Abstract
The irritable bowel syndrome (IBS) is a complex disorder in which psychosocial, cultural and biological factors, interact. Recent knowledge in the pathophysiology of IBS, seem to combine issues such as a low grade inflammation or immune activation and dysbiosis that can trigger or exacerbate IBS. On the other hand, stress mediated through the hypothalamic-pituitary-adrenal axis can produce motility abnormalities that can modify the microbiota as well, with the subsequent immune activation in the mucosa and stimulation of nerve terminals, generating symptoms of IBS. Also, we speculate that, stress, dysbiosis or an underlying genetic predisposition, may increase the epithelial permeability leading to a contact between pathogens-associated molecular patterns and toll-like receptors in the deeper layers of the gut, developing a host immunity response and IBS generation. We believe that the role of toll-like receptors in IBS and elucidating the communication processes between the immune and the nervous system, warrant future research.
Keywords: Immunity, Mucosal; Irritable bowel syndrome; Stress, psychological; Toll-like receptors
Introduction
Traditionally, irritable bowel syndrome (IBS) has been considered a functional gastrointestinal disorder (FGID), characterized by pain or discomfort associated with changes in bowel habit, such as diarrhea, constipation or alternating/mixture.1 As with other FGIDs, in IBS there is no evidence of organic, biochemical or structural abnormalities; however, recent data are shaping new concepts. Currently, IBS is diagnosed based on clinical symptoms by the Rome criteria, in the absence of alarm signs; but patients usually undergo exhaustive diagnostic evaluations looking for organic gastrointestinal pathologies before the diagnosis is accepted by both patients and physicians.2 Despite this, IBS is the most common FGID and may affect up to 20% of the adult population in industrialized and non industrialized countries. For example, in non industrialized countries, a 16% prevalence of IBS has been reported in the community, and up to 35% among volunteers.3-5 Furthermore, it is the first reason for consultation to gastroenterologists.5
In recent years, new concepts have changed the main interest in IBS, energizing a growing group of gastroenterologists and scientists around the world. On the one hand, data from several groups have begun to blur the division between functional and organic disorders.6 For example, the recognition of inflammation in IBS, albeit its low grade,7 the participation of this inflammatory response on gut motility and sensitivity8 and finally the fact that IBS could represent an abnormal mucosal response to an altered intestinal microbiota, echoing current theories on inflammatory bowel disease (IBD),9 could answer the long time considered convergence between IBD, celiac disease and IBS.7 Additionally, there is evidence implicating gastrointestinal infections in the pathogenesis of IBS, the so called post-infectious IBS (PI-IBS).10 With this information, a rapidly growing body of evidence has supported a low grade inflammation11-13 and an altered fecal microbiota condition related to the pathogenesis of IBS.14,15 On the other hand, some antibiotics and probiotics have proven to reduce symptoms, leading to a new understanding of IBS.
Issues of Interest in the Pathophysiology of IBS
1. Genetic factors
During the last decade, studies on familial aggregation16 and on twins17 have supported the involvement of hereditary factors in the pathogenesis of IBS. However, the lack of consistent findings that support a specific genetic mechanism in IBS based on symptom phenotypes, has lead to a different approach: the examination candidate genes associations with FGIDs and with the underlying abnormalities in gastrointestinal and colonic physiology.18 Examples of this approach include the association of IBS with IL-10, serotonin transporter, alpha-2 adrenergic receptors and G protein functions. Gonsalkorale et al19 and van der Veek et al20 reported an association of IL-10 low producer polymorphism with the presence of IBS in different populations.21 The above findings have suggested a genetic predisposition for a reduced anti-inflammatory response, mediated by a lower production of this regulatory cytokine. Additionally, this pro-inflammatory condition could be synergistically stimulated, if patients have a higher production of tumor necrosis factor-alpha (TNF-α), as has been related by high producer polymorphisms in patients compared to healthy controls.20
Recently, as a consequence of a gastroenteritis outbreak follow-up, Villani et al22 evaluated the genetic risk factors in patients with PI-IBS, reporting statistically significant relationships with several genes. The most striking features were the relationships between the immunity related toll-like receptor 9 (TLR9), IL-6 and adhesion E-cadherin 1 polymorphism genes, as risk factors.
2. Stress
Since the stress concept was borrowed from physics, Hans Selye23 identified the gut, along with the endocrine and immune systems, as the main targets altered by chemical and physical challenges. Recently, there have been several studies relating stressful events (psychological stressors) with the development of FGIDs such as IBS or symptom exacerbation,24-26 which intend to elucidate the stress related mechanisms that influence the pathophysiology of this disorder.
The release of corticotrophin-releasing factor (CRF) from the hypothalamus, in response to diverse physical and psychological stressors, plays a major role in orchestrating the behavioral, neuroendocrine and autonomic responses to stress.27,28 The hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system are the 2 core branches of this stress response system. Abnormalities in the stress response in the form of changes in adrenocorticotrophin hormone, cortisol and catecholamine levels, have been reported in IBS.29 In fact, the importance of CRF has led research to develop CRF antagonists as possible treatments for IBS.30
Additionally, alterations in the HPA axis have received much attention due to its possible implication in a non-standard immune activation in the gut wall,31 and in symptom exacerbation in different IBS subgroups.32 This non-standard immune activation is represented by an abnormal mucosal response to an altered microbiota including an increase infiltration of mononuclear, mast and enteroendocrine cells in the colonic mucosa, but without a frank histological inflammation by light microscopy and absence of clinical and laboratory markers of inflammation.9
Also, in order to solve the therapeutic needs related to these factors, several groups have turned their scope to exploring the efficacy of probiotics, mainly due to the potential effects that can be developed by gut derived flora, in symptom improvement (pain and discomfort) and low grade inflammation in the intestinal mucosa, probably through several mechanisms and interactions, including possible modifications of the HPA axis balance.33
3. IBS and the involved immunological response
Several groups have been reporting evidences that suggest the development of low grade inflammation in the gut mucosa (Table 1), mainly in patients with PI-IBS.34 Almost 20 years ago, Salzmann et al35 suggested an increased infiltration of mononuclear cells in the lamina propria of patients with IBS. On the other hand, the majority of individuals that suffer an acute gastroenteritis recover completely after the infectious agent is cleared, but a significant group develops FGIDs, such as IBS, functional dyspepsia or both.6,36 It is remarkable that non-gastrointestinal infections have also been reported as risk factors for the development of IBS.37
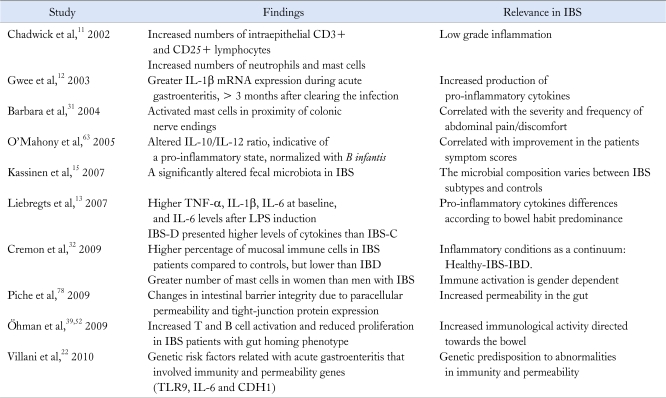
Table 1.
Immunological Abnormalities and Microbiota-Gut Interactions in Irritable Bowel Syndrome
IBS, irritable bowel syndrome; LPS, lipopolysaccharide; IBS-D, diarrhea predominant-IBS; IBS-C, constipation predominant-IBS; IBD, inflammatory bowel disease; TLR9, toll-like receptor 9; CDH1, E-cadherin 1.
1) Low grade inflammation-immune activation
The concept of low grade inflammation has been coined by several groups, and as previously mentioned, it is described as an increased infiltration in the colonic mucosa of T lymphocytes, mast cells and enteroendocrine cells (Fig. 1).31,38,39 However, the term low immune activation is probably more appropriate. The higher number of mast cells present in colonic biopsies, which has been reported as a key immunological trait of IBS patients, is an important issue.31,32,40,41 In fact, a higher number of mast cells in proximity to the colon sensory nerves31 have been correlated with the severity and frequency of abdominal pain,40 bloating,32 and probably with rectal hypersensitivity.38 It is noteworthy that Cremon et al32 reported higher numbers of T and mast cells in about 50% of the IBS patients. Their study compared the percentage of colonic mucosa infiltrated by immune cells in patients with IBS and those with truly inflammatory conditions, such as IBD (eg, microscopic colitis, inactive and active ulcerative colitis) and healthy controls, finding intermediate infiltration levels in IBS patients compared to controls, but lower levels when compared to patients with IBD. The authors concluded that inflammation could be a common pathophysiological factor triggering each one of these disorders, where IBS and IBD might represent the 2 ends of a wide spectrum of chronic inflammatory conditions.
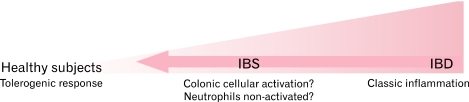
Figure 1.
Possible immunological responses in the gut mucosa related to developing bowel disorders. In healthy controls there is a tolerogenic reaction towards commensal microorganisms. However, it is possible that bacterial populations present in some patients or a genetic predisposition, may shift the response towards an immune activation in irritable bowel syndrome (IBS) or to a full-blown inflammatory response in inflammatory bowel disease (IBD).
2) Mast cells
Mast cells are distributed along the digestive tract and in all layers of the intestinal wall, and might be in a permanent state of alert or activation due to the continuous presence of potential antigens within the gastrointestinal lumen.42 Furthermore, mast cell functions are diverse. Traditionally, they have been considered as the key effectors cells of allergic reactions. These cells could also be of major importance in recruiting and activating eosinophils, B and T cells in activated state (presence of CD4+ and CD25+ markers).43,44 On the other hand, mast cells have been involved in the communication with intrinsic and extrinsic nerves to modulate sensory and motility functioning.45 It is important to note that the resulting reactions from the mediators released by the above mentioned cells include responses in the enteric nervous system, visceral hiperalgesia and an increased mucosal permeability, as well as the generation of symptoms, such as changes in bowel habit and abdominal pain.33,44
3) Enteroendocrine and other immune cells
Other immune cell types have also been observed to be increased in patients with IBS. That is the case for enteroendocrine cells, which have been related to PI-IBS.46 In this subgroup of patients, enteroendocrine cells have been reported to be present in higher numbers than in any other IBS subgroup.47 Additionally, neutrophils have been reported to be augmented in the gut mucosa of patients and in animal models, but unlike the above immune cells (mast cells and enteroendocrine cells) these observations seem to be scarce11,48 and possibly this cellular population is not an important mediator of the low immune activation present in IBS. In addition, the presence of neutrophils would imply a full-blown inflammatory process (Fig. 1).49
4) Lymphocytes
Chadwick et al,11 reported increased numbers of T cells expressing CD25+ in the lamina propria of the colon that could be related with the low immune activation in the bowel.50 Recently, it has been hypothesized that CD4+ CD25+ T regulatory (Treg) cells are reduced in numbers and in their functional capabilities, producing an inadequate suppression of intestinal inflammatory activity.51 However, research data from Holmén et al51 did not find a significant difference in the mRNA expression of the FoxP3 - a specific marker of Treg cells -, between IBS patients and healthy controls, or in the suppressive activity of the peripheral CD4+ CD25+ cells from patients and controls. Despite the above research, the study was not able to rule out the possibility that the colonic Treg cells are dysfunctional in vivo or that alteration of other mucosal immunological cells with suppressive activity could be associated with the low grade inflammation that is found in the gut of patients with IBS.51
Recently, Öhman et al,39 reported several alterations in peripheral blood T cells, such as the presence of CD69+ lymphocytes (early activation) and the integrin alpha4beta7, receptor (intestinal homing) (Fig. 2). The same group52 also described a state of B cell activation, finding increased CD80 and CD86 expression, at least in a subpopulation of IBS patients. In addition, the slight but significant difference in the over expression of IgG and alpha4beta7, together with the increased expression of CD80 and CD86, suggests that IBS patients also have increased B cell activation in the extra-intestinal lymph nodes.52 In order to explain this cellular infiltrate, Ohman and Simrén47 suggested 2 possible explanations: (1) It could be due to an unregulated mild inflammation or (2) It can reflect a potent inflammatory suppressive process led by activated T lymphocytes. However, as of today, the profile of cytokines released by mast cells, enteroendocrine cells, T and B lymphocytes and Treg cells in IBS, as well as the level of activation of the infiltrated lymphocytes in the gut mucosa of these patients, is unknown.
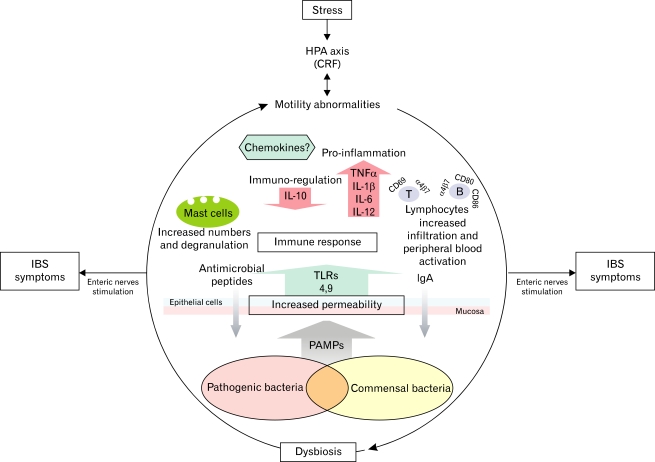
Figure 2.
Integrative model of irritable bowel syndrome (IBS) pathophysiology. IBS can develop from centrally dominant factors such as stress or luminal factors like dysbiosis triggering an altered immune response. Stress alters gastrointestinal motility mediated through the hypothalamicpituitary-adrenal (HPA) axis, and these motility abnormalities can modify the microbiota with the subsequent immune activation in the mucosa and stimulation of nerve terminals, generating symptoms of IBS. On the other hand, dysbiosis related to gastrointestinal infections, small intestine bacterial overgrowth or antibiotics may increase the epithelial permeability leading to contact between pathogens-associated molecular patterns (PAMPs) and toll-like receptors (TLRs) in the deeper layers of the gut with the subsequent host immunity response and IBS generation or symptom exacerbation. CRF, corticotrophin-releasing factor.
5) IBS and microscopic colitis
The finding of increased numbers of T cells in the lamina propria of the colon in a subgroup of IBS patients,11 may need to be differentiated from the presence of microscopic colitis. Microscopic colitis is a syndrome of chronic watery diarrhea with a chronic inflammatory cell infiltrate in the colonic mucosa, a normal colonic appearance in the colonoscopy and no laboratory abnormalities, therefore, the clinical presentation may be indistinguishable from diarrhea predominant-IBS (IBS-D).53,54 In fact, Limsui et al55 have reported that 41% to 56% of patients with mcroscopic colitis, met symptom criteria for IBS. Also, Chey et al56 found that the overall prevalence of microscopic colitis in suspected IBS patients is 1.5% and 2.3% in those older than 45 years. On the other hand, classic microscopic colitis encompases 2 subgroups, collagenous colitis and lymphocytic colitis.53 The first one has an abnormally thickened subepithelial collagen band and the second one, has increased intraepithelial lymphocytes (IELs).53 Therefore, lymphocitic colitis may be similar with IBS respect to low grade inflammation, however, in microscopic colitis the IELs are increased from 5 per 100 surface epithelial cells in normal subjects, to 20 or more per 100 epithelial cells.57 Hence, colonoscopy with multiple biopsies is crucial to rule out microscopic colitis53,54 and to differentiate it from IBS. Furthermore, very recent data presented in abstract form have confirm that there is no difference between IBS and controls in the number of IELs from the cecum, transverse and sigmoid colon, and both groups have less than 10 IELs×100 enterocytes,58 confirming the last statement.
4. Immune response molecules
The gastrointestinal tract, as well as other organs lined with mucosal tissue, is the first line of defense against luminal antigens and environmental pathogens. The epithelial cells in the mucosa form a layer that avoids the paracellular transit of substances with high molecular weight, such as pathogens or antigens. Also these cells produce large amounts of mucus and secrete anti-microbial peptides which limit bacterial populations.59 Under physiological conditions this epithelial barrier is not completely impermeable to all bacteria, developing a tolerogenic immune response.60 On the other hand, bacteria or other antigens pass through the paracellular space, leading to a local and transitory pro-inflammatory cytokine production that can slightly inhibit invasion60,61 by micro organisms (Fig. 2). This response occurs rapidly and does not trigger a measurable systemic pro-inflammatory state.61
1) Cytokines
Research on the immunological response in IBS has been led by the analysis of differences in cytokines expression. Cytokines are intercellular signaling proteins, mainly produced by immune cells (mast cells, macrophages, glial cells and lymphocytes in proximity with afferent primary non-adrenergic non-cholinergic nerves in the gut mucosa). Their main function is to regulate and coordinate the immunological response,62 but they could also play an important role in the development of motor dysfunction, visceral pain and anxiety or depression, mediated by the CNS.29,62 Gwee et al12 opened a research path, when finding higher levels of IL-1β mRNA expression in IBS patients both shortly after a gastrointestinal infection and three months after the infection was cleared. Later, O'Mahony et al63 reported an abnormal ratio of immunoregulatory/proinflammatory IL-10/IL-12 cytokines in non-stimulated peripheral blood mononuclear cells from patients with IBS, indicating an inflammatory state in these patients. In addition, the authors related the normalization of this cytokine ratio and the significant symptomatic relief to the use of a probiotic, the Bifidobactium infantis 35624. Recently, Liebregts et al13 reported a markedly higher basal and lipopolysacharide stimulated levels of pro-inflammatory cytokines (IL-1β , TNF-α and IL-6) released from peripheral blood mononuclear cells in patients with IBS. Moreover, the authors suggested that pro-inflammatory cytokines can partly explain symptom manifestations.13 More recently, O'Mahony et al64 evidenced a higher IFN-γ and TNF-α production from whole blood stimulated with lipopolysacharide in an animal model relevant for IBS (rat maternal separation) compared to non-stressed rats. Also, Clarke et al65 using the same rat model, showed correlations between the IL-6 and the polyunsaturated fatty acid profile (negative correlations with omega-3 total levels, and positive correlations with the omega-6/omega-3 ratio), suggesting a phenotype that is primed for immune activation. Also, several other pro-inflammatory (IL-2, IL-12, IL-17 and IL-18) and anti-inflammatory (IL-4 and IL-13) cytokines have been shown to be present in a lesser extent in IBS.62
2) Role of cytokines
The specific roles displayed by cytokines in IBS patients are not fully established. Microarray studies have supported the hypothesis of an immune activation, as they have shown a reduced expression of immunoregulatory cytokines together with an over expression of the pro-inflammatory ones.66 However, the cytokines are able to start several processes in different cellular types. For example, in vitro studies have demonstrated neuronal activation, probably mediated by glial cells, based on the fact these cells have a high density of receptors for cytokines and chemokines.67 The above suggests neuronal excitation mediated by intermediate cellular populations (glial cells). When stimulated by cytokines, the glial and endothelial cells locally release nitric oxide, arachidonic acid, prostaglandins, oxygen reactive species and excitatory aminoacids (mainly glutamate), which could modify the neuronal activity.68 Also, glial cells can produce chemokines which in turn can induce attraction of immunological cells, amplifying the inflammatory signals.68,69
3) Chemokines
However, the roles played by chemokines in IBS are not yet fully understand, and only a small number of studies have reported changes in these molecules. For example, Dinan et al29,70 reported increased levels of the chemokine IL-8/CXCL8. At the same time, MacSharry et al66 reported reduced levels in both the expression and secretion levels of chemokines (IL-8/CXCL8; CXCL9 and monocyte chemotactic protein-1) in the colonic mucosa of IBS patients. The above findings are controversial and warrant the search of additional markers (eg, lactoferrin, calprotectin, reactive C protein and myeloperoxidase) that can become biomarkers in IBS and that can explain the underlying biological mechanisms as well.71
5. Toll-like receptors
The innate immune system is the first line of defense and is promptly activated against pathogens or tissue damage, after the recognition of several pathogens-associated molecular patterns (PAMPs) or danger associated molecular patterns (DAMPs).72 The most studied receptors for PAMPs and DAMPs are the TLRs, which have a significantly specific response in the innate immunity.73 Phylogenetic analysis classifies the TLRs in three main branches, which seem to correspond with the recognized ligands: (1) TLRs 1, 2, 4, 6 and 10 are involved with lipids recognition, (2) TLRs 5 and 11 recognize proteins and (3) TLRs 3, 7, 8 and 9 are sensitive to nucleic acids.74 TLRs display differential expression levels through the gut mucosal layers, with a spatial and cellular specific allocation probably related to their function.
It is remarkable how changes in the integrity of the epithelial barrier or permeability dysfunction lead to contact between PAMPs and TLRs in the deeper layers of the gut and the subsequent host immunity response (Fig. 2).75 However, despite the fact that there is not yet a study that has related mucosal permeability with TLRs in IBS patients, research has suggested that permeability abnormalities are related to IBS generation and symptom exacerbation, especially in the PI-IBS and IBS-D subgroups.10,76,77 In fact, ex vivo assays have shown an augmented permeability in all IBS subgroups based on bowel habit predominance,78 although the mechanisms involved in these changes are unknown. Recent reports have suggested alterations in the tight junction proteins, and their physiological processes are the possible underlying mechanisms of these changes.79
Despite the fact that the TLRs are deeply related with some gastrointestinal chronic inflammatory disorders such as ulcerative colitis or Crohn's disease,75,80 available data regarding their participation in the pathophysiology of IBS is quite limited. Recently, Brint et al,81 reported a 5-fold up-regulation of TLR4 in women with IBS compared to healthy controls, while reporting a reduced expression of TLR7 and TLR8 in the same patients.
On the other hand, McKernan et al,82 based on a rat model for IBS, reported many differences in the TLRs expression in chronically stressed rats compared to controls. The authors used 2 different models (maternal separation and stress susceptible Wistar-Kyoto rats), showing a significantly higher expression of the TLR3, 4 and 5 on both, while the Wistar-Kyoto which are genetically susceptible to stress, also had a significant over expression in the TLRs 7, 8 and 9 compared to the Sprague-Dawley which are not genetically susceptible to stress. The reasons involved in these differences are not clear; however, alterations in the gut microbiota could be related.
More recently, Villani et al22 evidenced the complex genetic scenario present in the gut mucosa of IBS patients. In this key study, the presence of genetic variants involved with TLR9, IL-6 and intestinal permeability E-cadherin, were found to be independent risk factors for developing IBS following an episode of acute gastroenteritis.
6. Microbiota interactions
The role of TLRs in the pathophysiology of IBS must be linked to the extremely taxonomically complex and ecologically dynamic community inside the gut, which comprises around 1,000 different species,83 the microbiota84 and the fine immune response, developing a tolerogenic reaction towards these commensal microorganisms in addition to the strongly combative response against the pathogenic agents.85 An abnormal or unbalanced response is currently considered a relevant issue in explaining the mechanisms underlying IBD. Although the relationship between the gut mucosal response and microbiota has not been completely elucidated in IBS, microbiota alterations due to the use of systemic antibiotics,86 small intestine bacterial overgrowth (SIBO)87-89 or acute gastroenteritis,90 are extensively considered risk factors for the development of IBS. In contrast, the use of some probiotics has proven to be effective in improving symptoms of IBS.34
Despite the complexity of the gut flora ecosystem, some commensal microorganisms have been significantly related to IBS, including increased Clostridium perfringens (or more diverse Clostridium spp. in general) or decreased Bifidobacteria.91-93 Similarly, Codling et al94 reported higher gut microbial instability and diversity in controls compared to patients. In addition to the related immune mechanisms, very recently Tana et al95 also introduced the concept of the association of organic acids produced by bacterial fermentation derived from higher levels of Veillonella and Lactobacillus spp. with IBS. In summary, the available data has suggested the presence of a dysbiotic microbiota, especially in patients with IBS-D, even though the overall structure is similar between patients and healthy controls.96
Finally, Collins et al97 have proposed that contrary to what occurs in normal subjects following a gastrointestinal infection or after the use of antibiotics, where the changes of microbiota are temporary and completely restored, in patients with IBS a vicious cycle is developed, where intestinal physiological changes, could lead to modifications of the bacterial diversity and richness. In addition, modifications in the microbiota, may trigger changes in the motor function and gut sensitivity, and perhaps produce a positive feedback sustaining the motility abnormalities and visceral hypersensitivity (Fig. 2).
Conclusion
In recent years there has been an increase in the knowledge of the pathophysiological mechanisms related to IBS. However, the evidence obtained from other chronic pain syndromes, where biological, psychological and cultural factors interact in a multifactorial fashion similar to what occurs in IBS, suggests that the data from research studies must be used with caution.98 Several elements are involved in this complex disorder, including an unbalanced immune response, dysbiosis or abnormalities in the intestinal permeability, all of them interacting to develop IBS. We must highlight that the mechanisms underlying the microbiota and host interaction warrant further research to elucidate the communication processes between the immune and the nervous system. We believe that the TLRs and their subsequent cellular activation and production of messenger molecules in IBS need to be investigated. In addition, the single contribution of each TLR and the interaction between 2 or more of these receptors to induce cellular activation and the mechanisms by which this event can affect the pathophysiology of IBS, also deserves future research.
Footnotes
Financial support: This study was supported in part by grant PAPIIT, IN-210010 of DGAPA, Universidad Autónoma de México (UNAM).
Conflicts of interest: None.
References
- 1.Schmulson M. Síndrome de intestino irritable. In: Schmulson M, editor. Clínicas de Gastroenterología de México, Motilidad y Trastornos Funcionales Digestivos. Asociación Mexicana de Gastroenterología. 1st ed. Volume 1. Mexico DF: Editorial Alfil SA de CV; 2008. pp. 109–128. [Google Scholar]
- 2.Cash BD, Schoenfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol. 2002;97:2812–2819. doi: 10.1111/j.1572-0241.2002.07027.x. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Colombo A, Bravo-Gonzalez D, Corona-Lopez A, et al. First community-based study of functional gastrointestinal disorders (FGID) in México using the Rome II modular questionnaire. Gastroenterology. 2006;130(suppl 2):A508. [Google Scholar]
- 4.Schmulson M, Ortiz O, Santiago-Lomeli M, et al. Frequency of functional bowel disorders among healthy volunteers in Mexico City. Dig Dis. 2006;24:342–347. doi: 10.1159/000092887. [DOI] [PubMed] [Google Scholar]
- 5.Schmulson M, Adeyemo M, Gutiérrez-Reyes G, et al. Differences in gastrointestinal symptoms according to gender in Rome II positive IBS and dyspepsia in a Latin American population. Am J Gastroenterol. 2010;105:925–932. doi: 10.1038/ajg.2010.58. [DOI] [PubMed] [Google Scholar]
- 6.Gwee KA. Post-infectious irritable bowel syndrome, an inflammation-immunological model with relevance for other IBS and functional dyspepsia. J Neurogastroenterol Motil. 2010;16:30–34. doi: 10.5056/jnm.2010.16.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quigley EM. Irritable bowel syndrome and inflammatory bowel disease: interrelated diseases? Chin J Dig Dis. 2005;6:122–132. doi: 10.1111/j.1443-9573.2005.00202.x. [DOI] [PubMed] [Google Scholar]
- 8.Bercik P, Verdú EF, Collins SM. Is irritable bowel syndrome a low grade inflammatory disease? Gastroenterol Clin North Am. 2005;34:235–245. doi: 10.1016/j.gtc.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Keohane J, O'Mahony C, O'Mahony L, O'Mahony S, Quigley EM, Shanahan F. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: a real association or reflection of occult inflammation? Am J Gastroenterol. 2010;105:1788, 1789–1794. doi: 10.1038/ajg.2010.156. [DOI] [PubMed] [Google Scholar]
- 10.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-infectious irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 12.Gwee KA, Collins SM, Read NW, et al. Increased rectal mucosal expression of interleukin 1 beta in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52:523–526. doi: 10.1136/gut.52.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 14.Malinen E, Rinttilä T, Kajander K, et al. Analysis of fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 15.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Saito YA, Zimmerman JM, Harmsen WS, et al. Irritable bowel syndrome aggregates strongly in families: a family-based case-control study. Neurogastroenterol Motil. 2008;20:790–797. doi: 10.1111/j.1365-2982.2007.1077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bengtson MB, Ronning T, Vatn MH, Harris JR. Irritable bowel syndrome in twins: genes and environment. Gut. 2006;55:1754–1759. doi: 10.1136/gut.2006.097287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camilleri M. Genetics and irritable bowel syndrome: from genomics to intermediate phenotype and pharmacogenetics. Dig Dis Sci. 2009;54:2318–2324. doi: 10.1007/s10620-009-0903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonsalkorale WM, Perrey C, Pravica V, Whorwell PJ, Hutchinson IV. Interleukin 10 genotypes in irritable bowel syndrome: evidence for an inflammatory component? Gut. 2003;52:91–93. doi: 10.1136/gut.52.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Veek PP, van den Berg M, de Kroon YE, Verspaget HW, Masclee AA. Role of tumor necrosis factor-alpha and interleukin-10 gene polymorphisms in irritable bowel syndrome. Am J Gastroenterol. 2005;100:2510–2516. doi: 10.1111/j.1572-0241.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez-Reyes G, Martínez-García R, Morales-Rochlin N, Gonzalez M, Corona de Lau C, Schmulson M. Interleukin-10 genotypes in IBS-Rome II subjects in México. Gastroenterology. 2006;130(suppl 2):A512. [Google Scholar]
- 22.Villani AC, Lemire M, Thabane M, et al. Genetic risk factors for post-infectious irritable bowel syndrome following a waterborne outbreak of gastroenteritis. Gastroenterology. 2010;138:1502–1513. doi: 10.1053/j.gastro.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 23.Selye H. A syndrome produced by diverse nocuous agents. J Neuropsychiatry Clin Neurosci. 1998;10:230–231. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 24.Barreau F, Cartier C, Leveque M, et al. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J Physiol. 2007;580:347–356. doi: 10.1113/jphysiol.2006.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taché Y, Brunnhuber S. From Hans Selye's discovery of biological stress to the identification of corticotropin-releasing factor signaling pathways implication in stress-related functional bowel diseases. Ann N Y Acad Sci. 2008;1148:29–41. doi: 10.1196/annals.1410.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stengel A, Taché Y. Neuroendocrine Control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol. 2009;71:219–239. doi: 10.1146/annurev.physiol.010908.163221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fish EW, Shahrokh D, Bagot R, et al. Epigenetic programming of stress responses through variations in maternal care. Ann N Y Acad Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- 28.Chang L, Sundaresh S, Elliott J, et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinan TG, Quigley EM, Ahmed SM, et al. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 30.Martinez V, Taché Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr Pharm Des. 2006;12:4071–4088. doi: 10.2174/138161206778743637. [DOI] [PubMed] [Google Scholar]
- 31.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 32.Cremon C, Gargano L, Morselli-Labate AM, et al. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 33.Quigley EM. Probiotics in functional gastrointestinal disorders: what are the facts? Curr Opin Pharmacol. 2008;8:704–708. doi: 10.1016/j.coph.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Rijnierse A, Nijkamp FP, Kraneveld AD. Mast cells and nerves tickle in the tummy: implications for inflammatory bowel disease and irritable bowel syndrome. Pharmacol Ther. 2007;116:207–235. doi: 10.1016/j.pharmthera.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Salzmann JL, Peltier-Koch F, Bloch F, Petite JP, Camilleri JP. Morphometric study of colonic biopsies: a new method of estimating inflammatory diseases. Lab Invest. 1989;60:847–851. [PubMed] [Google Scholar]
- 36.Barbara G, Cremon C, Pallotti F, De Giorgio R, Stanghellini V, Corinaldesi R. Postinfectious irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48(suppl 2):S95–S97. doi: 10.1097/MPG.0b013e3181a15e2e. [DOI] [PubMed] [Google Scholar]
- 37.McKeown ES, Parry SD, Stansfield R, Barton JR, Welfare MR. Postinfectious irritable bowel syndrome may occur after non-gastrointestinal and intestinal infection. Neurogastroenterol Motil. 2006;18:839–843. doi: 10.1111/j.1365-2982.2006.00821.x. [DOI] [PubMed] [Google Scholar]
- 38.Park JH, Rhee PL, Kim G, et al. Enteroendocrine cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:539–546. doi: 10.1111/j.1365-2982.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 39.Öhman L, Isaksson S, Lindmark AC, et al. T-Cell activation in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:1205–1212. doi: 10.1038/ajg.2009.116. [DOI] [PubMed] [Google Scholar]
- 40.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 41.Walker MM, Talley NJ, Prabhakar M, et al. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2009;29:765–773. doi: 10.1111/j.1365-2036.2009.03937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Argenzio RA. Neuro-immune pathobiology of infectious enteric disease. Adv Exp Med Biol. 1997;412:21–29. doi: 10.1007/978-1-4899-1828-4_2. [DOI] [PubMed] [Google Scholar]
- 43.Nakae S, Suto H, Kakurai M, Sedgwick J, Tsai M, Galli S. Mast cells enhance T cell activation: importance of mast cell-derived TNF. PNAS. 2005;102:6467–6472. doi: 10.1073/pnas.0501912102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- 45.Barbara G, Stanghellini V, De Giorgio G, Corinaldesi R. Functional gastrointestinal disorders and mast cells: implications for therapy. Neurogastroenterol Motil. 2006;18:6–17. doi: 10.1111/j.1365-2982.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- 46.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 47.Ohman L, Simrén M. New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig Liver Dis. 2007;39:201–215. doi: 10.1016/j.dld.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Bradesi S, Schwetz I, Ennes HS, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–G53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 49.Collins SM. A case for an immunological basis for irritable bowel syndrome. Gastroenterology. 2002;122:2078–2080. doi: 10.1053/gast.2002.34097. [DOI] [PubMed] [Google Scholar]
- 50.Foell D, Wittkowski H, Roth J. Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut. 2009;58:859–868. doi: 10.1136/gut.2008.170019. [DOI] [PubMed] [Google Scholar]
- 51.Holmén N, Isaksson S, Simrén M, Sjövall H, Öhman L. CD4+ CD25+ regulatory T cells in irritable bowel syndrome patients. Neurogastroenterol Motil. 2007;19:119–125. doi: 10.1111/j.1365-2982.2006.00878.x. [DOI] [PubMed] [Google Scholar]
- 52.Öhman L, Lindmark AC, Isaksson S, et al. B-cell activation in patients with irritable bowel syndrome (IBS) Neurogastroenterol Motil. 2009;21:644, 650–e27. doi: 10.1111/j.1365-2982.2009.01272.x. [DOI] [PubMed] [Google Scholar]
- 53.Chang F, Deere H, Vu C. Atypical forms of microscopic colitis: morphological features and review of the literature. Adv Anat Pathol. 2005;12:203–211. doi: 10.1097/01.pap.0000175115.63165.6b. [DOI] [PubMed] [Google Scholar]
- 54.Fernández-Bañares F. How much symptom overlap is there between microscopic colitis and IBS? Nat Clin Pract Gastroenterol Hepatol. 2007;4:304–305. doi: 10.1038/ncpgasthep0814. [DOI] [PubMed] [Google Scholar]
- 55.Limsui D, Pardi DS, Camilleri M, et al. Symptomatic overlap between irritable bowel syndrome and microscopic colitis. Inflamm Bowel Dis. 2007;13:175–181. doi: 10.1002/ibd.20059. [DOI] [PubMed] [Google Scholar]
- 56.Chey WD, Nojkov B, Rubenstein JH, Dobhan RR, Greenson JK, Cash BD. The yield of colonoscopy in patients with non-constipated irritable bowel syndrome: results from a prospective, controlled US trial. Am J Gastroenterol. 2010;105:859–865. doi: 10.1038/ajg.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lazenby AJ, Yardley JH, Giardiello FM, Bayless TM. Pitfalls in the diagnosis of collagenous colitis: experience with 75 cases from a registry of collagenous colitis at the Johns Hopkins Hospital. Hum Pathol. 1990;21:905–910. doi: 10.1016/0046-8177(90)90173-3. [DOI] [PubMed] [Google Scholar]
- 58.Walker MM, Warwick A, Ung C, et al. The Popcol Study: Epidemiology by endoscopy in a swedish adult random population. Intraepithelial lymphocyte and eosinophil counts in the normal colon and irritable bowel syndrome. Gastroenterology. 2010;138(suppl 1):S585. [Google Scholar]
- 59.Kraehenbuhl JP, Corbett M. Immunology. Keeping the gut at bay. Science. 2004;303:1624–1625. doi: 10.1126/science.1096222. [DOI] [PubMed] [Google Scholar]
- 60.Al-Sadi R, Boivin M, Ma Y. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci. 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiank C, Taché Y, Larauche M. Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: role of corticotropin-releasing factor receptors. Brain Behav Immun. 2010;24:41–48. doi: 10.1016/j.bbi.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kraneveld AD, Rijnierse A, Nijkamp FP, Garssen J. Neuro-immune interactions in inflammatory bowel disease and irritable bowel syndrome: Future therapeutic targets. Eur J Pharmacol. 2008;585:361–374. doi: 10.1016/j.ejphar.2008.02.095. [DOI] [PubMed] [Google Scholar]
- 63.O'Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 64.O'Mahony SM, Marchesi JR, Scully P, et al. Early life stress alters behavior, immunity and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 65.Clarke G, O'Mahony SM, Hennessy AA, et al. Chain reactions: early-life stress alters the metabolic profile of plasma polyunsaturated fatty acids in adulthood. Behav Brain Res. 2009;205:319–321. doi: 10.1016/j.bbr.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 66.Macsharry J, O'Mahony L, Fanning A, et al. Mucosal cytokine imbalance in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1467–1476. doi: 10.1080/00365520802276127. [DOI] [PubMed] [Google Scholar]
- 67.Milligan ED, Sloane EM, Watkins LR. Glia in pathological pain: a role for fractalkine. J Neuroimmunol. 2008;198:113–120. doi: 10.1016/j.jneuroim.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guyon A, Massa F, Rovére C, Nahon JL. How cytokines can influence the brain: a role for chemokines? J Neuroimmunol. 2008;198:46–55. doi: 10.1016/j.jneuroim.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 69.Guyon A, Nahon JL. Multiple actions of the chemokine stroma cell-derived factor1 alpha on neuronal activity. J Mol Endocrinol. 2007;38:365–376. doi: 10.1677/JME-06-0013. [DOI] [PubMed] [Google Scholar]
- 70.Dinan TG, Clarke G, Quigley EM, et al. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol. 2008;103:2570–2576. doi: 10.1111/j.1572-0241.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- 71.Clarke G, Quigley EM, Cryan JF, Dinan TG. Irritable bowel syndrome: towards biomarker identification. Trends Mol Med. 2009;15:478–489. doi: 10.1016/j.molmed.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Fukata M, Vamadevan AS, Abreu MT. Toll-like receptors (TLRs) and Nod-like receptors in inflammatory disorders. Semin Immunol. 2009;21:242–253. doi: 10.1016/j.smim.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 73.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rakoff-Nahoum S, Medzhitov R. Role of Toll-Like receptors in tissue repair and tumorigenesis. Biochemistry (Mosc) 2008;73:555–561. doi: 10.1134/s0006297908050088. [DOI] [PubMed] [Google Scholar]
- 75.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 76.Marshall JK, Thabane M, Garg AX, et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20:1317–1322. doi: 10.1111/j.1365-2036.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 77.Dunlop SP, Hebden J, Campbell E, et al. Abnomal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288–1294. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 78.Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196–201. doi: 10.1136/gut.2007.140806. [DOI] [PubMed] [Google Scholar]
- 79.Coëffier M, Gloro R, Boukhettala N, et al. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. Am J Gastroenterol. 2010;105:1181–1188. doi: 10.1038/ajg.2009.700. [DOI] [PubMed] [Google Scholar]
- 80.Himmel ME, Hardenberg G, Piccirillo CA, Steiner TS, Levings MK. The role of T-regulatory T cells and Toll-like receptors in the pathogenesis of the human inflammatory bowel disease. Immunology. 2008;125:145–153. doi: 10.1111/j.1365-2567.2008.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brint E, MacSharry J, Shanahan F, Quigley EM. 400 Differential expression of Toll-like receptors (TLRS) in Irritable bowel syndrome (IBS) Gastroenterology. 2009;136(suppl 1):A67. doi: 10.1038/ajg.2010.438. [DOI] [PubMed] [Google Scholar]
- 82.McKernan DP, Nolan A, Brint EK, et al. Toll-like receptor mRNA expression is selectively increased in the colonic mucosa of two animal models relevant to irritable bowel syndrome. PLoS One. 2009;4:e8226. doi: 10.1371/journal.pone.0008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hattori M, Taylor TD. The human intestinal microbiome: a new frontier of human biology. DNA Res. 2009;16:1–12. doi: 10.1093/dnares/dsn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Honda K, Takeda K. Regulatory mechanisms of immune responses to intestinal bacteria. Mucosal Immunol. 2009;2:187–196. doi: 10.1038/mi.2009.8. [DOI] [PubMed] [Google Scholar]
- 86.Di Stefano M, Corazza GR. The rationale for antibiotics in IBS. Am J Gastroenterol. 2008;103:2652. doi: 10.1111/j.1572-0241.2008.02074_1.x. [DOI] [PubMed] [Google Scholar]
- 87.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–3506. doi: 10.1111/j.1572-0241.2000.03368.x. [DOI] [PubMed] [Google Scholar]
- 88.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. A double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 89.Pimentel M, Lin HC, Enayati P, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2005;290:G1089–G1095. doi: 10.1152/ajpgi.00574.2004. [DOI] [PubMed] [Google Scholar]
- 90.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 91.Si JM, Yu YC, Fan YJ, Chen SJ. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J Gastroenterol. 2004;10:1802–1805. doi: 10.3748/wjg.v10.i12.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mättö J, Maunuksela L, Kajander K, et al. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome - a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol. 2005;43:213–222. doi: 10.1016/j.femsim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 93.Maukonen J, Satokari R, Mättö J, Söderlung H, Mattilla-Sandholm T, Saarela M. Prevalence and temporal stability of selected clostridial groups in irritable bowel syndrome in relation to predominant faecal bacteria. J Med Microbiol. 2006;55:625–633. doi: 10.1099/jmm.0.46134-0. [DOI] [PubMed] [Google Scholar]
- 94.Codling C, O'Mahony L, Shanahan F, Quigley EM, Marchesi JR. A molecular analysis of fecal and mucosal bacterial communities in irritable bowel syndrome. Dig Dis Sci. 2010;55:392–397. doi: 10.1007/s10620-009-0934-x. [DOI] [PubMed] [Google Scholar]
- 95.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–519. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 96.Krogius-Kurikka L, Lyra A, Malinen E, et al. Microbial community analysis releals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. doi: 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Collins SM, Denou E, Verdu EF, Bercik P. The putative role of the intestinal microbiota in the irritable bowel syndrome. Dig Liver Dis. 2009;41:850–853. doi: 10.1016/j.dld.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 98.Bielefeldt K, Levinthal D. Pieces of a puzzle: Permeability, proinflammatory pathways and pain? Pain. 2009;146:7–8. doi: 10.1016/j.pain.2009.06.008. [DOI] [PubMed] [Google Scholar]