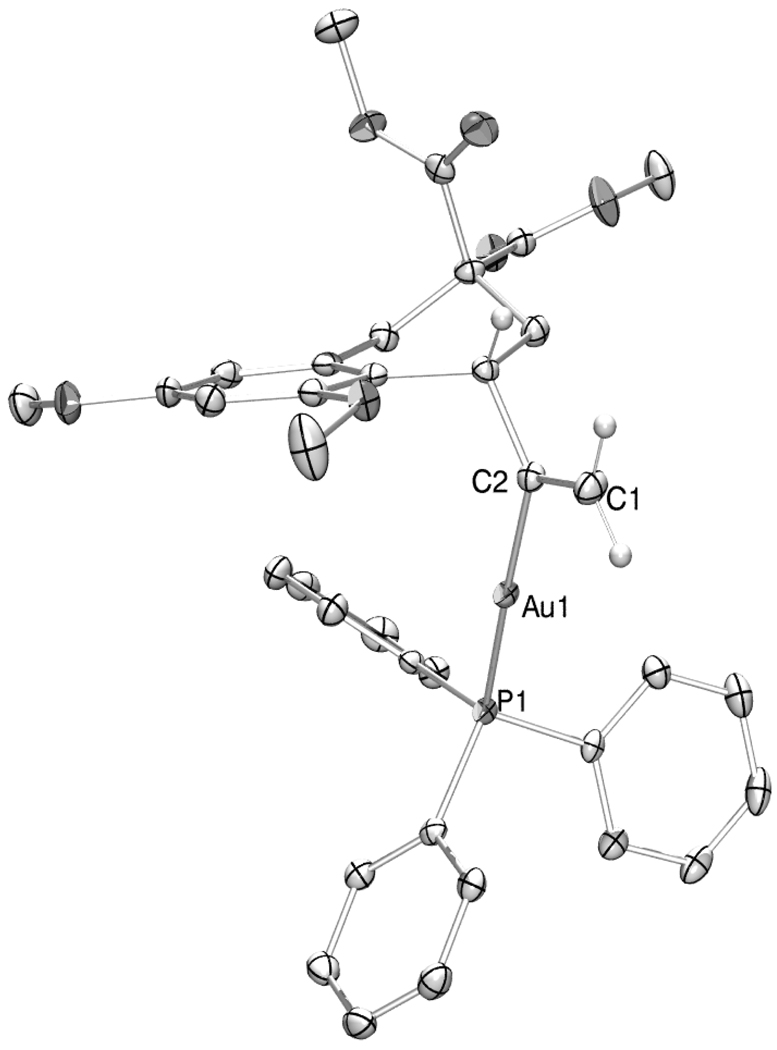
Compound 5a was air stable and amenable to aqueous workup without protodeauration; however attempts to purify it through Florisil® or silica led to immediate decomposition and elution of 2. On the other hand, neutral alumina cleanly converted 5a to 3, whose monometalated nature was confirmed by NMR[11] and X-Ray crystallography (Figure 1).[12,13] In contrast to 5a, the 1H-NMR showed large upfield shifts of the syn (6.43 ppm (5a) to 4.97 ppm (3)) and anti (5.91 ppm (5a) to 5.62 ppm (3)) vinyl hydrogens, suggesting significantly different chemical environments in the two compounds.
Figure 1.
ORTEP diagram of intermediate 3 with 50% probability ellipsoids; most hydrogen atoms omitted for clarity. Key bond lengths [Å] include: Au1-P1 [2.2913(5)], Au1-C2 [2.050(2)], and C1-C2 [1.324(4)].[13]
The reactivity of 3 and 5a also differed greatly.[3b–d,4] Treatment with excess acetic acid (pKa=4.76) immediately protodeaurated 3 (to 2),[14] while 5a was untouched and unreactive with even α-bromo-acetic acid (pKa=2.86) (12 h). Excess TFA (pKa=−0.25), however, resulted in a rapid decomposition to only traces of 2. The second equivalent of Ph3PAu+ in 5a therefore hinders protodeauration.
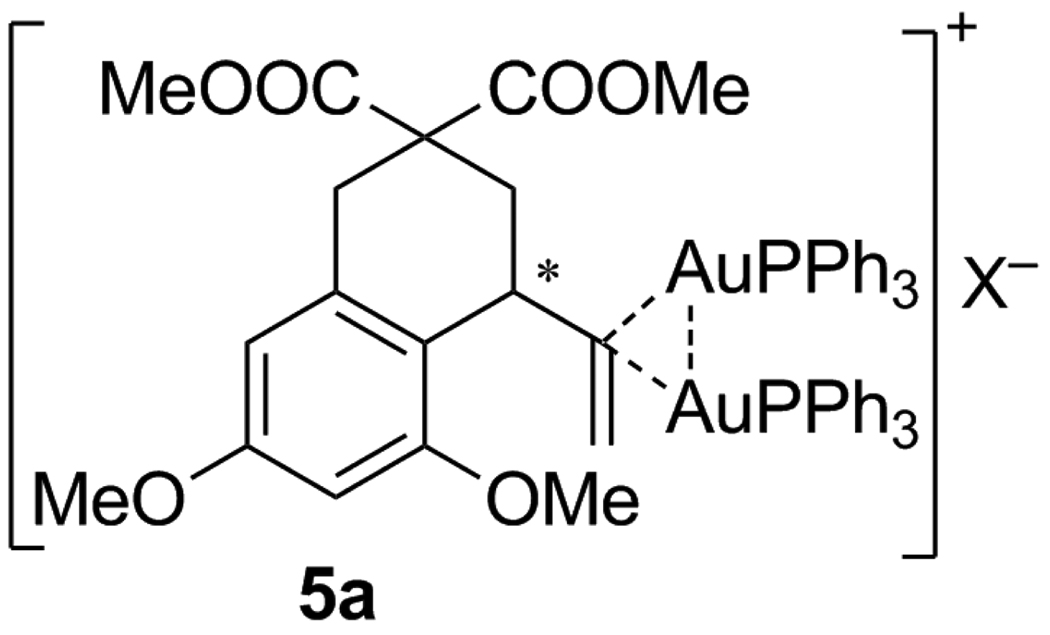
These spectroscopic and reactivity observations coupled with the work of Grandberg,[4] Nesmeyanov,[15b,c] and Schmidbaur[3g] on arylgold(I) organometallic complexes suggested that 5a was a diaurated structure that engaged the vinyl anion in a bridging 3-center-2-electron mode and was stabilized by a Au-Au interaction (Scheme 2). X-ray crystal structures of numerous bridging aryl compounds are known,[15] and a recent computational study implicated the intermediacy of a bridging vinyl in the cycloisomerization of ene-ynes.[5b] The phosphines in such a chiral structure would be diastereotopic and account for the inequivalent resonances in the 31P-NMR.
Scheme 2.
Proposed structure of resting state 5a (X=NTf2).
Grandberg has reported the geminally diaurated parent species [(Ph3PAu)2CH=CH2]+[BF4]− to be unstable and characterizable only by its IR and reactivity;[16] the more crowded nature of 5a presumably contributes to its thermal stability. The enhanced acid stability of a bridging vinyl can be rationalized by noting that the three-center two-electron bond should be more electron deficient and crowded than the traditional vinyl-gold(I) complex 3. The stabilizing Au-Au bond interactions are well established and measured to be worth 5–10 kcal mol−1.[17]
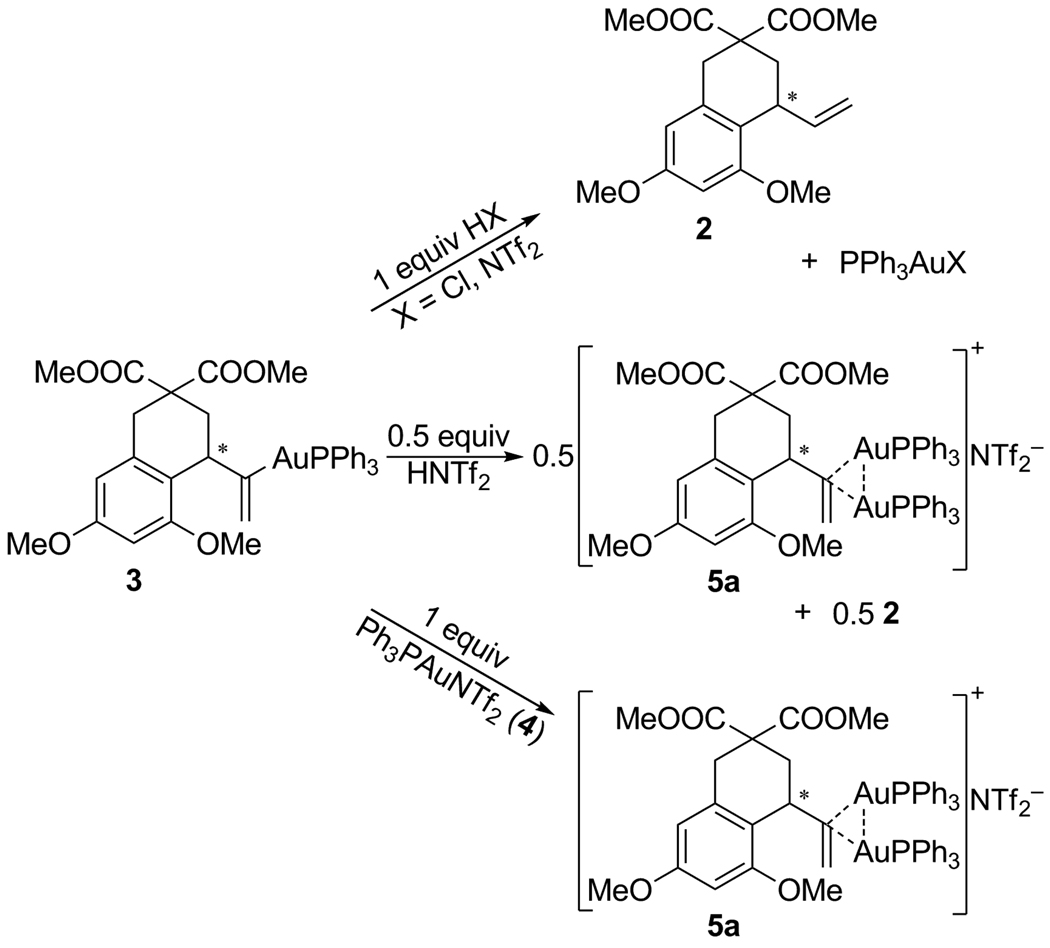
The isolation of 3 and 5a provided a rare opportunity to study the comparative reactivity of bone fide reaction intermediates. Compound 3 was found to readily react with stoichiometric quantities of HX (X = Cl, NTf2) to yield 2 and the expected gold(I) species (Scheme 3).[18] When only 0.5 equivalent of HNTf2 was added, clean conversion to 5a and 2 occurred. Similarly clean and rapid was the conversion of 3 and 4 to 5a, a process that presumably competes favourably with protodeauration under catalysis conditions.
Scheme 3.
Reactivity of intermediate 3.
In reactivity paralleling that of Nesmeyanov and Grandberg in diaurated aryl compounds, 5a extrudes Ph3PAuL and 3 upon reaction with a suitable ligand [Eq. 1]. Although 5a and 3 were expectedly unreactive with 1, they could each be activated with HNTf2 to reveal 4. The subsequent conversion of 1 to 2 showed the viability of both 3 and 5a as catalytic intermediates [Eq. 2].
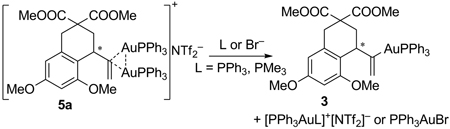 |
(1) |
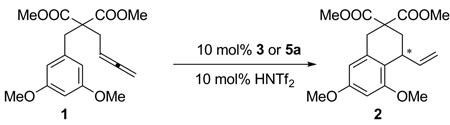 |
(2) |
To elucidate whether the resting of the catalyst at 5a was dependent on NTf2−, different silver salts were investigated in an in situ activation protocol (Table 1). In each case, 5a–e was observed by 31P-NMR suggesting a purely outer sphere role for the anions with the possible exception of OTf− which generated a broadened singlet at 36 ppm.
Table 1.
Activation protocol with different silver salts.
| Entry | Catalyst/Activator | Resting State[a] |
|---|---|---|
| 1 | Ph3PAuNTf2 | 5a |
| 2 | Ph3PAuCl / AgNTf2 | 5a |
| 3 | Ph3PAuCl / AgPF6 | 5b[b] |
| 4 | Ph3PAuCl / AgSbF6 | 5c[c] |
| 5 | Ph3PAuCl / AgOTf | 5d[d] |
| 6 | Ph3PAuCl / AgBF4 | 5e[e] |
Monitored by 31P-NMR.
X = PF6 (5b) Signals at 35.4 ppm and 35.1 ppm were observed.
X = SbF6 (5c) Signals at 35.7 ppm and 35.4 ppm were observed.
X = OTf (5d) A broad peak 35.8 ppm was observed instead of two sharp singlets.
X = BF4 (5e) Signals at 35.9 ppm and 35.6 ppm were observed.
The above results indicate that 3 and 5a are each viable intermediates in the intramolecular hydroarylation of allenes and that an additional structure needs to be added to the mechanism in Scheme 1. The bridging structure in 5a was found to be considerably more stable than 3, and served to sequester the key Lewis acidic species Ph3PAu+ more quickly than the latter promoted allene activation. Although 5a was less reactive to Brønsted acids than 3, both could be activated by HNTf2 to generate catalytically active 4. It is not yet clear whether 5a operates on or off the cycle under true catalytic conditions, but it is clear that the mechanism is more complex than previously envisioned.
In conclusion, this report describes the synthesis, characterization, and reactivity of two intermediates in the Ph3PAu+-catalyzed cycloisomerization of arene-allenes. It reports, for the first time, the experimental viability of the computationally predicted diaurated reaction intermediates, and therefore provides important insights into the burgeoning field of gold catalysis.
Experimental Section
For experimental procedures and data see the supporting information.
Supplementary Material
Footnotes
We thank Prof. Dr. A. Stephen K. Hashmi for fruitful discussions and Dr. Peter White for assistance with crystallography (pwhite@unc.edu for correspondence). The Fulbright Foreign Student Program (for Dieter Weber) is gratefully acknowledged as is the National Institute of General Medicine (GM-60578).
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.a) Li Z, Brouwer C, He C. Chem.Rev. 2008;108:3239–3265. doi: 10.1021/cr068434l. [DOI] [PubMed] [Google Scholar]; b) Widenhoefer RA. Chem. Eur. J. 2008;14:5382–5391. doi: 10.1002/chem.200800219. [DOI] [PubMed] [Google Scholar]; c) Gorin DJ, Sherry BD, Toste FD. Chem. Rev. 2008;108:3351–3378. doi: 10.1021/cr068430g. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Arcadi A. Chem. Rev. 2008;108:3266–3325. doi: 10.1021/cr068435d. [DOI] [PubMed] [Google Scholar]; e) Jiménez-Núñez E, Echavarren AM. Chem. Rev. 2008;108:3326–3350. doi: 10.1021/cr0684319. [DOI] [PubMed] [Google Scholar]; f) Bongers N, Krause N. Angew. Chem. Int. Ed. 2008;47:2178–2181. doi: 10.1002/anie.200704729. [DOI] [PubMed] [Google Scholar]; g) Gorin DJ, Toste FD. Nature. 2007;446:395–403. doi: 10.1038/nature05592. [DOI] [PubMed] [Google Scholar]; h) Hashmi ASK. Chem. Rev. 2007;107:3180–3211. doi: 10.1021/cr000436x. [DOI] [PubMed] [Google Scholar]; i) Fürstner A, Davies PW. Angew. Chem. Int. Ed. 2007;46:3410–3449. doi: 10.1002/anie.200604335. [DOI] [PubMed] [Google Scholar]; j) Hashmi ASK, Hutchings GJ. Angew. Chem. Int. Ed. 2006;45:7896–7936. doi: 10.1002/anie.200602454. [DOI] [PubMed] [Google Scholar]
- 2.Recent examples of mechanistic studies on gold(I)-catalyzed reactions: Seidel G, Mynott R, Fürstner A. Angew. Chem. Int. Ed. 2009;48:2510–2513. doi: 10.1002/anie.200806059. Pérez AG, López CS, Marco-Contelles J, Faza ON, Soriano E, de Lera AR. J. Org. Chem. 2009;74:2982–2991. doi: 10.1021/jo802516k. Mauleón P, Krinsky JL, Toste FD. J. Am. Chem. Soc. 2009;131:4513–4520. doi: 10.1021/ja900456m.
- 3.Selected examples of X-ray crystal structures of proposed gold(I)-intermediates: Gold(I)-carbodicarbene-complex: Fürstner A, Alcarazo M, Goddard R, Lehmann CW. Angew. Chem. Int. Ed. 2008;47:3210–3214. doi: 10.1002/anie.200705798. Vinyl-gold(I)-complexes: Hashmi ASK, Ramamurthi TD, Rominger F. J. Organomet. Chem. 2009;694:592–597. Shi Y, Ramgren SD, Blum SA. Organometallics. 2009;28:1275–1277. Mohr F, Falvello LR, Laguna M. Eur. J. Inorg. Chem. 2006:833–838. and references therein; Fañanás-Mastral M, Aznar F. Organometallics. 2009;28:666–668. Aryl-gold(I)-complexes: Partyka DV, Updegraff JB, III, Zeller M, Hunter AD, Gray TG. Organometallics. 2007;26:183–186. Porter KA, Schier A, Schmidbaur H. Organometallics. 2003;22:4922–4927. Raubenheimer HG, Desmet M, Kruger GJ. J. Chem. Soc. Dalton Trans. 1995:2067–2071. π-arene-gold(I) complex: Herrero-Gómez E, Nieto-Oberhuber C, López S, Benet-Buchholz J, Echavarren AM. Angew. Chem. Int. Ed. 2006;45:5455–5459. doi: 10.1002/anie.200601688. Chelated η2-alkene-gold(I) complex: Shapiro ND, Toste FD. Proc. Natl. Acad. Sci. USA. 2008;105:2779–2782. Cationic gold(I) π-alkene complex: Brown TJ, Dickens MG, Widenhoefer RA. J. Am. Chem Soc. 2009 doi: 10.1021/ja9015827.
- 4.a) Grandberg KI, Dyadchenko VP. J. Organomet. Chem. 1994;474:1–21. [Google Scholar]; b) Grandberg KI. Russ. Chem. Rev. 1982;51:249–262. [Google Scholar]
- 5. Liu L, Xu B, Mashuta MS, Hammond GB. J. Am. Chem. Soc. 2008;130:17642–17643. doi: 10.1021/ja806685j. For DFT studies see: Cheong PH, Morganelli P, Luzung MR, Houk KN, Toste FD. J. Am. Chem. Soc. 2008;130:4517–4526. doi: 10.1021/ja711058f.
- 6. Tarselli MA, Gagné MR. J. Org. Chem. 2008;73:2439–2441. doi: 10.1021/jo7024948. Tarselli MA, Liu A, Gagné MR. Tetrahedron. 2009;65:1785–1789. doi: 10.1016/j.tet.2008.10.110. . Other examples: Zhang Z, Liu C, Kinder RE, Han X, Qian H, Widenhoefer RA. J. Am. Chem. Soc. 2006;128:9066–9073. doi: 10.1021/ja062045r. Liu Z, Wasmuth AS, Nelson SG. J. Am. Chem. Soc. 2006;128:10352–10353. doi: 10.1021/ja0629110. Park C, Lee PH. Org. Lett. 2008;10:3359–3362. doi: 10.1021/ol801196g. Hashmi ASK, Schwarz L, Choi J, Frost TM. Angew. Chem. Int. Ed. 2000;39:2285–2288. doi: 10.1002/1521-3773(20000703)39:13<2285::aid-anie2285>3.0.co;2-f.
- 7.Gandon V, Lemière G, Hours A, Fensterbank L, Malacria M. Angew. Chem. Int. Ed. 2008;47:7534–7538. doi: 10.1002/anie.200802332. [DOI] [PubMed] [Google Scholar]
- 8.For this specific substrate regioselective elimination is observed, however some ene-allenes eliminate to a regioisomer mixture; see Tarselli MA, Chianese AR, Lee SJ, Gagné MR. Angew. Chem. Int. Ed. 2007;46:6670–6673. doi: 10.1002/anie.200701959.
- 9.Mézailles N, Ricard L, Gagosz F. Org. Lett. 2005;7:4133–4136. doi: 10.1021/ol0515917. [DOI] [PubMed] [Google Scholar]
- 10.Similar reactivity studies by 31P-NMR: Grisé CM, Barriault L. Org. Lett. 2006;8:5905–5908. doi: 10.1021/ol062582g. Grisé CM, Rodrigue EM, Barriault L. Tetrahedron. 2008;64:797–808.
- 11.The 31P-NMR showed the expected singlet at 43.0 ppm for a classical Ph3PAu-vinyl; see reference [3d].
- 12.Compound 3 is one of only a few vinyl-gold(I) species not stabilized by an electron withdrawing group; see reference [3d].
- 13.The observed distances are similar to those published by Laguna; see reference [3d]. X-ray crystal structure data for 3 at 100K: C36H36AuO6P, Mr =792.61 g mol−1, triclinic, space group P-1, a = 8.7814(5)Å, b = 13.7359(7)Å, c = 14.3110(7)Å, α = 105.099(2)°, β = 96.500(2)°, γ = 97.267(2)°, V = 1633.85(15)Å3, Z = 2, ρcalcd = 1.611 g cm−3, R1 = 0.0186 (0.0188), wR2 = 0.0448 (0.0449), for 5692 reflections with I>2σ(I) (for 5759 reflections (Rint = 0.0294) with a total of 27480 measured reflections), goodness of fit on F2 = 1.121, largest diff. peak (hole) = 0.0930 (−0.725 eÅ−3); see supporting information.
- 14.Compound 3 also protodeaurated on flash silica and only partially survives short plugs of alumina; see references [3c] and [5a] for comparison.
- 15.See reference [3g]; Usón R, Laguna A, Fernandez EJ, Media A, Jones PG. J. Organomet. Chem. 1988;350:129–138. Nesmeyanov AN, Perevalova EG, Grandberg KI, Lemenovskii DA, Baukova TV, Afanassova OB. J. Organomet. Chem. 1974;65:131–144. Nesmeyanov AN, Perevalova EG, Grandberg KI, Lemenovskii DA. Izv. Akad. Nauk SSSR, Ser. Khim. 1974;5:1124–1137. and references therein.
- 16.Grandberg KI, Smyslova EI, Kosina AN. Izv. Akad. Nauk SSSR, Ser. Khim. 1973;12:2787–2789. [Google Scholar]
- 17.Schmidbaur H. Chem. Soc. Rev. (London) 1995:391–400. [Google Scholar]
- 18.Products were not isolated but characterized by 1H- and 31P-NMR and compared to literature values (see supporting information).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.