Abstract
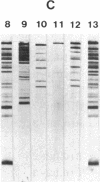
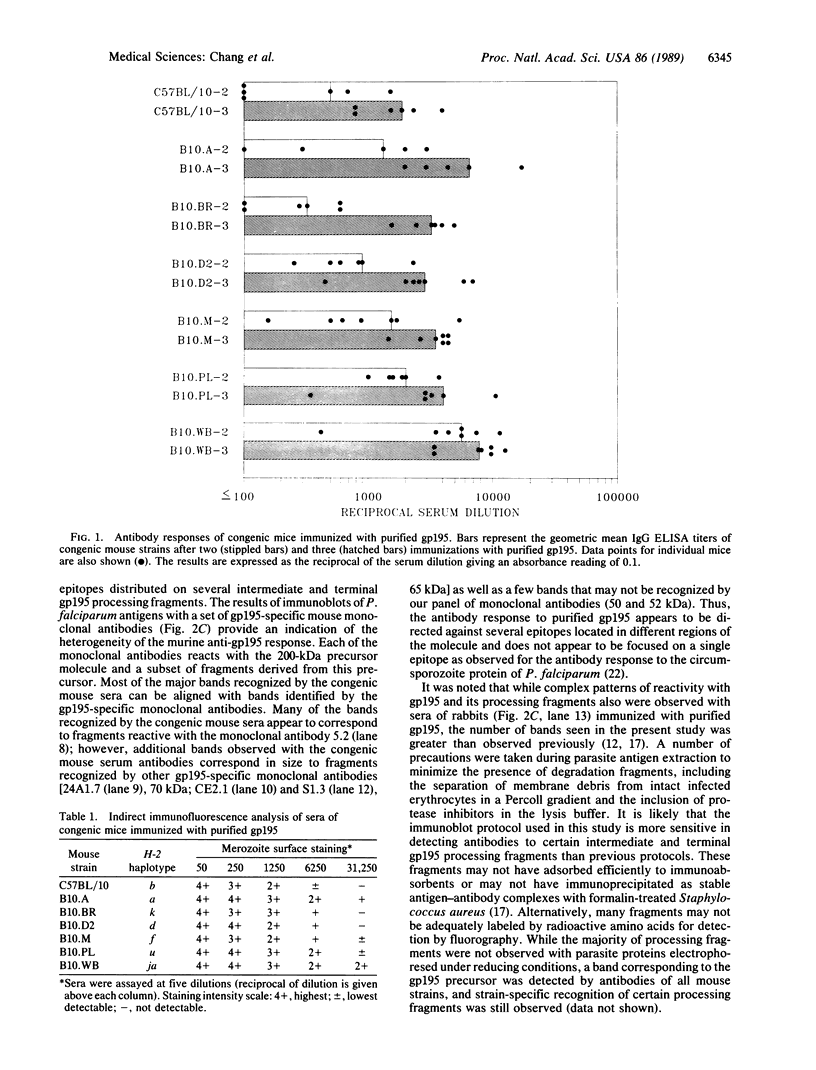
The antibody response to the Plasmodium falciparum major merozoite surface antigen (gp195) of congenic mouse strains differing in H-2 haplotype has been examined. All seven strains of mice were capable of producing gp195-specific antibodies. Generalized immune recognition of gp195 by mice of diverse H-2 haplotypes distinguished gp195 from the P. falciparum circumsporozoite protein and the 230-kDa and 48/45-kDa gamete surface antigens. However, the H-2 genetic locus appeared to influence the specificity of gp195-specific antibodies. Immunoblot patterns of mouse sera with parasite antigens revealed a complex pattern of reactivity with terminal and intermediate processing fragments of gp195. The majority of immunoblot bands observed were similar for all of the mouse strains; however, there were several strains that additionally recognized a few unique fragments or displayed more intense reactivities with specific processing fragments. These results suggest that while individuals of diverse major histocompatibility complex makeup are capable of recognizing the gp195 antigen, the recognition of specific gp195 B-cell and T-cell epitopes may be under control of the major histocompatibility complex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berzofsky J. A., Richman L. K., Killion D. J. Distinct H-2-linked Ir genes control both antibody and T cell responses to different determinants on the same antigen, myoglobin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4046–4050. doi: 10.1073/pnas.76.8.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle D. B., Newbold C. I., Smith C. C., Brown K. N. Monoclonal antibodies that protect in vivo against Plasmodium chabaudi recognize a 250,000-dalton parasite polypeptide. Infect Immun. 1982 Oct;38(1):94–102. doi: 10.1128/iai.38.1.94-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J., Whittle H. C., Berzins K., Howard R. J., Marsh K., Sjoberg K. Inhibition of Plasmodium falciparum growth by IgG antibody produced by human lymphocytes transformed with Epstein-Barr virus. Clin Exp Immunol. 1986 Jan;63(1):135–140. [PMC free article] [PubMed] [Google Scholar]
- Campos-Neto A., Levine H., Schlossman S. F. Immune response gene control of antibody specificity. Cell Immunol. 1982 May 1;69(1):128–137. doi: 10.1016/0008-8749(82)90057-0. [DOI] [PubMed] [Google Scholar]
- Carter R., Graves P. M., Quakyi I. A., Good M. F. Restricted or absent immune responses in human populations to Plasmodium falciparum gamete antigens that are targets of malaria transmission-blocking antibodies. J Exp Med. 1989 Jan 1;169(1):135–147. doi: 10.1084/jem.169.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecka J. M., Stratton J. A., Miller A., Sercarz E. Structural aspects of immune recognition of lysozymes. III. T cell specificity restriction and its consequences for antibody specificity. Eur J Immunol. 1976 Sep;6(9):639–646. doi: 10.1002/eji.1830060909. [DOI] [PubMed] [Google Scholar]
- Chizzolini C., Dupont A., Akue J. P., Kaufmann M. H., Verdini A. S., Pessi A., Del Giudice G. Natural antibodies against three distinct and defined antigens of Plasmodium falciparum in residents of a mesoendemic area in Gabon. Am J Trop Med Hyg. 1988 Aug;39(2):150–156. doi: 10.4269/ajtmh.1988.39.150. [DOI] [PubMed] [Google Scholar]
- Coppel R. L. Prospects for a malaria vaccine. Microbiol Sci. 1986 Oct;3(10):292–295. [PubMed] [Google Scholar]
- Crisanti A., Müller H. M., Hilbich C., Sinigaglia F., Matile H., McKay M., Scaife J., Beyreuther K., Bujard H. Epitopes recognized by human T cells map within the conserved part of the GP190 of P. falciparum. Science. 1988 Jun 3;240(4857):1324–1326. doi: 10.1126/science.2453924. [DOI] [PubMed] [Google Scholar]
- Del Giudice G., Cooper J. A., Merino J., Verdini A. S., Pessi A., Togna A. R., Engers H. D., Corradin G., Lambert P. H. The antibody response in mice to carrier-free synthetic polymers of Plasmodium falciparum circumsporozoite repetitive epitope is I-Ab-restricted: possible implications for malaria vaccines. J Immunol. 1986 Nov 1;137(9):2952–2955. [PubMed] [Google Scholar]
- Del Giudice G., Engers H. D., Tougne C., Biro S. S., Weiss N., Verdini A. S., Pessi A., Degremont A. A., Freyvogel T. A., Lambert P. H. Antibodies to the repetitive epitope of Plasmodium falciparum circumsporozoite protein in a rural Tanzanian community: a longitudinal study of 132 children. Am J Trop Med Hyg. 1987 Mar;36(2):203–212. doi: 10.4269/ajtmh.1987.36.203. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Epstein N., Miller L. H., Kaushel D. C., Udeinya I. J., Rener J., Howard R. J., Asofsky R., Aikawa M., Hess R. L. Monoclonal antibodies against a specific surface determinant on malarial (Plasmodium knowlesi) merozoites block erythrocyte invasion. J Immunol. 1981 Jul;127(1):212–217. [PubMed] [Google Scholar]
- Gabra M. S., Grossiord D., Perrin L. H., Shaw A., Cheung A., McGregor I. A. Defined Plasmodium falciparum antigens in malaria serology. Bull World Health Organ. 1986;64(6):889–896. [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Berzofsky J. A., Maloy W. L., Hayashi Y., Fujii N., Hockmeyer W. T., Miller L. H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986 Aug 1;164(2):655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Miller L. H., Kumar S., Quakyi I. A., Keister D., Adams J. H., Moss B., Berzofsky J. A., Carter R. Limited immunological recognition of critical malaria vaccine candidate antigens. Science. 1988 Oct 28;242(4878):574–577. doi: 10.1126/science.2902690. [DOI] [PubMed] [Google Scholar]
- Graves P. M., Carter R., Burkot T. R., Quakyi I. A., Kumar N. Antibodies to Plasmodium falciparum gamete surface antigens in Papua New Guinea sera. Parasite Immunol. 1988 Mar;10(2):209–218. doi: 10.1111/j.1365-3024.1988.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Hall R., Hyde J. E., Goman M., Simmons D. L., Hope I. A., Mackay M., Scaife J., Merkli B., Richle R., Stocker J. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. 1984 Sep 27-Oct 3Nature. 311(5984):379–382. doi: 10.1038/311379a0. [DOI] [PubMed] [Google Scholar]
- Hall R., Osland A., Hyde J. E., Simmons D. L., Hope I. A., Scaife J. G. Processing, polymorphism, and biological significance of P190, a major surface antigen of the erythrocytic forms of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Apr;11:61–80. doi: 10.1016/0166-6851(84)90055-0. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Biosynthesis and processing of a Plasmodium falciparum schizont antigen recognized by immune serum and a monoclonal antibody. J Exp Med. 1982 Nov 1;156(5):1528–1538. doi: 10.1084/jem.156.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981 Nov 26;294(5839):361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J Exp Med. 1984 Aug 1;160(2):624–629. doi: 10.1084/jem.160.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui G. S., Siddiqui W. A. Serum from Pf195 protected Aotus monkeys inhibit Plasmodium falciparum growth in vitro. Exp Parasitol. 1987 Dec;64(3):519–522. doi: 10.1016/0014-4894(87)90068-3. [DOI] [PubMed] [Google Scholar]
- Kramer K. J., Kan S. C., Siddiqui W. A. Concentration of Plasmodium falciparum-infected erythrocytes by density gradient centrifugation in Percoll. J Parasitol. 1982 Apr;68(2):336–337. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lyon J. A., Geller R. H., Haynes J. D., Chulay J. D., Weber J. L. Epitope map and processing scheme for the 195,000-dalton surface glycoprotein of Plasmodium falciparum merozoites deduced from cloned overlapping segments of the gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2989–2993. doi: 10.1073/pnas.83.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon J. A., Haynes J. D. Plasmodium falciparum antigens synthesized by schizonts and stabilized at the merozoite surface when schizonts mature in the presence of protease inhibitors. J Immunol. 1986 Mar 15;136(6):2245–2251. [PubMed] [Google Scholar]
- Majarian W. R., Daly T. M., Weidanz W. P., Long C. A. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J Immunol. 1984 Jun;132(6):3131–3137. [PubMed] [Google Scholar]
- Milich D. R., Leroux-Roels G. G., Louie R. E., Chisari F. V. Genetic regulation of the immune response to hepatitis B surface antigen (HBsAg). IV. Distinct H-2-linked Ir genes control antibody responses to different HBsAg determinants on the same molecule and map to the I-A and I-C subregions. J Exp Med. 1984 Jan 1;159(1):41–56. doi: 10.1084/jem.159.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. H., Howard R. J., Carter R., Good M. F., Nussenzweig V., Nussenzweig R. S. Research toward malaria vaccines. Science. 1986 Dec 12;234(4782):1349–1356. doi: 10.1126/science.2431481. [DOI] [PubMed] [Google Scholar]
- Nardin E. H., Barr P. J., Heimer E., Etlinger H. M. Genetic restriction of the murine humoral response to a recombinant Plasmodium vivax circumsporozoite protein. Eur J Immunol. 1988 Jul;18(7):1119–1122. doi: 10.1002/eji.1830180722. [DOI] [PubMed] [Google Scholar]
- Pal R. The present status of insecticide resistance in anopheline mosquitoes. J Trop Med Hyg. 1974 Feb;77(2):28–41. [PubMed] [Google Scholar]
- Patarroyo M. E., Amador R., Clavijo P., Moreno A., Guzman F., Romero P., Tascon R., Franco A., Murillo L. A., Ponton G. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature. 1988 Mar 10;332(6160):158–161. doi: 10.1038/332158a0. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Merkli B., Loche M., Chizzolini C., Smart J., Richle R. Antimalarial immunity in Saimiri monkeys. Immunization with surface components of asexual blood stages. J Exp Med. 1984 Aug 1;160(2):441–451. doi: 10.1084/jem.160.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirson P. J., Perkins M. E. Characterization with monoclonal antibodies of a surface antigen of Plasmodium falciparum merozoites. J Immunol. 1985 Mar;134(3):1946–1951. [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Brown J., Whittle H., Lin P. S. Human-human hybridomas secreting monoclonal antibodies to the Mr 195,000 Plasmodium falciparum blood stage antigen. J Exp Med. 1986 Jan 1;163(1):179–188. doi: 10.1084/jem.163.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui W. A., Tam L. Q., Kan S. C., Kramer K. J., Case S. E., Palmer K. L., Yamaga K. M., Hui G. S. Induction of protective immunity to monoclonal-antibody-defined Plasmodium falciparum antigens requires strong adjuvant in Aotus monkeys. Infect Immun. 1986 Apr;52(1):314–318. doi: 10.1128/iai.52.1.314-318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui W. A., Tam L. Q., Kramer K. J., Hui G. S., Case S. E., Yamaga K. M., Chang S. P., Chan E. B., Kan S. C. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci U S A. 1987 May;84(9):3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigaglia F., Takacs B., Jacot H., Matile H., Pink J. R., Crisanti A., Bujard H. Nonpolymorphic regions of p190, a protein of the Plasmodium falciparum erythrocytic stage, contain both T and B cell epitopes. J Immunol. 1988 May 15;140(10):3568–3572. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala F., Cochrane A. H., Nardin E. H., Nussenzweig R. S., Nussenzweig V. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J Exp Med. 1983 Jun 1;157(6):1947–1957. doi: 10.1084/jem.157.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]