Abstract
Hepatocellular carcinoma (HCC) is a significant worldwide health concern that is associated with discrete etiological events, encompassing viral infection, metabolic stress and genotoxic compounds. In particular, exposure to the genotoxic hepatocarcinogen aflatoxin B1 (AFB1) is a significant factor in the genesis of human liver cancer. Presumably, genetic events associated with HCC could influence the effect of environmental insults, yielding a predilection for tumor development. The retinoblastoma (RB) tumor suppressor pathway is functionally inactivated in HCC through discrete mechanisms; however, the role of RB in suppressing tumorigenesis in this disease is poorly understood. Therefore, we analysed how RB status affects the response to AFB1 in reference to acute exposures and tumor development reflective of chronic exposure. Liver-specific Rb deletion resulted in an aberrant proliferative response to AFB1. This cell-cycle induction was associated with increased levels of secondary genetic damage and failure in appropriate cell-cycle coupling. This effect of RB loss was unique to AFB1 and involved the induction of a non-canonical proliferative pathway, and was not merely reflective of the overall cell-cycle deregulation or aberrant regenerative responses. The acute responses to AFB1 exposure presaged aberrations in hepatocyte nuclear morphology and ploidy with RB loss. Correspondingly, RB-deficient livers showed significantly enhanced susceptibility to liver tumorigenesis initiated by AFB1. Combined, these studies show that RB has a critical role in mediating checkpoint responses in liver tissue to maintain genome integrity and in suppressing tumorigenesis.
Keywords: retinoblastoma tumor suppressor, aflatoxin B1, carbon tetrachloride, DNA damage, cell cycle, hepatocellular carcinoma
Introduction
Liver cancer is a major worldwide health concern. In excess of 500 000 cases are diagnosed throughout the world annually, and nearly 680 000 deaths will be attributed to liver cancer in 2007 (Jemal et al., 2007). Unfortunately, there is a significant mortality rate associated with the disease, with a 5-year survival rate of only 11% in developed countries (Farazi and DePinho, 2006; Jemal et al., 2007; McGivern and Lemon, 2008) Furthermore, there are few successful interventions for advanced disease and although new therapeutics are being deployed in the clinic, their overall efficacy has been limited. Most cases of liver cancer can be traced to specific etiological events (Yu and Yuan, 2004; Farazi and DePinho, 2006). For example, the majority of liver cancer cases in Eastern Asia are associated with hepatitis B infection, whereas in Western Europe hepatitis C infection represents a likely primary causal event (Thorgeirsson and Grisham, 2002; Yu and Yuan, 2004; Farazi and DePinho, 2006; McGivern and Lemon, 2008) In addition to viral infection, it is well appreciated that environmental toxins strongly predispose to the development of liver cancer. In particular, the aflatoxins (for example, aflatoxin B1 (AFB1)), which are present in moldy grains, are key etiological factors for hepatocellular carcinoma (HCC) in multiple geographical locales (Yu and Yuan, 2004; Farazi and DePinho, 2006; McGivern and Lemon, 2008). In spite of the potency of AFB1, chronic exposure is a key facet of progression to HCC, suggesting that genetic events common to HCC may modulate the response and ultimately enhance to tumorigenesis when induced by such agents (Bioulac-Sage et al., 2003; Farazi and DePinho, 2006; Laurent-Puig and Zucman-Rossi, 2006; McGivern and Lemon, 2008).
Loss of the retinoblastoma (RB) tumor suppressor pathway represents a relatively common event in liver cancer (Bioulac-Sage et al., 2003; Farazi and DePinho, 2006; Laurent-Puig and Zucman-Rossi, 2006). Specifically, the region of the Rb locus (13q14) is subject to loss of heterozygosity at relatively high frequency in liver cancer, and histological loss of RB protein expression occurs in a subset of tumors (Zhang et al., 1994; Azechi et al., 2001; Laurent-Puig and Zucman-Rossi, 2006; Skawran et al., 2008) RB can be functionally inactivated through a variety of mechanisms, including deregulated phosphorylation and direct sequestration by oncoproteins. In liver cancer, cyclin D1 deregulation or loss of the p16ink4a tumor suppressor is hypothesized to contribute to RB inactivation (Zhang et al., 1993; Azechi et al., 2001; Edamoto et al., 2003) In addition, the oncoprotein gankyrin and specific hepatitis virus-encoded proteins (for example, NS5B, NS5A) have been shown to promote the functional inactivation of RB (Edamoto et al., 2003; Munakata et al., 2005, 2007; McGivern and Lemon, 2008). Thus, through these combined mechanisms it has been postulated that the loss of RB function is a common event in liver cancer and is associated with tumor development in addition to poor prognosis of the disease.
RB functions as a cell-cycle regulatory factor to modulate proliferation in response to mitogenic and anti-mitogenic signals (Cobrinik, 2005; Knudsen and Knudsen, 2006; Burkhart and Sage, 2008). In quiescent cells, RB is hypophosphorylated and assembles protein complexes that repress the activity of genes regulated by the E2F family of transcription factors. The target genes of E2F encompass a wide range of factors critical for progression through S phase (for example, cyclin A, MCM7 and PCNA) and mitotic progression (for example, cyclin B1, cdk1, Plk1 and Cdc20) (Markey et al., 2007). Thus, loss of RB function is commonly associated with a deregulation of such targets. Typically, RB loss is viewed as promoting unchecked proliferation in tissue and contributing specifically to the initiation of tumor development. However, studies in multiple models have recently suggested that the effect of RB loss is more subtle and is a particularly significant modifier of the response to environmental or oncogenic stresses (Cobrinik, 2005; DeGregori and Johnson, 2006; Wikenheiser-Brokamp, 2006; Burkhart and Sage, 2008).
In this study we analysed how RB status modulates the response to AFB1 in exposed animals. These studies show a striking requirement for RB in mediating appropriate cell-cycle control after AFB1 exposure, limiting secondary DNA damage and suppressing tumorigenesis. Together, these findings implicate RB loss as a cooperative event in hepatocarcinogenesis driven by AFB1 exposure.
Results
Retinoblastoma (RB) loss deregulates the response to genotoxic carcinogens
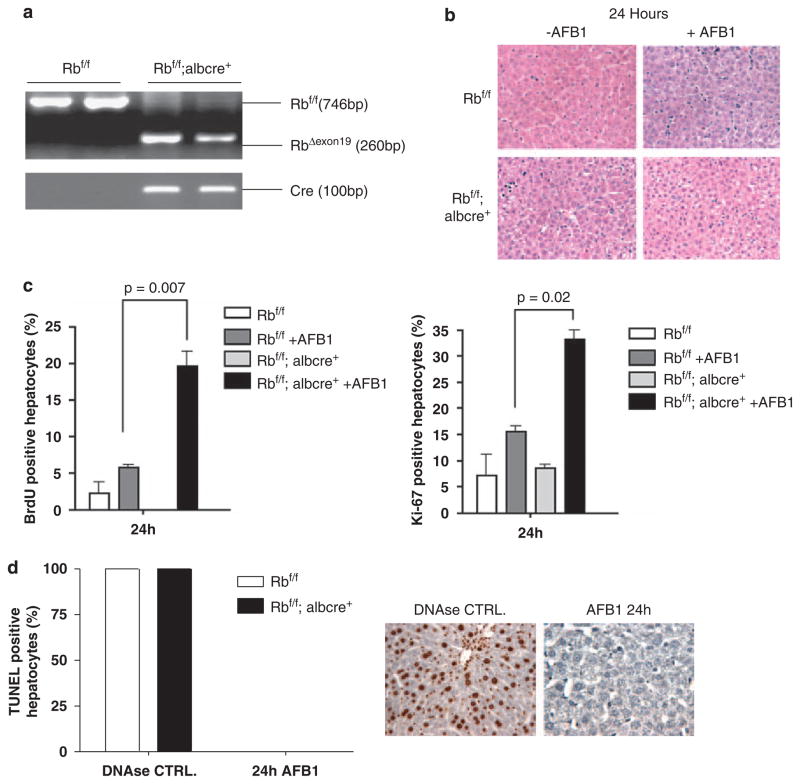
It is believed that AFB1 functions in the liver predominantly as a genotoxic carcinogen, and RB has been shown to have a significant role in the checkpoint response to DNA-damaging agents in cell culture models (Knudsen et al., 2000, 2006; Knudsen and Knudsen, 2008). Therefore, initially the acute effects of AFB1 exposure were explored in the context of animals harboring either RB-proficient or RB-deficient livers. As shown in Figure 1a, the RB allele was efficiently recombined in the male neonatal mice used in these studies (Figure 1a). AFB1 was administered through a single intraperitoneal injection and the mice were killed at 24 h after injection. Immediately after killing, the liver weight was determined and expressed as a percentage of the total body weight. AFB1 exposure had little overall influence on liver weight in RB-proficient and RB-deficient mice (data not shown). Furthermore, histological analyses at 24 h after treatment revealed little qualitative difference between conditions (Figure 1b). As there is basal proliferation occurring in neonatal liver tissue, the potential effect of AFB1 or RB on proliferation in liver tissue was analysed by 5-bromo-2-deoxyuridine (BrdU) incorporation. Interestingly, in neonatal animals RB deficiency had no discernible effect on proliferation in the vehicle-treated animals. Thus, at this age of development RB status has little effect on basal proliferation. Interestingly, AFB1 exposure did not suppress the basal BrdU incorporation occurring in RB-proficient livers. Thus, classical checkpoint responses are not invoked by the dose of AFB1 used in these studies. However, RB-deficient livers showed a striking fourfold increase in BrdU incorporation after AFB1 exposure (Figure 1c). These findings indicate that RB loss has a profound effect on the acute response to the genotoxic hepatocarcinogen AFB1. As these effects on BrdU incorporation could reflect repair synthesis associated with DNA damage, Ki67 staining was also performed. These data showed that consistent with the BrdU incorporation, an aberrant proliferative response was occurring specifically in the context of RB-deficient livers (Figure 1c). Furthermore, TdT-mediated dUTP nick end labeling assay was performed to determine the levels of apoptosis; however, there was little evidence that suggested cell death in any condition (Figure 1d). Altogether, these data show that RB loss has a key role in suppressing an aberrant proliferative response mediated by AFB1. Thus, similar to in vitro studies these findings suggest that RB has a key role in mediating DNA damage checkpoints to restrain cell-cycle progression in vivo.
Figure 1.
Retinoblastoma (RB) loss deregulates the response to genotoxic carcinogens. (a) Cre-mediated recombination of the floxed Rb locus (RbexonΔ19) was detected by PCR analysis of genomic DNA using primers surrounding the loxP sites flanking Rb exon 19. (b) Formalin-fixed, paraffin-embedded (FFPE) sections from the left lobe of 24-h post-treated and non-treated livers. Representative images were obtained to show histological features between conditions. (c) 5-bromo-2-deoxyuridine (BrdU) was detected and the percentage of BrdU-positive hepatocytes in each condition was calculated. Ki-67 was detected and the percentage of Ki-67-positive hepatocytes in each condition was calculated. (d) TdT-mediated dUTP nick end labeling (TUNEL) assay, deoxyribonuclease (DNAse)-treated livers were used as a positive control.
Regeneration in the neonate liver is independent of retinoblastoma (RB) status
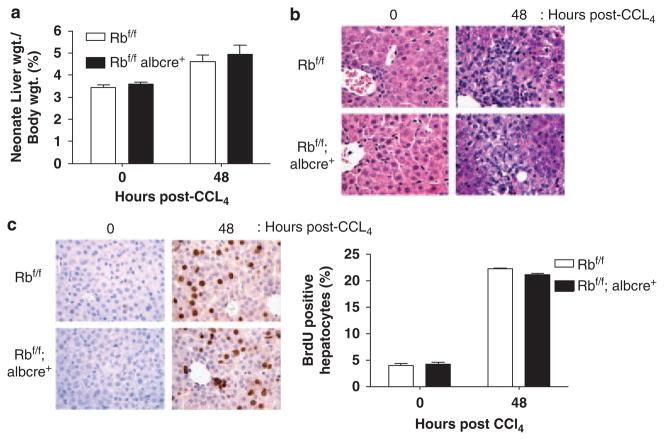
To analyse whether the effects observed with AFB1 treatment were a manifestation of overall aberrant cell-cycle control, we explored the effect of RB loss on liver regeneration. Specifically, day 7 Rbf/f and Rbf/f albcre+ littermates (same age as used for the AFB1 analyses) were injected with a 10% solution of carbon tetrachloride ligand 4 and corn oil to induce liver damage, thus, yielding a compensatory regenerative response (Lazareva, 1981). At 48 h after injection, the mice were excised and the livers were extracted. Immediately after killing, the livers were weighed and expressed as a percentage of body weight, as shown in Figure 2a. There was a transient increase in weight at 48 h after injection regardless of RB status when compared with control livers. Furthermore, liver histology at 48 h after injection showed that approximately 50% of the tissue represented large necrotic areas especially in proximity to the portal veins (Figure 2b). To quantify regenerative response, BrdU incorporation was examined at 48 h after injection. A fourfold induction in BrdU levels was observed when compared with controls (Figure 2c). However, it was apparent that RB status had no effect on cell-cycle entry in this model. Similar results were observed in adult animals, in which RB loss had no effect on either cell-cycle entry or exit (data not shown). Therefore, the aberrant RB-dependent response to AFB1 is not reflective of a general breakdown in cell-cycle control mechanisms, which are engaged during liver regenerative processes.
Figure 2.
Regeneration in the neonatal liver is independent of retinoblastoma (RB) status. (a) Immediately after killing, the livers were weighed and expressed as a percentage of the total body weight. (b) Formalin-fixed, paraffin-embedded (FFPE) sections from the left lobe of 0 and 48 h post-treated livers. Representative images were obtained to show histological features between conditions. (c) 5-bromo-2-deoxyuridine (BrdU) was detected and the percentage of BrdU-positive hepatocytes in each condition was calculated.
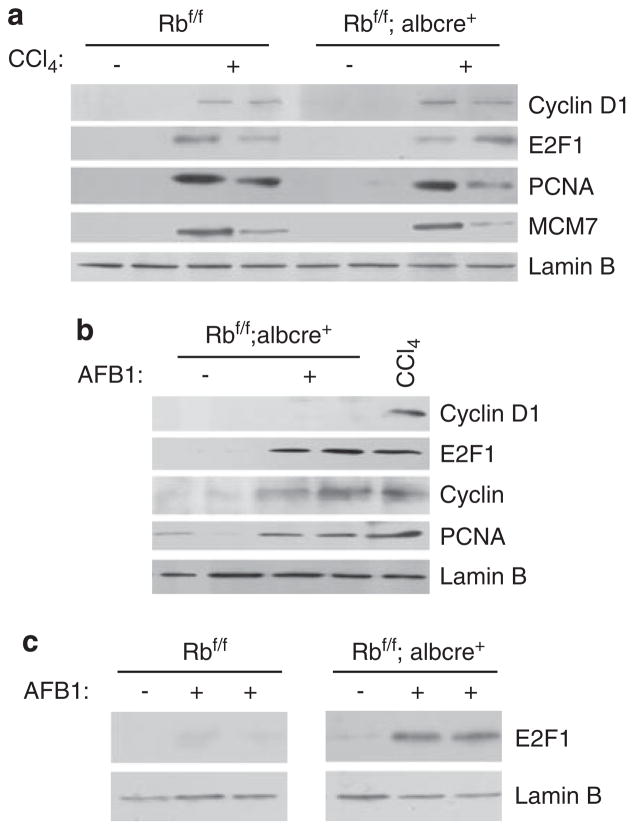
On the basis of the findings described above, we analysed the underlying mechanisms of regeneration and AFB1-mediated proliferation. In regenerating livers, the induction of cyclin D1 is associated with the proliferation event along with the stimulation of E2F1 expression and the expression of RB/E2F targets, such as PCNA and MCM7 (Figure 3a). In contrast with regenerative signaling, the levels of cyclin D1 were undetectable in RB-deficient AFB1-treated livers in spite of the profound cell-cycle entry occurring in this model. Rather, the induction of E2F1 was observed in RB-deficient livers treated with AFB1 when compared with AFB1-treated RB-proficient livers. As this event was specific to Rb-deficient livers, we analysed other RB/E2F targets. In coincidence with E2F1 levels, we see a subsequent induction of downstream target genes, cyclin A and PCNA (Figure 3b). Multiple laboratories have shown that E2F1 can be induced by DNA damage (Stevens and La Thangue, 2003) and our findings indicate that RB specifically keeps the levels of E2F1 in check to inhibit aberrant proliferation. Thus, the cell-cycle response to AFB1 treatment is clearly distinct mechanistically from canonical cyclin D1-mediated proliferation that is engaged during regeneration.
Figure 3.
Distinct mechanisms define the critical role for retinoblastoma (RB) in hepatic cell-cycle control among unique stresses. (a) Equal total protein was separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE), and the levels of cyclin D1 and RB/EF2 target genes were analysed. Lamin B was used as a control. (b) Equal total protein was separated using SDS–PAGE, and the levels of E2F1 were analysed. Lamin B was used as a control. (c) Equal amount of protein was separated using SDS–PAGE, and the levels of E2F1 were analyzed in both RB-proficient and RB-deficient samples. Lamin B was used as a control.
Increased double-stranded breaks (DSBs) and failure in mitotic progression in retinoblastoma (RB)-deficient livers exposed to aflatoxin B1 (AFB1)
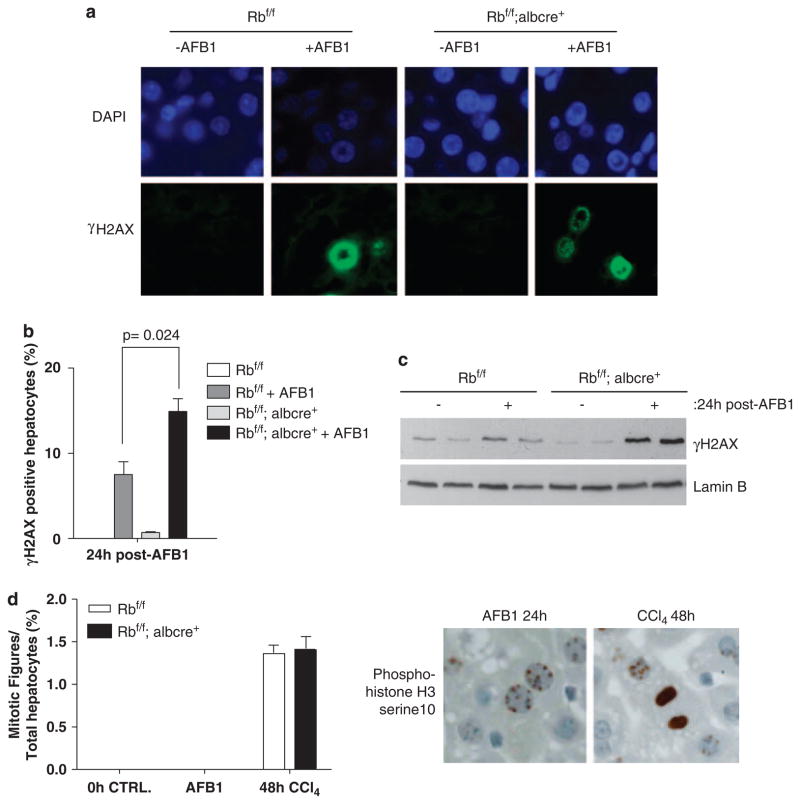
In the liver, AFB1 is metabolized, which leads to oxidative damage and adduct formation. It is well appreciated that DNA replication through such DNA damage lesions can lead to secondary DNA damages, most notably double-stranded breaks (DSBs). To determine whether RB loss led to additional secondary genomic damage, the abundance of the phospho-histone variant H2AX (γ-H2AX) was determined using immuno fluorescence (IF) staining (Figure 4a). RB-proficient AFB1 livers did show a modest increase in levels of γ-H2AX; however, the percentage of cells positive for γ-H2AX was dramatically enhanced in RB-deficient AFB1-treated mice (Figure 4b). In accordance with these findings, we observed increased protein levels of γ-H2AX in Rb-deficient treated tissue (Figure 4c). Thus, loss of RB leads to a significant increase in the magnitude of secondary DNA damage after AFB1 treatment.
Figure 4.
Increased double stranded breaks (DSBs) and failure in mitotic progression in retinoblastoma (RB)-deficient livers exposed to aflatoxin B1 (AFB1). (a) Representative images of γ-H2AX were taken. (b) Plots depicting total positive hepatocytes for γ-H2AX. (c) Equal total protein was separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE), and the levels of γ-H2AX were detected. (d) Plot depicting mitotic figures present in AFB1-treated conditions, and carbon tetrachloride ligand 4 (CCl4) samples used as positive control. The images represent phospho-histone H3 serine10 stain.
Typically, such DNA damage has a negative effect on cell-cycle transitions, particularly the G2/M transition. To determine whether the hepatocytes were capable of progressing into mitosis, phospho-histone H3 serine10 reactivity was examined. At this stage there was a striking lack of phospho-histone H3 mitotic figures in livers exposed to AFB1, regardless of RB status (Figure 4d). These data suggest that the induction of proliferative signals, as achieved with RB loss, elicits additional secondary DNA damage and leads to an uncoupling between cell-cycle entry and mitotic division.
Retinoblastoma (RB)-deficient mice livers show an increased susceptibility to hepatocellular carcinoma (HCC) induced by aflatoxin B1 (AFB1)
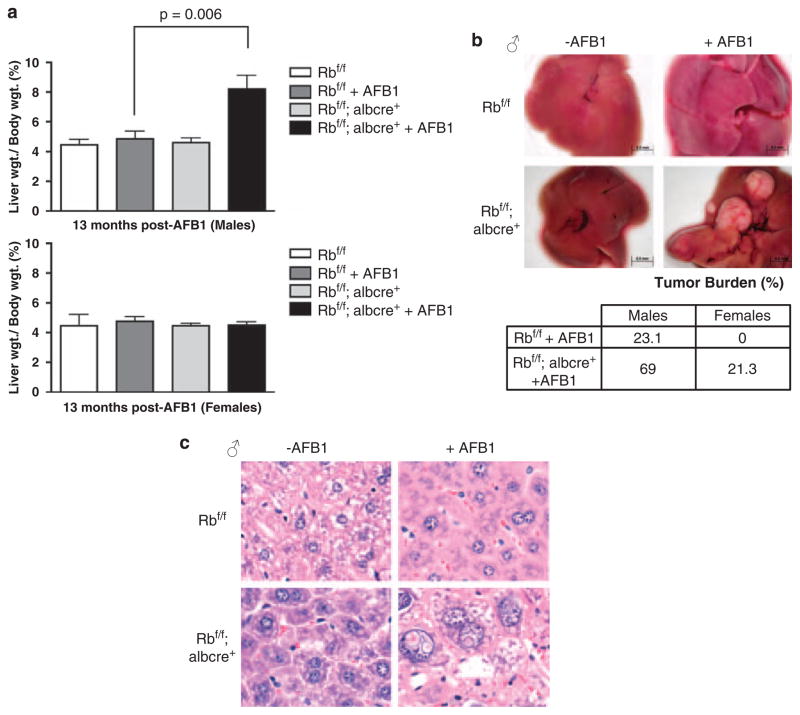
Owing to the profound association of AFB1 with human liver tumorigenesis, (Fujimoto et al., 1994; Skawran et al., 2008) and the striking influence of RB loss on the acute response to AFB1, the effect of RB status on AFB1-mediated tumor development was determined. For these analyses, Rbf/f and Rbf/f albcre+ mice were aged to 13 months after AFB1 exposure. Following this protocol, RB-deficient male mice exposed to AFB1 showed a significant increase in liver weight, as compared with RB-proficient males (Figure 5a). This difference in liver weight was a reflection of the increased tumor incidence and burden in these mice (Figure 5b). Histology of male liver tissue indicated that Rbf/f albcre+ livers exposed to AFB1 expressed a heterogeneous spectrum of abnormalities, including centrilobular pleomorphism, large adenomas and HCC (Figure 5c). Female Rbf/f and Rbf/f albcre+ mice showed little difference in liver weight and showed minimal tissue damage, consistent with the relative resistance of female organisms to liver tumorigenesis. Specifically, although male mice with RB-deficient livers showed a significant increase in tumor burden, female Rbf/f albcre+ did show an increase in tumor susceptibility, but these data were not statistically significant because of the low penetrance in the cohort.
Figure 5.
Retinoblastoma (RB)-deficient mice livers show an increased susceptibility to hepatocellular carcinoma (HCC) when induced by aflatoxin B1 (AFB1). (a) After killing, the livers were excised, weighed and expressed as a percentage of total body weight for both males and females (Rbf/f n = 5, Rbf/f+AFB1 n = 10, Rbf/f albcre+/− n = 8, Rbf/f albcre+/−+AFB1 n = 13 MALES; n = 3 or 5 in each condition for FEMALES). (b) Representative images of excised livers and a table expressing percentages of tumor burden in both female and male livers. (c) Male formalin-fixed, paraffin-embedded (FFPE) liver tissue sections were stained with hematoxylin and eosin (H and E) and representative images were taken to show histology.
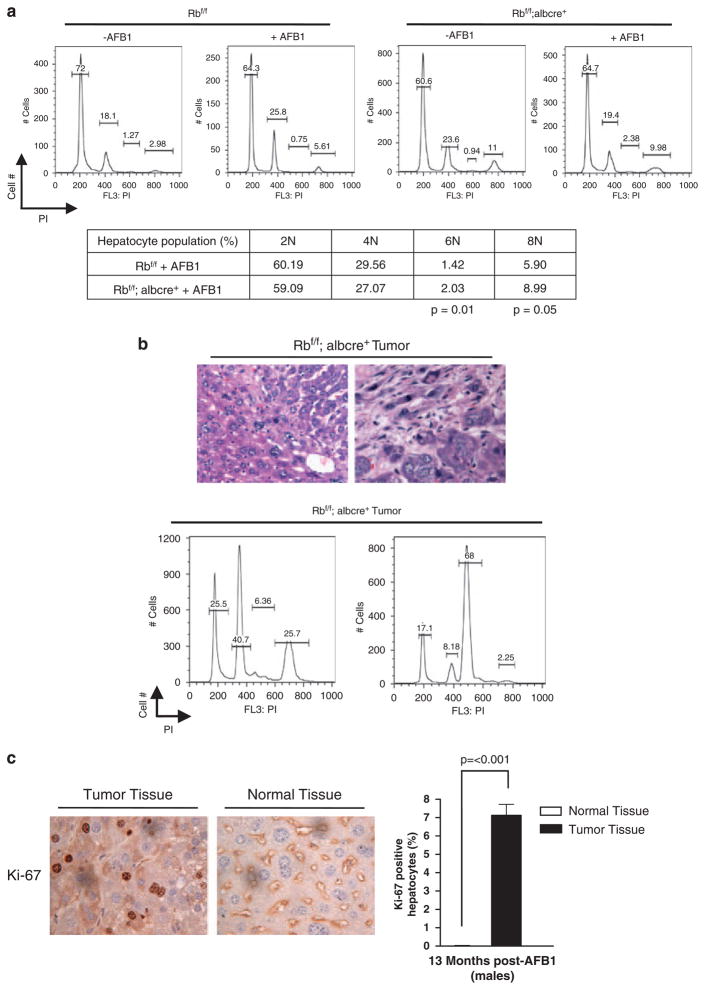
The remnant, non-neoplastic tissue in the livers was analysed to decipher the likely basis for the predisposition to tumorigenesis with AFB1. Although the liver tissue harbored a highly abnormal histology, the tissue was non-proliferative, as determined using Ki-67 staining (Figure 6c). Thus, loss of RB does not lead to a general deregulation of proliferation in the liver of aged mice after AFB1 exposure. However, the liver tissue showed a significant change in ploidy with the loss of RB and treatment with AFB1, as increases in 6 and 8N populations were observed between Rbf/f and Rbf/f albcre+ tissue (Figure 6a). The tumors arising in the Rbf/f albcre+ mice were highly heterogeneous and showed a variety of distinct predominant ploidies, including basal 2, 4 and 6N states (Figure 6b). Such tumors were highly proliferative, as indicated by Ki-67 and the presence of mitotic figures (Figure 6c). Altogether, these data suggest that RB loss has a particularly important role in the response to genotoxic carcinogens, and the basis of this response is likely associated with the aberrant acute response to such agents and genomic damage leading to a breakdown in genome integrity.
Figure 6.
Retinoblastoma (RB)-deficient mice livers and tumors show genome instability. (a) Representative Flow cytometry traces for each condition. The table represents mean of each hepatocyte population for 2, 4, 6 and 8N, expressed as a percentage. (b) Images of tumor sections stained with hematoxylin and eosin (H and E) to show heterogeneity of tissue. Furthermore, representative Flow cytometry traces of Rb-deficient aflatoxin B1 (AFB1)-treated tumors, and representative images of mitotic figures in AFB1-treated Rb-deficient tumors. (c) Images of non-neoplastic tissue and tumor tissue stained against Ki-67. The plot depicts tumor and normal tissue levels of Ki-67.
Discussion
Retinoblastoma deficiency is a common event in liver tumorigenesis. In this study we explored the interaction between the loss of RB and exposure to AFB1, a highly relevant genotoxic carcinogen implicated in human liver tumorigenesis. Interestingly, loss of RB has been observed in human liver tumors that have an etiological history of AFB1 exposure (Fujimoto et al., 1994). This finding suggests that loss of RB occurring in the context of chronic AFB1 exposure could contribute to aberrant acute responses to AFB1, ultimately leading to tumorigenesis.
In cell culture models, DNA damage elicits cell-cycle checkpoint responses. Such checkpoints are typically associated with an inhibition of cell-cycle progression in wild-type proliferative cells (Knudsen et al., 2000, 2006). Interestingly, in the context of neonatal livers, AFB1 exposure does not show this inhibition. Such findings have been observed with other genotoxic carcinogens in liver tissue (Yang et al., 2003). Thus, classical checkpoint responses are not observed with such genotoxic carcinogens, and perhaps this observation represents a contributory aspect of tumorigenesis induced by such agents. Strikingly, an exposure to AFB1 shows distinct effects in RB-proficient versus RB-deficient liver tissue. Specifically, AFB1 treatment leads to a profound induction of cell-cycle entry in RB-deficient livers. Moreover, this phenomenon is associated not only with BrdU incorporation, but also with other facets of proliferation, including Ki67 induction. Interestingly, this response is transient and does not contribute to increases in liver weight or any aspects of hyperplasia. Particularly striking is the absence of histologically mitotic cells. Thus, although the in vivo response to AFB1 does not conform to classical checkpoint responses, loss of RB cooperates with AFB1 to yield an aberrant proliferative response with a significant uncoupling between cell-cycle entry and mitosis. It has been suggested that in the presence of DNA damage, E2F1 is induced and stabilized (Stevens and La Thangue, 2003). In the context of neonatal livers treated with AFB1, RB status has a key role in regulating E2F1 levels, the levels of E2F targets (for example, PCNA and Cyclin A) and ultimately progression of the cell cycle. However, this event was unique to AFB1 exposure and, more importantly, to RB deficiency. In regenerating neonatal livers the proliferative response is controlled by cyclin D1, which leads to the stimulation of E2F activity irrespective of endogenous RB.
Classically, it is viewed that DNA damage checkpoints function, at least in part, to limit deleterious forms of secondary damage and mutations that could arise by attempting to replication and divide damaged genetic material (Yu and Yuan, 2004; Farazi and DePinho, 2006; Laurent-Puig and Zucman-Rossi, 2006). AFB1 induces oxidative damage, which yields relatively few DSBs as a primary mechanism. However, replication through such damage can lead to DSBs. In this context, RB-deficient liver incurred significant increases in such secondary DNA damage as assessed by the used γ-H2AX reactivity as a marker of DSBs. Thus, these findings indicate that the loss of RB does compromises an S-phase checkpoint function and increases the relative burden/type of DNA damage incurred by AFB1. However, in spite of Rb-deficient cells progressing into S phase, there was an overall lack of mitotic progression. Thus, the induction of proliferative signals from RB loss and the increase in secondary damages ultimately led to a disconnect between cell-cycle entry and mitotic division.
It is widely believed that checkpoint responses are critical for the suppression of tumorigenesis (Donehower, 1997). As shown here, we found that RB loss rendered the liver significantly more susceptible to AFB1-mediated tumorigenesis. Liver cancer susceptibility bifurcates by sex, and male mice with RB-deficient livers were most sensitive to tumorigenesis, although RB-deficient female mice were as susceptible to tumor development as RB-proficient male mice. Interestingly, although focal disease was apparent in all models, the remnant liver in the absence of RB showed extensive hallmarks of neoplastic disease (for example, nuclear pleomorphism). However, such tissue was clearly distinguishable from tumor tissue based on the absence of proliferation. Thus, potent RB-independent mechanisms must remain in place to limit deregulated proliferation from occurring throughout the liver. As such, it would be expected that tumor-specific mutations or epigenetic alterations must contribute to the development of aberrant proliferation associated with HCC. Consistent with RB having a role in maintaining genomic integrity, we observed that loss of RB contributed to aberrant ploidy of hepatocytes (>4 N) in the remnant liver, and those tumors arising in the absence of RB harbored heterogeneous DNA contents. Putatively, such alterations in genome structure could be reflective of the initial checkpoint dysfunction observed with acute responses to AFB1 in RB-deficient livers.
When combined, these findings indicate that RB loss influences the acute response to AFB1 and contributes to liver tumorigenesis induced by long-term insult. These findings support further investigation in the role of RB pathway in the context of AFB1-induced HCC in human populations.
Materials and methods
Animals
The generation and genotyping of the Rbf/f and Rbf/f albcre+ mice have been previously discussed (Mayhew et al., 2005). All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Thomas Jefferson University.
Animal treatments
For acute AFB1 studies, 7-day-old Rbf/f and Rbf/f albcre+ littermates were given a single intraperitoneal injection of the genotoxic carcinogen AFB1 (Sigma-Aldrich, St Louis, MO, USA) suspended in Trycaprylin (Sigma-Aldrich) at 10 mg/kg body weight. Mice were then killed at 24 h and 13 months after AFB1 exposure. For carbon tetrachloride ligand 4 studies, 7-day-old Rbf/f and Rbf/f albcre+ littermates were given a single intraperitoneal injection of a solution consisting of 10% carbon tetrachloride ligand 4 mixed with corn oil. Mice were then killed at 0 and 48 h after injection. All mice were given a single intraperitoneal injection of 150 mg/kg Brdu (Sigma-Aldrich) dissolved in 0.9% saline, 1 h before killing to label S-phase hepatocytes. Directly after killing, the livers were excised, weighed and in some cases, photographed to show gross histology and tumor burden. The left lobe was fixed in 10% neutral buffered formalin for 24 h, embedded in paraffin and sectioned for histological assessment. Moreover, a small section of the tissue was saved for genotyping. The remaining liver tissue was flash frozen in liquid nitrogen and stored at −80 °C until further use. In some experiments tumors were excised, flash frozen and stored at −80 °C until further use.
Preparation of liver nuclei, protein analysis and ploidy determination
Liver nuclei, protein extraction, immunoblotting procedures and flow cytometric determination of ploidy levels were preformed exactly as previously described (Mayhew et al., 2005, 2007). Immunoblotting was preformed using commercially available antibodies, PCNA (P-10, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and MCM7 (141.2, Santa Cruz Biotechnology, Inc.). Polyclonal antibodies included E2F1 (C-20, Santa Cruz Biotechnology, Inc.), cyclin D1 (Ab-3, Neomarkers, Billerica, MA, USA), cyclin A (C-19, Santa Cruz, Biotechnology, Inc.), phospho-histone H2AX (Ser139, Upstate Biotechnology, Billerica, MA, USA) and lamin B (M-20), purchased from Santa Cruz. In all cases, each condition was represented by two different samples.
Histological and Immunohistochemistry and Immunofluorescence (IF)
For histological evaluation, liver tissue embedded in paraffin was sectioned at 4 μm, slides were then deparaffinized and stained with hematoxylin and eosin. To show DNA synthesis, Brdu incorporation was measured in liver sections as previously described (Mayhew et al., 2005). Commercially available monoclonal and polyclonal antibodies against, Ki67 (rat monoclonal anti-ki67, 1:100 dilution; Dako North America, Inc., Real Carpinteria, CA, USA), γH2AX (mouse monoclonal anti-γH2AX, 1:200 dilution; Upstate Biotechnology), and phospho-histone H3 serine10 (rabbit polyclonal anti-phospho-histone H3 serine 10, 1:1000 dilution; Upstate Biotechnology) were used for immunohistochemistry. Ki-67 and phospho-histone H3 (S10) stained sections were deparaffinized through a series of xylene washes and rehydrated through ethanol/water gradients. Slides were then boiled in antigen unmasking solution (Dako North America, Inc.) using a microwave (5 min at 100% power followed by 20 min at 30% power). Slides were then quenched in 0.3% hydrogen peroxide for 10 min and washed with phosphate buffered saline. Sections were then blocked in 10% normal goat serum for 30 min. Primary antibodies respectively were diluted in blocking solution and left in incubation either overnight at 4 °C or for 1 h at 37 °C. Following a series of phosphate buffered saline washes, the slides were placed in Biotinylayted secondary antibody and streptavidin preoxidase conjugate for 30 min and developed in 3,3′diaminobenzidine solution (Vector Laboratories, Burlingame, CA, USA) for 1 to 2 min and then washed in double distilled water. Slides were counterstained with hematoxylin and dehydrated through a series of ethanol/water gradients and washed in xylene, and then mounted. γH2AX sections were stained in the same manner, except that primary antibodies were diluted in IF buffer and Alexa Floura 488 goat anti-mouse (Invitrogen Corporation, Carlsbad, CA, USA) was used as the secondary antibody, also diluted in IF buffer. 4,6-diamidino-2-phenylindole (Sigma-Aldrich) was used to stain nuclei diluted at 1:1000 in IF buffer for 5 min.
Statistical analysis and quantitation data
Slides were scored in a blind manner for at least 600 hepatocytes from several fields for each staining. For histological assessment, a veterinary pathologist scored slides in a blind manner (AW). Statistical significance between groups was calculated using a two-tailed unpaired t-test. Differences were classified as statistically significant at P < 0.05.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Azechi H, Nishida N, Fukuda Y, Nishimura T, Minata M, Katsuma H, et al. Disruption of the p16/cyclin D1/retinoblastoma protein pathway in the majority of human hepatocellular carcinomas. Oncology. 2001;60:346–354. doi: 10.1159/000058531. [DOI] [PubMed] [Google Scholar]
- Bioulac-Sage P, Laurent-Puig P, Balabaud C, Zucman-Rossi J. Genetic alterations in hepatocellular adenomas. Hepatology. 2003;37:480. doi: 10.1053/jhep.2003.50058. author reply 480–1. [DOI] [PubMed] [Google Scholar]
- Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer. 2008;8:671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–2809. doi: 10.1038/sj.onc.1208619. [DOI] [PubMed] [Google Scholar]
- DeGregori J, Johnson DG. Distinct and overlapping roles for E2F family members in transcription, proliferation and apoptosis. Curr Mol Med. 2006;6:739–748. doi: 10.2174/1566524010606070739. [DOI] [PubMed] [Google Scholar]
- Donehower LA. Genetic instability in animal tumorigenesis models. Cancer Surv. 1997;29:329–352. [PubMed] [Google Scholar]
- Edamoto Y, Hara A, Biernat W, Terracciano L, Cathomas G, Riehle HM, et al. Alterations of RB1, p53 and Wnt pathways in hepatocellular carcinomas associated with hepatitis C, hepatitis B and alcoholic liver cirrhosis. Int J Cancer. 2003;106:334–341. doi: 10.1002/ijc.11254. [DOI] [PubMed] [Google Scholar]
- Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- Fujimoto Y, Hampton LL, Wirth PJ, Wang NJ, Xie JP, Thorgeirsson SS. Alterations of tumor suppressor genes and allelic losses in human hepatocellular carcinomas in China. Cancer Res. 1994;54:281–285. [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Knudsen KE, Booth D, Naderi S, Sever-Chroneos Z, Fribourg AF, Hunton IC, et al. RB-dependent S-phase response to DNA damage. Mol Cell Biol. 2000;20:7751–7763. doi: 10.1128/mcb.20.20.7751-7763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen ES, Knudsen KE. Retinoblastoma tumor suppressor: where cancer meets the cell cycle. Exp Biol Med (Maywood) 2006;231:1271–1281. doi: 10.1177/153537020623100713. [DOI] [PubMed] [Google Scholar]
- Knudsen ES, Knudsen KE. Tailoring to RB: tumour suppressor status and therapeutic response. Nat Rev Cancer. 2008;8:714–724. doi: 10.1038/nrc2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen ES, Sexton CR, Mayhew CN. Role of the retinoblastoma tumor suppressor in the maintenance of genome integrity. Curr Mol Med. 2006;6:749–757. doi: 10.2174/1566524010606070749. [DOI] [PubMed] [Google Scholar]
- Laurent-Puig P, Zucman-Rossi J. Genetics of hepatocellular tumors. Oncogene. 2006;25:3778–3786. doi: 10.1038/sj.onc.1209547. [DOI] [PubMed] [Google Scholar]
- Lazareva MN. Alpha-fetoprotein production by the synchronized regenerating murine liver. Its independence on the phases of the mitotic cycle. Oncodev Biol Med. 1981;2:89–99. [PubMed] [Google Scholar]
- Markey MP, Bergseid J, Bosco EE, Stengel K, Xu H, Mayhew CN, et al. Loss of the retinoblastoma tumor suppressor: differential action on transcriptional programs related to cell cycle control and immune function. Oncogene. 2007;26:6307–6318. doi: 10.1038/sj.onc.1210450. [DOI] [PubMed] [Google Scholar]
- Mayhew CN, Bosco EE, Fox SR, Okaya T, Tarapore P, Schwemberger SJ, et al. Liver-specific pRB loss results in ectopic cell cycle entry and aberrant ploidy. Cancer Res. 2005;65:4568–4577. doi: 10.1158/0008-5472.CAN-04-4221. [DOI] [PubMed] [Google Scholar]
- Mayhew CN, Carter SL, Fox SR, Sexton CR, Reed CA, Srinivasan SV, et al. RB loss abrogates cell cycle control and genome integrity to promote liver tumorigenesis. Gastroenterology. 2007;133:976–984. doi: 10.1053/j.gastro.2007.06.025. [DOI] [PubMed] [Google Scholar]
- McGivern DR, Lemon SM. Tumor suppressors, chromosomal instability, and hepatitis C virus-associated liver cancer. Annu Rev Pathol. 2008;4:399–415. doi: 10.1146/annurev.pathol.4.110807.092202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata T, Nakamura M, Liang Y, Li K, Lemon SM. Down-regulation of the retinoblastoma tumor suppressor by the hepatitis C virus NS5B RNA-dependent RNA polymerase. Proc Natl Acad Sci USA. 2005;102:18159–18164. doi: 10.1073/pnas.0505605102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata T, Liang Y, Kim S, McGivern DR, Huibregtse J, Nomoto A, et al. Hepatitis C virus induces E6AP-dependent degradation of the retinoblastoma protein. PLoS Pathog. 2007;3:1335–1347. doi: 10.1371/journal.ppat.0030139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skawran B, Steinemann D, Becker T, Buurman R, Flik J, Wiese B, et al. Loss of 13q is associated with genes involved in cell cycle and proliferation in dedifferentiated hepatocellular carcinoma. Mod Pathol. 2008;21:1479–1489. doi: 10.1038/modpathol.2008.147. [DOI] [PubMed] [Google Scholar]
- Stevens C, La Thangue NB. A new role for E2F-1 in checkpoint control. Cell Cycle. 2003;5:435–437. [PubMed] [Google Scholar]
- Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- Wikenheiser-Brokamp KA. Retinoblastoma family proteins: insights gained through genetic manipulation of mice. Cell Mol Life Sci. 2006;63:767–780. doi: 10.1007/s00018-005-5487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Tang J, Rogler CE, Stanley P. Reduced hepatocyte proliferation is the basis of retarded liver tumor progression and liver regeneration in the lacking N-acetylglucosaminyltransferase III. Cancer Res. 2003;63:7753–7759. [PubMed] [Google Scholar]
- Yu MC, Yuan JM. Environmental factors and risk for hepatocellular carcinoma. Gastroenterology. 2004;127:S72–S78. doi: 10.1016/j.gastro.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Jiang W, Chen CJ, Lee CS, Kahn SM, Santella RM, et al. Amplification and overexpression of cyclin D1 in human hepatocellular carcinoma. Biochem Biophys Res Commun. 1993;196:1010–1016. doi: 10.1006/bbrc.1993.2350. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xu HJ, Murakami Y, Sachse R, Yashima K, Hirohashi S, et al. Deletions of chromosome 13q, mutations in retinoblastoma 1, and retinoblastoma protein state in human hepatocellular carcinoma. Cancer Res. 1994;54:4177–4182. [PubMed] [Google Scholar]