Desmoid tumors are rare, benign, slow-growing, fibroblastic neoplasms that originate from musculoaponeurotic and fascial structures throughout the body. These tumors do not have the ability to metastasize, but they are locally aggressive, with a high rate of recurrence even after surgical resection. Desmoids can cause severe clinical sequelae, including mortality due to infiltration of nearby organs. In this case report, we discuss a patient who presented with right lower quadrant abdominal pain and extraluminal colonic obstruction secondary to a desmoid tumor.
Case Report
A 46-year-old woman with a past medical history of rheumatoid arthritis, who was on weekly methotrexate therapy, presented to her primary care physician complaining chiefly of right lower quadrant abdominal pain of 4 weeks' duration. She described the pain as a progressive, dull ache lasting over the preceding 6 months, with intermittent episodes of sharp, severe pain. The pain occasionally radiated to the back and left lower quadrant, but it generally remained in the right lower quadrant. The patient experienced some associated nausea but denied having any associated vomiting, constipation, diarrhea, hematochezia, or weight loss. She had tried taking over-the-counter analgesics and, eventually, simethicone and hyoscyamine (Levsin, Schwarz Pharma), as prescribed by her primary care physician. At a follow-up visit 6 months later, the patient reported no relief with the above therapies and was experiencing progressive symptoms of dull pain and pressure in the right lower quadrant. Her past medical history was significant for a ton-sillectomy as a child and for rheumatoid arthritis, which had been diagnosed in 2005 and for which she was receiving weekly methotrexate injections (20 mg/wk). Besides methotrexate, she was also taking folic acid. Her family history was significant for gallstones and kidney stones, but she did not have any history of autoimmune diseases, inflammatory bowel disease, or malignancy. She did not use tobacco, alcohol, or recreational drugs.
On examination, the patient was afebrile with stable vital signs. She was alert and in no acute distress. Her conjunctivae were anicteric, her mucus membranes were moist, and her oropharynx was clear. She had a normal thyroid examination and no cervical or supraclavicular lymphadenopathy. Her lungs were clear to auscultation, and her heart showed regular rhythm with no murmurs, rubs, or gallops. Abdominal examination revealed a normal-appearing abdomen, which was nontender, nondistended without any peritoneal signs of rebound or guarding, and without appreciable hepatosplenomegaly. Bowel sounds were present in all 4 quadrants. The remainder of her examination was unremarkable.
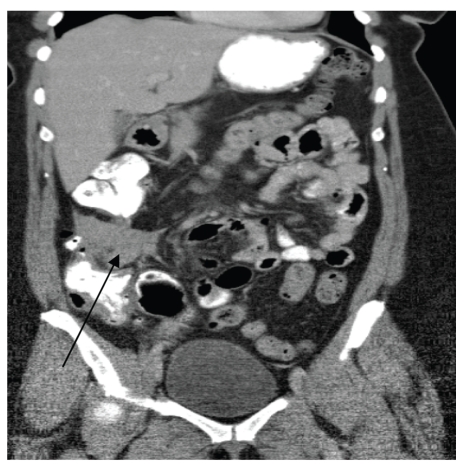
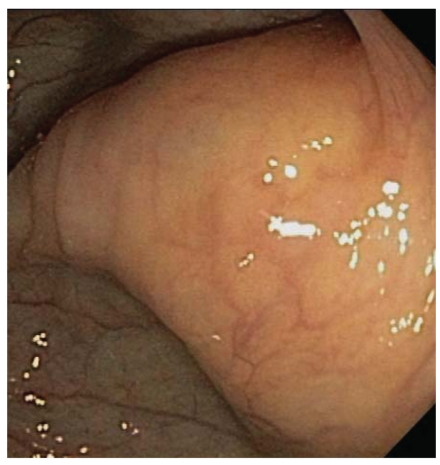
Given the progressive nature of her symptoms, a computed tomography (CT) scan of the abdomen and pelvis was ordered with oral and intravenous contrast. The CT scan revealed a 6.4-cm × 3.1-cm soft-tissue density adjacent to the ascending colon (Figure 1). Circumferential wall thickening of the adjacent ascending colon was seen, as was a left adnexal mass measuring 4.0 cm × 3.1 cm, which was consistent with an ovarian cyst. Otherwise, the remainder of the examination was unremarkable. The radiologist proposed that the right lower quadrant mass represented an ascending colon neoplasm with adjacent lymph node enlargement or a primary extracolonic mass. Colonoscopy was thus recommended and revealed a 5-mm polyp in the transverse colon and an impassable segment in the mid- to distal ascending colon, corresponding to the location of the abnormality on the patient's CT scan (Figure 2). The endoscopist noted a twist in the bowel at this segment, which raised concerns for a possible chronic cecal volvulus. A barium enema study was ordered to further evaluate these findings.
Figure 1.
Computed tomography scan revealing a right lower quadrant mass adjacent to the ascending colon (arrow).
Figure 2.
Impassable segment of the ascending colon seen during colonoscopy.
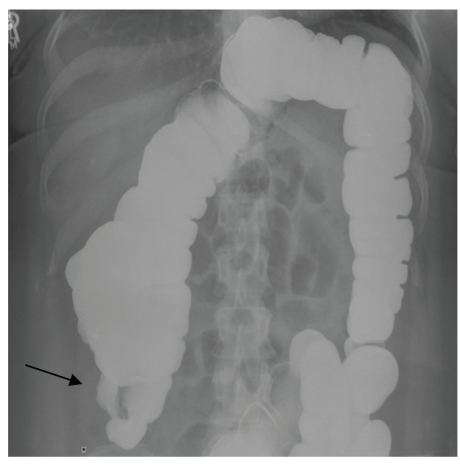
Despite multiple maneuvers by the barium technician, contrast did not reach the cecum. The barium enema study confirmed that this obstruction was secondary to a very irregular and narrowed colon in the region of the hepatic flexure and ascending colon, with associated dilation of the proximal bowel (Figure 3). Based upon the study, it was not possible to determine whether this finding was caused by an intraluminal growth or extrinsic compression. Given these findings, the patient was referred for surgical evaluation for definitive diagnosis and possible resection.
Figure 3.
Barium enema study revealing obstruction of the ascending colon (arrow) caused by either an intraluminal or extracolonic mass.
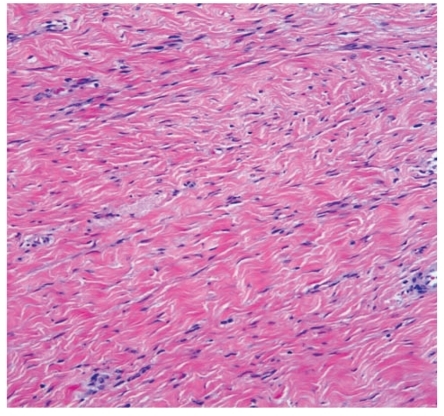
The patient underwent incisional biopsy of the mass and subsequent right hemicolectomy with ileocecectomy, appendectomy, and associated removal of pericolonic fat and mesentery, which included the mass in question. The gross specimen included a 6.2-cm × 6-cm × 3.7-cm well-defined, tan white, firm, trabecular, and slightly whorled mass that was completely free of surgical margins (Figure 4). The mass grossly extended to involve the serosal and muscularis layer of the right colon but did not grossly involve the mucosa. On histologic evaluation, the mass consisted of fibroblastic proliferation with generally low cellularity, bland cytology, and no appreciable mitotic activity. The mass was also densely collagenized. The tumor cells stained positive for desmin and beta-catenin but negative for CD34, CD117, S-100 protein, and keratin AE1/3, which was consistent with a diagnosis of desmoid tumor (fibromatosis).
Figure 4.
Desmoid tumor with fibroblastic proliferation, low cellularity, and dense collagenization (hematoxylin and eosin stain).
The patient tolerated the procedure without complications. At a follow-up visit 3 months later, she reported complete resolution of her right lower quadrant pain. Plans for further follow-up included serial clinical and radiographic evaluation (CT scans) every 6 months.
Discussion
In this case report, our patient presented with right lower quadrant abdominal pain of 6 months' duration, and work-up eventually revealed a desmoid tumor. These tumors typically appear as well-differentiated growths of fibrocollagenous tissue that are locally infiltrative and aggressive. Although they do not have malignant potential or the ability to metastasize, their aggressive nature often causes significant morbidity and mortality due to infiltration of vital organs and structures.1 Given their tendency to recur after resection or medical management, desmoid management can be quite challenging for clinicians.
Pathophysiology
The cell of origin for desmoid tumors is the myofbroblast, which explains their typical occurrence in musculo-aponeurotic tissue. The currently understood mechanism for development of desmoid tumors involves mutations in the adenomatous polyposis coli (APC) gene on chromosome 5q. The APC gene codes for the beta-catenin binding/degradation protein (APC protein). Beta-catenin is responsible for activating transcription factor Tcf-4, an important protein involved in fibroblast proliferation. The APC protein prevents accumulation of beta-catenin by mediating its phosphorylation and degradation.2 Thus, mutations in the APC gene result in beta-catenin over-expression and resultant dysregulation of mesenchymal and fibroblast cell proliferation and invasiveness.3 This explains the high rate of desmoid occurrence in patients with familial adenomatous polyposis (FAP) or Gardner syndrome. These disorders, known to cause early and aggressive development of colorectal adenomatous polyps and cancer as well as soft- and hard-tissue neoplasms, are secondary to point mutations in the APC gene. Up to 20% of patients with FAP may be afflicted by desmoid tumors.4 Similarly, patients who have had previous surgery or antecedent trauma are at increased risk for desmoid development, as beta-catenin levels are elevated during the proliferative phase of wound healing.5 Alternatively, a link has been postulated between desmoid development and high estrogen states such as pregnancy, as estrogen has been shown to promote fibroblast proliferation.6
Epidemiology
Desmoid tumors are extremely rare, causing 0.03% of all neoplasms and less than 3% of all soft-tissue tumors. They typically develop in young and middle-aged adults (10–60 years of age) but can be occasionally found in young children as well as the elderly. There is a slight female predominance with this disease, but no racial or ethnic trends have been observed.7
Clinical Presentation
Given the extensive distribution of musculoskeletal and stromal tissues, desmoids can arise almost anywhere in the body. However, there are several common sites of development: the trunk and extremities (usually the shoulder or pelvic girdle), the abdominal wall (typically arising from the rectus muscle), and inside the abdomen (from the bowel or mesentery). Depending upon the location, most desmoids present as slow-growing, painless (or minimally painful) masses. Intra-abdominal desmoids may cause bowel obstruction or ischemia, whereas extremity desmoids may cause nerve entrapment or lymphatic obstruction. Given the slow-growing nature of intraabdominal desmoids, pain associated with these tumors typically indicates significant visceral involvement.8 Due to a link with wound healing, sites of prior trauma or surgery are often involved. On examination, the typical finding consists of a firm, smooth, and mobile mass that is adherent to its surrounding structures. Usually, the overlying skin is not affected.
Diagnostic Evaluation
Initial work-up typically consists of imaging of the affected anatomic site to confirm the cause. Although both CT and magnetic resonance imaging (MRI) are adequate for evaluation, MRI is preferred, particularly for extremity desmoids, as it better reveals involvement of surrounding tissues. As neither imaging modality can distinguish a desmoid from a malignant soft-tissue tumor, histologic evaluation is needed to confirm the diagnosis. A biopsy can confirm monoclonal growth of fibroblast cells, which appear as a bundle of spindle cells in a fibrous stroma. Cellularity is typically low. Distinguishing desmoids from fibrosarcomas is particularly important and necessitates an incisional biopsy, rather than fine-needle aspiration. Pathologically, this is accomplished by observing that the cells in the desmoids lack nuclear and cytoplasmic features of malignancy (ie, there are few mitotic bodies, and necrosis is absent). Immunohistochemical staining can aid in the process of diagnosis, as can nuclear betacatenin staining. Additionally, spindle cells often contain vimentin and smooth muscle actin but rarely stain for desmin, cytokeratins, and S-100.9
Treatment
Early referral to a center that specializes in multimodality care of sarcomas is warranted. In asymptomatic patients, close observation is often the preferred strategy. Patients with symptoms are typically treated, given the inevitably progressive growth of desmoid tumors. Surgery with a wide margin of resection is the preferred treatment whenever feasible, particularly for intra-abdominal desmoids.10 Limitations include tumor involvement of vital anatomic structures, where resection would lead to functional limitation or significant morbidity and mortality. Similarly, desmoids frequently recur at a high rate (16–39%), even when surgical margins are free of tumor, and these recurrences are often more aggressive than the initial tumor.11
Radiation therapy has been shown to be an effective alternative for definitive care in patients who refuse surgery, patients who are not good surgical candidates, or patients in whom surgery carries a high risk.12 Additionally, trials are being conducted to evaluate neoadjuvant radiation prior to surgery, which has shown promise.
For patients with extra-abdominal tumors as well as those with recurrence despite local therapy, systemic medical therapy is often prescribed. Options include tamoxifen, which is thought to suppress desmoid growth due to the presence of estrogen receptor beta on tumor cells, particularly extra-abdominal desmoids. Similarly, nonsteroidal anti-inflammatory drugs (NSAIDs) such as sulindac, alone or with tamoxifen, have shown promising response rates. With either anti-estrogen or NSAID therapy, tumor shrinkage is often slow and delayed by many months.13
Finally, in patients who do not respond to non-cytotoxic therapy, trials have shown good response to treatment with either doxorubicin-based regimens or methotrexate with vinca alkaloids.14
Post-treatment surveillance includes clinical examination and radiographic assessment every 6 months for at least 3 years and then yearly thereafter.
summary
This case report highlights the incidence of desmoid tumors, a rare but clinically significant cause of neoplastic disease. Although desmoids are uncommon, the asymptomatic nature of their progression and their locally invasive and aggressive behavior require clinicians to be aware of them, as they can cause significant functional impairment as well as morbidity and mortality if vital structures are involved. Surgical resection is the preferred therapy, though recurrence is high despite complete resection. Radiation therapy, as well as medical therapy with noncytotoxic and cytotoxic agents, has a role as an alternative to surgical therapy or experimentally in the neoadjuvant setting. Close follow-up of these patients is warranted, given the high rate of recurrence.
References
- 1.Schlemmer M. Desmoid tumors and deep fibromatoses. Hematol Oncol Clin North Am. 2005;19:565–571. doi: 10.1016/j.hoc.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Bapat B, Alman BA. Adenomatous polyposis coli gene mutation alters proliferation through its beta-catenin-regulatory function in aggressive fibromatosis (desmoid tumor) Am J Pathol. 1998;153:709–714. doi: 10.1016/s0002-9440(10)65614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tejpar S, Nollet F, Li C, et al. Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor) Oncogene. 1999;18:6615–6620. doi: 10.1038/sj.onc.1203041. [DOI] [PubMed] [Google Scholar]
- 4.Hizawa K, Iida M, Mibu R, Aoyagi K, Yao T, Fujishima M. Desmoid tumors in familial adenomatous polyposis/Gardner's syndrome. J Clin Gastroenterol. 1997;25:334–337. doi: 10.1097/00004836-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Cheon SS, Cheah AY, Turley S, et al. Beta-catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci U S A. 2002;99:6973–6978. doi: 10.1073/pnas.102657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Way JC, Culham BA. Desmoid tumour. The risk of recurrent or new disease with subsequent pregnancy: a case report. Can J Surg. 1999;42:51–54. [PMC free article] [PubMed] [Google Scholar]
- 7.Shields CJ, Winter DC, Kirwan WO, Redmond HP. Desmoid tumours. Eur J Surg Oncol. 2001;27:701–706. doi: 10.1053/ejso.2001.1169. [DOI] [PubMed] [Google Scholar]
- 8.Easter DW, Halasz NA. Recent trends in the management of desmoid tumors. Summary of 19 cases and review of the literature. Ann Surg. 1989;210:765–769. doi: 10.1097/00000658-198912000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson JW, Fletcher CD. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cell lesions: analysis of a series and review of the literature. Histopathology. 2007;51:509–514. doi: 10.1111/j.1365-2559.2007.02794.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith AJ, Lewis JJ, Merchant NB, Leung DH, Woodruff JM, Brennan MF. Surgical management of intra-abdominal desmoid tumours. Br J Surg. 2000;87:608–613. doi: 10.1046/j.1365-2168.2000.01400.x. [DOI] [PubMed] [Google Scholar]
- 11.Melis M, Zager JS, Sondak VK. Multimodality management of desmoid tumors: how important is a negative surgical margin? J Surg Oncol. 2008;98:594–602. doi: 10.1002/jso.21033. [DOI] [PubMed] [Google Scholar]
- 12.Nuyttens JJ, Rust PF, Thomas CR, Jr, Turrisi AT., 3rd Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: a comparative review of 22 articles. Cancer. 2000;88:1517–1523. [PubMed] [Google Scholar]
- 13.Janinis J, Patriki M, Vini L, Aravantinos G, Whelan JS. The pharmacological treatment of aggressive fibromatosis: a systematic review. Ann Oncol. 2003;14:181–190. doi: 10.1093/annonc/mdg064. [DOI] [PubMed] [Google Scholar]
- 14.Patel SR, Benjamin RS. Desmoid tumors respond to chemotherapy: defying the dogma in oncology. J Clin Oncol. 2006;24:11–12. doi: 10.1200/JCO.2005.03.6566. [DOI] [PubMed] [Google Scholar]