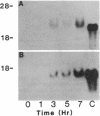
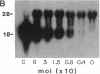
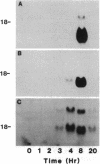
Abstract
Rat astrocytes, immunologically competent glial cells of the central nervous system (CNS), released a variety of cytokines after activation. Lipopolysaccharide-stimulated astrocytes produced tumor necrosis factor (TNF) as demonstrated by Northern blot analysis using a mouse TNF probe and by functional assay. Biological activity of rat astrocyte-derived TNF was neutralized by rabbit antiserum against recombinant murine TNF. Stimulation of astrocytes by lipopolysaccharide also activated the interleukin 1 and interleukin 6 genes. We have also investigated whether a neurotropic paramyxovirus, Newcastle disease virus, triggers cytokine production by astrocytes. This virus induced astrocytes to produce TNF, lymphotoxin, interleukin 6, and alpha- and beta-interferons. Thus, stimulation by endotoxin and virus activated distinct, yet overlapping, sets of cytokine genes. We propose that astrocytes and the cytokines they produce may play a significant role in the pathogenesis of immunologically and/or virally mediated CNS disease, in CNS intercellular communication, and in the interactions between the nervous and immune systems.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Holtmann H., Toker L., Hahn T., Wallach D. Tumor necrosis factor induction by Sendai virus. J Immunol. 1986 Apr 15;136(8):2938–2942. [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Fierz W., Endler B., Reske K., Wekerle H., Fontana A. Astrocytes as antigen-presenting cells. I. Induction of Ia antigen expression on astrocytes by T cells via immune interferon and its effect on antigen presentation. J Immunol. 1985 Jun;134(6):3785–3793. [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Fontana A., Kristensen F., Dubs R., Gemsa D., Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982 Dec;129(6):2413–2419. [PubMed] [Google Scholar]
- Frei K., Bodmer S., Schwerdel C., Fontana A. Astrocytes of the brain synthesize interleukin 3-like factors. J Immunol. 1985 Dec;135(6):4044–4047. [PubMed] [Google Scholar]
- Fujita T., Ohno S., Yasumitsu H., Taniguchi T. Delimitation and properties of DNA sequences required for the regulated expression of human interferon-beta gene. Cell. 1985 Jun;41(2):489–496. doi: 10.1016/s0092-8674(85)80022-2. [DOI] [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Hotta H., Yamanishi K., Taniguchi T. Interferon-beta gene regulation: tandemly repeated sequences of a synthetic 6 bp oligomer function as a virus-inducible enhancer. Cell. 1987 May 8;49(3):357–367. doi: 10.1016/0092-8674(87)90288-1. [DOI] [PubMed] [Google Scholar]
- Harper H. D., Pitha P. M. Effect of concanavalin A on interferon induction by poly IC. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1220–1226. doi: 10.1016/0006-291x(73)90595-0. [DOI] [PubMed] [Google Scholar]
- Hirsch M. R., Wietzerbin J., Pierres M., Goridis C. Expression of Ia antigens by cultured astrocytes treated with gamma-interferon. Neurosci Lett. 1983 Oct 31;41(1-2):199–204. doi: 10.1016/0304-3940(83)90247-1. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., McArthur J. C., Narayan O. The neurobiology of human immunodeficiency virus infections. FASEB J. 1988 Nov;2(14):2970–2981. doi: 10.1096/fasebj.2.14.2846395. [DOI] [PubMed] [Google Scholar]
- Johnson R. T. Viruses and chronic neurological diseases. Johns Hopkins Med J. 1982 Apr;150(4):132–140. [PubMed] [Google Scholar]
- Koski C. L., Ramm L. E., Hammer C. H., Mayer M. M., Shin M. L. Cytolysis of nucleated cells by complement: cell death displays multi-hit characteristics. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3816–3820. doi: 10.1073/pnas.80.12.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs E. J., Radzioch D., Young H. A., Varesio L. Differential inhibition of IL-1 and TNF-alpha mRNA expression by agents which block second messenger pathways in murine macrophages. J Immunol. 1988 Nov 1;141(9):3101–3105. [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Li C. B., Gray P. W., Lin P. F., McGrath K. M., Ruddle F. H., Ruddle N. H. Cloning and expression of murine lymphotoxin cDNA. J Immunol. 1987 Jun 15;138(12):4496–4501. [PubMed] [Google Scholar]
- Lévi-Strauss M., Mallat M. Primary cultures of murine astrocytes produce C3 and factor B, two components of the alternative pathway of complement activation. J Immunol. 1987 Oct 1;139(7):2361–2366. [PubMed] [Google Scholar]
- Massa P. T., Brinkmann R., ter Meulen V. Inducibility of Ia antigen on astrocytes by murine coronavirus JHM is rat strain dependent. J Exp Med. 1987 Jul 1;166(1):259–264. doi: 10.1084/jem.166.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa P. T., Schimpl A., Wecker E., ter Meulen V. Tumor necrosis factor amplifies measles virus-mediated Ia induction on astrocytes. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7242–7245. doi: 10.1073/pnas.84.20.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa P. T., ter Meulen V., Fontana A. Hyperinducibility of Ia antigen on astrocytes correlates with strain-specific susceptibility to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4219–4223. doi: 10.1073/pnas.84.12.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M., Fujita T., Kimura Y., Maruyama M., Harada H., Sudo Y., Miyata T., Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988 Sep 9;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- Paul N. L., Ruddle N. H. Lymphotoxin. Annu Rev Immunol. 1988;6:407–438. doi: 10.1146/annurev.iy.06.040188.002203. [DOI] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Analysis of interferon mRNA in human fibroblast cells induced to produce interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7426–7430. doi: 10.1073/pnas.78.12.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robbins D. S., Shirazi Y., Drysdale B. E., Lieberman A., Shin H. S., Shin M. L. Production of cytotoxic factor for oligodendrocytes by stimulated astrocytes. J Immunol. 1987 Oct 15;139(8):2593–2597. [PubMed] [Google Scholar]
- Ryals J., Dierks P., Ragg H., Weissmann C. A 46-nucleotide promoter segment from an IFN-alpha gene renders an unrelated promoter inducible by virus. Cell. 1985 Jun;41(2):497–507. doi: 10.1016/s0092-8674(85)80023-4. [DOI] [PubMed] [Google Scholar]
- Sariban E., Imamura K., Luebbers R., Kufe D. Transcriptional and posttranscriptional regulation of tumor necrosis factor gene expression in human monocytes. J Clin Invest. 1988 May;81(5):1506–1510. doi: 10.1172/JCI113482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakamura S., Taga T., Matsuda T., Hirano T., Kishimoto T., Kaziro Y. Induction of neuronal differentiation in PC12 cells by B-cell stimulatory factor 2/interleukin 6. Mol Cell Biol. 1988 Aug;8(8):3546–3549. doi: 10.1128/mcb.8.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K. W., Raine C. S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988 Apr;23(4):339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Suzumura A., Lavi E., Bhat S., Murasko D., Weiss S. R., Silberberg D. H. Induction of glial cell MHC antigen expression in neurotropic coronavirus infections. Characterization of the H-2-inducing soluble factor elaborated by infected brain cells. J Immunol. 1988 Mar 15;140(6):2068–2072. [PubMed] [Google Scholar]
- Suzumura A., Lavi E., Weiss S. R., Silberberg D. H. Coronavirus infection induces H-2 antigen expression on oligodendrocytes and astrocytes. Science. 1986 May 23;232(4753):991–993. doi: 10.1126/science.3010460. [DOI] [PubMed] [Google Scholar]
- Taniguchi T. Regulation of cytokine gene expression. Annu Rev Immunol. 1988;6:439–464. doi: 10.1146/annurev.iy.06.040188.002255. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman B. Mechanisms in multiple sclerosis. Nature. 1985 Nov 14;318(6042):104–105. doi: 10.1038/318104a0. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Clark S. C. Multiple actions of interleukin 6 within a cytokine network. Immunol Today. 1988 May;9(5):137–139. doi: 10.1016/0167-5699(88)91200-5. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Bartlett P. F., Clark-Lewis I., Battye F., Schrader J. W. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984 Aug 23;310(5979):688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]