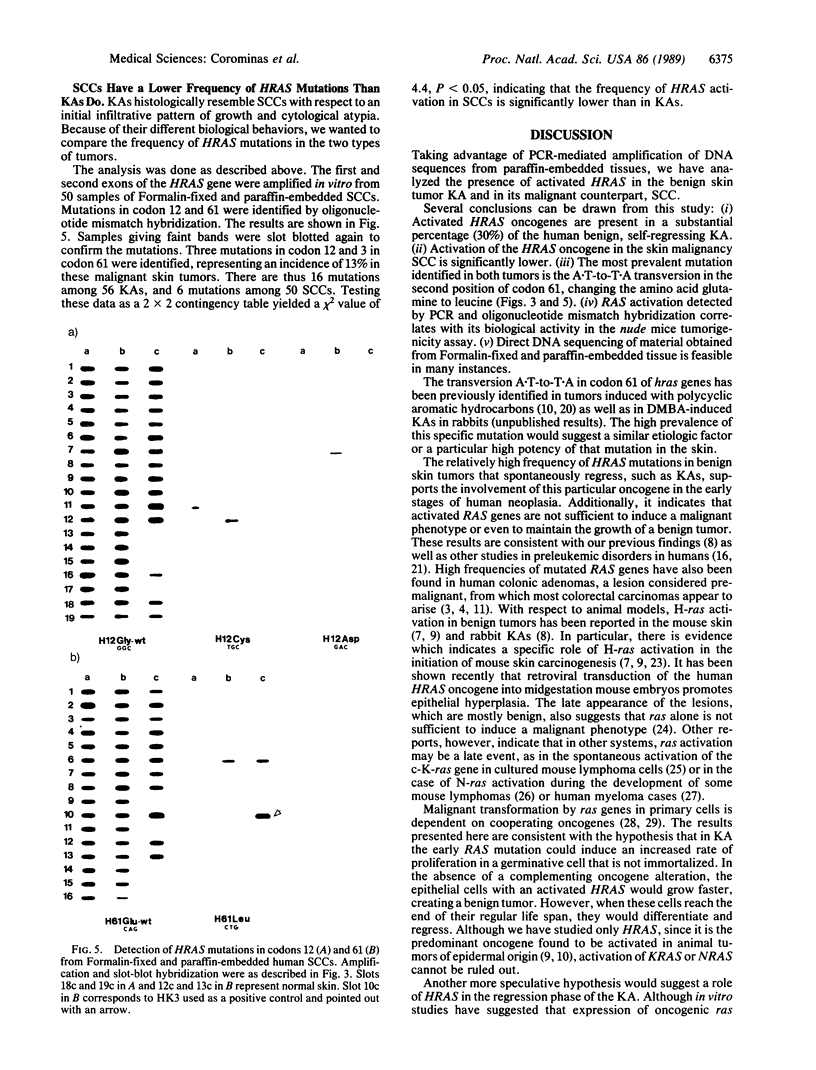
Abstract
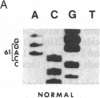
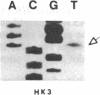
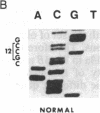
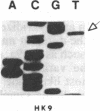
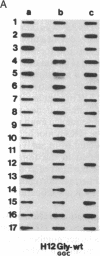
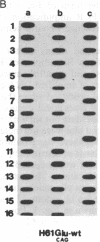
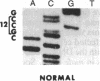
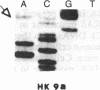
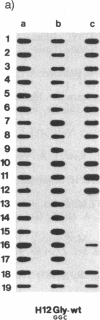
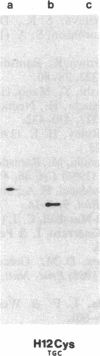
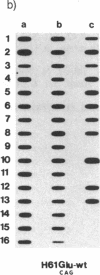
In vitro DNA amplification followed by oligonucleotide mismatch hybridization was used to study the frequency of HRAS mutations in the benign self-regressing skin tumors keratoacanthomas and in squamous cell carcinomas. We used freshly obtained keratoacanthomas as well as Formalin-fixed paraffin-embedded tissues from both types of tumors. DNA from 50 samples of each tumor type was analyzed for activating mutations involving codons 12 and 61. A relatively high percentage (30%) of HRAS mutations was found in the keratoacanthomas compared with 13% in the squamous cell carcinomas. The most frequent mutation identified is the A-T-to-T.A transversion in the second position of codon 61. The present findings demonstrate the involvement of the HRAS oncogene in human benign tumors. Moreover, they indicate that an activated HRAS oncogene is not sufficient to maintain a neoplastic phenotype and argue against a role of HRAS in the progression of skin tumorigenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Balmain A., Pragnell I. B. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983 May 5;303(5912):72–74. doi: 10.1038/303072a0. [DOI] [PubMed] [Google Scholar]
- Balmain A., Ramsden M., Bowden G. T., Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984 Feb 16;307(5952):658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985 Oct;42(3):841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bizub D., Wood A. W., Skalka A. M. Mutagenesis of the Ha-ras oncogene in mouse skin tumors induced by polycyclic aromatic hydrocarbons. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6048–6052. doi: 10.1073/pnas.83.16.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L. The ras gene family and human carcinogenesis. Mutat Res. 1988 May;195(3):255–271. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- Brown K., Quintanilla M., Ramsden M., Kerr I. B., Young S., Balmain A. v-ras genes from Harvey and BALB murine sarcoma viruses can act as initiators of two-stage mouse skin carcinogenesis. Cell. 1986 Aug 1;46(3):447–456. doi: 10.1016/0092-8674(86)90665-3. [DOI] [PubMed] [Google Scholar]
- Compere S. J., Baldacci P. A., Sharpe A. H., Jaenisch R. Retroviral transduction of the human c-Ha-ras-1 oncogene into midgestation mouse embryos promotes rapid epithelial hyperplasia. Mol Cell Biol. 1989 Jan;9(1):6–14. doi: 10.1128/mcb.9.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L. E., Guerrero I., Pellicer A. Concomitant K- and N-ras gene point mutations in clonal murine lymphoma. Mol Cell Biol. 1988 May;8(5):2233–2236. doi: 10.1128/mcb.8.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Guerrero I., Wong H., Pellicer A., Burstein D. E. Activated N-ras gene induces neuronal differentiation of PC12 rat pheochromocytoma cells. J Cell Physiol. 1986 Oct;129(1):71–76. doi: 10.1002/jcp.1041290111. [DOI] [PubMed] [Google Scholar]
- Higuchi R., von Beroldingen C. H., Sensabaugh G. F., Erlich H. A. DNA typing from single hairs. Nature. 1988 Apr 7;332(6164):543–546. doi: 10.1038/332543a0. [DOI] [PubMed] [Google Scholar]
- Hirai H., Kobayashi Y., Mano H., Hagiwara K., Maru Y., Omine M., Mizoguchi H., Nishida J., Takaku F. A point mutation at codon 13 of the N-ras oncogene in myelodysplastic syndrome. Nature. 1987 Jun 4;327(6121):430–432. doi: 10.1038/327430a0. [DOI] [PubMed] [Google Scholar]
- Hirakawa T., Ruley H. E. Rescue of cells from ras oncogene-induced growth arrest by a second, complementing, oncogene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1519–1523. doi: 10.1073/pnas.85.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd V. J., Wallace R. B., Itakura K., Woo S. L. alpha 1-antitrypsin deficiency detection by direct analysis of the mutation in the gene. Nature. 1983 Jul 21;304(5923):230–234. doi: 10.1038/304230a0. [DOI] [PubMed] [Google Scholar]
- Kraus M. H., Yuasa Y., Aaronson S. A. A position 12-activated H-ras oncogene in all HS578T mammary carcinosarcoma cells but not normal mammary cells of the same patient. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5384–5388. doi: 10.1073/pnas.81.17.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Leon J., Kamino H., Steinberg J. J., Pellicer A. H-ras activation in benign and self-regressing skin tumors (keratoacanthomas) in both humans and an animal model system. Mol Cell Biol. 1988 Feb;8(2):786–793. doi: 10.1128/mcb.8.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E., Hjelle B., Morgan R., Hecht F., Bishop J. M. Mutations of the Kirsten-ras proto-oncogene in human preleukaemia. Nature. 1987 Nov 12;330(6144):186–188. doi: 10.1038/330186a0. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Mabry M., de Bustros A., Ihle J. N., Nelkin B. D., Baylin S. B. Introduction of v-Ha-ras oncogene induces differentiation of cultured human medullary thyroid carcinoma cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5923–5927. doi: 10.1073/pnas.84.16.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri A., Knowles D. M., Greco A., McCormick F., Dalla-Favera R. Analysis of RAS oncogene mutations in human lymphoid malignancies. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9268–9272. doi: 10.1073/pnas.85.23.9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Ko M., Ogura A., Liu D. G., Amano T., Takano T., Ikawa Y. Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature. 1985 Nov 7;318(6041):73–75. doi: 10.1038/318073a0. [DOI] [PubMed] [Google Scholar]
- Quintanilla M., Brown K., Ramsden M., Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986 Jul 3;322(6074):78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Ramselaar C. G., van der Meer J. B. The spontaneous regression of keratoacanthoma in man. Acta Derm Venereol. 1976;56(3):245–251. [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Noble M., Land H. Ras-mediated cell cycle arrest is altered by nuclear oncogenes to induce Schwann cell transformation. EMBO J. 1988 Jun;7(6):1635–1645. doi: 10.1002/j.1460-2075.1988.tb02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit V. T., Boot A. J., Smits A. M., Fleuren G. J., Cornelisse C. J., Bos J. L. KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res. 1988 Aug 25;16(16):7773–7782. doi: 10.1093/nar/16.16.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Vousden K. H., Marshall C. J. Three different activated ras genes in mouse tumours; evidence for oncogene activation during progression of a mouse lymphoma. EMBO J. 1984 Apr;3(4):913–917. doi: 10.1002/j.1460-2075.1984.tb01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa Y., Srivastava S. K., Dunn C. Y., Rhim J. S., Reddy E. P., Aaronson S. A. Acquisition of transforming properties by alternative point mutations within c-bas/has human proto-oncogene. Nature. 1983 Jun 30;303(5920):775–779. doi: 10.1038/303775a0. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Vass W., Scolnick E. Altered growth and differentiation of cultured mouse epidermal cells infected with oncogenic retrovirus: contrasting effects of viruses and chemicals. Cancer Res. 1983 Dec;43(12 Pt 1):6021–6030. [PubMed] [Google Scholar]