Introduction
Chronic low grade infection with Gram negative organisms, inferred from traces in plasma of endotoxin, or lipopolysaccharide (LPS), has been linked to ischemic vascular disease [1–3]. Low level bacteremia in nominally healthy individuals may be a normal state involving commensal organisms and can arise from activities as routine as tooth brushing [4]. Innate immune responses to Gram negative bacteria involve interaction of bacterial LPS with toll-like receptor 4 (TLR4), which occurs on the surface of most cells, including platelets [5]. TLR4 signaling in nucleated cells proceeds through the NF-κB pathway to stimulate diverse reactions, including production of cytokines such as TNFα [6]. While mechanisms are not clear, platelets contain an NF-κB family of signaling complexes [7], and appear to participate in innate immune responses [5, 8]. In these and other studies of platelet economy in inflammation, LPS has been administered in doses that emulate processes associated with sepsis. Much lower levels of LPS associated with low level bacteremia have received little attention, in spite of some evidence that it may be a factor in vascular disease.
Individuals with TLR4 deficiencies may be at increased risk for infection but at lower risk for cardiovascular disease [9, 10]. The evidence is epidemiological, but raises the hypothesis that chronic bacteremia may be pathogenic for atherosclerosis.
Platelets participate in atherogenesis [11] and show clear signs of increased activity in individuals with established cardiovascular and thrombotic disease [12, 13]. Platelets may also show changes in asymptomatic individuals at elevated risk, as platelet turnover is increased in asymptomatic relatives of individuals with documented vascular disease [14]. However, causal relationships have not been established between infection-associated inflammation and platelet reactivity and turnover. Experiments were designed to establish this relationship. An effect of low level infection- associated inflammation on platelets would provide a basis to re-examine the links between infection, innate immunity and either atherogenesis or thrombosis.
Materials and Methods
Materials
Ultra-pure E. coli lipopolysaccharide (LPS, 0111:B4 strain-TLR4 ligand, product number tlrl-pelps) was obtained from InvivoGen, San Diego, CA and prepared as suggested by the supplier. EZ-Link Sulfo-NHS-Biotin was purchased from Pierce Biotechnology, Rockford, IL. Phycoerythrin (PE) or fluorescein isothiocyanate (FITC)-conjugated hamster anti-mouse CD61, rat anti-mouse CD62P-FITC monoclonal antibodies, recombinant annexin V-FITC and streptavidin-PE (SA-PE) conjugates were obtained from BD PharMingen International, San Diego, CA. Chicken anti-human fibrinogen FITC polyclonal antibody was purchased from Accurate Chemical and Scientific Corporation, Westbury, NY. Paraformaldehyde (16% solution, EM grade) purchased from Electron Microscopy Sciences, Hatfield, PA. All other reagents and solvents used in this study were of analytical/reagent grade.
Animals
Four to six month old, female C57BL10SnJ mice (wild-type, WT) and female C57BL10ScN mice homozygous for deletion of TLR4 (dTLR4) were obtained from The Jackson Laboratory, Bar Harbor, Maine. These dTLR4 mice do not bear the IL-12Rβ2 mutation originally described in this strain [15]. Experiments were approved by the Institutional Animal Care and Use Committee, Mayo Clinic College of Medicine, Rochester, MN.
Experimental Design and Blood Collection
Blood (25–50 μL) was collected through the retro-orbital sinus plexus as previously described [16]. Mice were injected with 100 μL of either LPS (0.04–200ng/g body weight) or sterile saline through the tail vein. For in vivo labeling of platelets, sulfo-NHS-biotin (35 mg/kg body weight) was injected through the tail vein 3–4 hrs after LPS injection. In all animals, blood (25–50 μL/mouse) was collected from the retro-orbital sinus at 24 hr intervals for 5–6 days after the injection of saline or LPS.
Measurement of tumor necrosis factor-alpha (TNFα)
Blood (40–50 μL) was collected from the tail vein prior to and at 0.5, 1, 2 and 3 hrs following the LPS injection. Serum was collected and immediately frozen at −20°C for subsequent determination of serum TNFα by enzyme-linked immunosorbent assay (ELISA, R&D Systems, Minneapolis, MN) [16].
Platelet Measurements
Platelets were counted in whole blood diluted in physiological saline (1:10 dilution) as previously described [16, 17] To measure biotinylated platelets, blood was diluted (1:100) directly in Hanks’ balanced salts solution without NaH2CO3, but buffered (pH 7.4) with 20 mM HEPES, and supplemented with 1 mg/mL albumin, and 1 μM tick anticoagulant peptide to block prothrombin activation. Each diluted sample (100 μL) was incubated (30 min, 22°C) with 2 μL each of fluorescein-conjugated hamster anti-mouse CD61 monoclonal antibody and R-phycoerythrin conjugated streptavidin. Platelets were then fixed with 5 volumes of 1% paraformaldehyde for 10–15 min and analyzed by flow cytometry (FACSCalibur™ or FACSCanto™, Becton Dickinson) within 2 hrs. Platelets were identified by forward and side scatter and with CD61 (glycoprotein IIIa)-fluorescein antibody and 10,000 events were measured for each sample. Sulfo-NHS-biotin labels 85–95% of platelets within 24 hrs; the threshold for streptavidin-phycoerythrin positive platelets was set so that ≤5% positive for saline-injected mice. The platelet population was gated using the platelet marker CD61-fluorescein. Cell Quest or Diva software (BD Biosciences) was used for data acquisition and analysis.
The percentage of reticulated platelets (those containing RNA) as an indirect measure of platelet production was determined as described previously [16, 17].
Reactive platelet assays
Expression of P-selectin, fibrinogen receptor (glycoprotein IIb/IIIa) and phosphatidylserine (annexin V binding) on the platelet surface membrane were measured with a rat anti-mouse P-selectin-fluorescein, chicken anti-human fibrinogen- fluorescein and annexin V- fluorescein, respectively [17, 18].
Statistical Analysis
All values are presented as means or mean ± SD. Statistical significance was evaluated by Student’s t test and linear regression analysis. Differences at a level of P<0.05 were considered to be significant. All experiments were carried out independently using a minimum of three and maximum of nine individual mice/dose group or test from WT and dTLR4 colonies.
Results
Platelet counts and activation
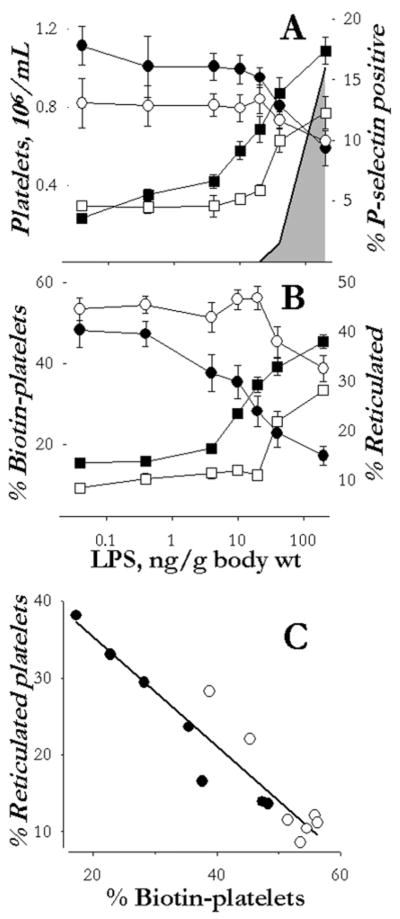
Platelet counts in dTLR4 mice were reproducibly 20% lower than in WT mice (P<0.001). Platelet counts did not change in either WT or dTLR4 between 15 min and 24 hrs (Figure 1A, circles) or up to five days (data not shown) after a single injection of LPS at doses < 40 ng/g body weight. However, at doses of LPS > 40ng/g body weight, platelet count decreased compared to saline injected animals 24h after the injection in both WT (P<0.001) and dTLR4 (P<0.0058) mice (Figure 1A, circles). The relative decrease in platelet counts with the highest doses of LPS was 25% in dTLR4 mice, and 50% in the WT. In both groups of animals, platelet counts returned to basal levels by day five (not shown).
Figure 1.
Changes in platelet count and characteristics following a single intravenous injection of saline or LPS in WT (filled symbols) and dTLR4 (open symbols) mice. Panel A: Platelet count (circles) and percentage of platelets positive for P-selectin (squares) 24 hrs after the injection. Panel B: Clearance of biotin-labeled platelets (circles) and appearance of reticulated plates (squares) three days after the injection. The shaded area in panel A shows the relative concentration of TNFα measured in the serum of WT mice one hr after the injection, which peaked at 2600 ng/ml and returned to below detection limit of assay by three hrs. TNFα was unchanged in the dTLR4 mice at all doses of LPS. Panel C: Relationship between the percentage of reticulated platelets and biotin-labeled platelets at three days after the injection from panel B, r2=0.89. Data are shown as mean ± SD in panels A and B of three to nine independent experiments. Data points in panel C are the mean values shown in panel B.
One day after LPS injection, the percentage of platelets staining positive for P-selectin increased significantly (P<0.001) at concentrations > 4 ng/g body weight in WT mice (Figure 1A, filled squares). In dTLR4 mice, significant increases (P<0.01) in the fraction of platelets expressing P-selectin were seen only with doses of LPS associated with decreases in platelet count, that is, doses >40 ng/g body weight. By three days post injection, the percentage of platelets expressing P-selectin had returned to 5% in both strains of mice, a percentage which was not different from that observed prior to the LPS injection. Expression of activated fibrinogen receptor (αIIBβ3*) and phosphatidylserine (annexin V binding) in platelets did not change following any dose of LPS in either WT or dTLR4 mice (not shown).
Serum makers of inflammation
Serum TNFα was not detected at any dose of LPS in dTLR4 mice. In WT mice, TNFα was not detected at LPS doses < 20 ng/g body weight (Figure 1, shaded area). However, at doses >20 ng/g body weight, TNFα increased and peaked within 1 hr of the injection reaching a maximum of 2600 ng/ml at peak with the 200 ng/g body weight dose of LPS (Figure 1). Whereas the rise in TNFα was congruent with the dose response for initial decrease in platelets, the rise in P-selectin positive platelets from WT mice was at least a log more sensitive than the rise in TNFα.
Platelet lifespan
The biotin label was detected on 85–90% of platelets in both strains of mice. In both strains of mice injected with saline, disappearance of these biotin-labeled platelets occurred within 5 days with a half-life of 3.2 days in WT and 3.6 days in dTLR4 mice (fit to single exponential). A single injection of LPS caused a significant (P<0.001) dose-dependent increase in biotin-platelet clearance in WT mice (Figure 1B, filled circles) with a threshold dose of 4 ng/g body weight. This dose was 1/10 that needed to cause thrombocytopenia, or a rise in TNF-α. The threshold for increase in platelet clearance was not congruent with TNFα or sustained thrombocytopenia in WT mice. In dTLR4 mice, the significant (P< 0.01) increase in platelet clearance was congruent with sustained thrombocytopenia at concentrations of LPS >40 ng/g body weight.
Platelet production
Injection of low doses of LPS, 4 and 20 ng/g body weight significantly increased (P<0.001) the percentage of reticulated platelets at day three in WT mice but not dTLR4 mice, which showed no response below doses needed to decrease platelet counts at day one (> 20 ng/g body weight; Figure 1B, squares). The percentage of reticulated platelet peaked at 3 days post-injection in both strains of mice but remained elevated to 20% of the platelets in both strains of mice injected with the highest doses of LPS at day 5. The increased appearance of reticulated platelets showed a negative, linear correlation (r2 = 0.89) with platelet clearance at day three (Figure 1C).
Discussion
Results of this study document changes in platelet activation and turnover that occur at doses of LPS below those causing either changes in platelet count or acute increases in the canonical response marker, TNFα. Prior studies employing substantially higher LPS dosage, but via intraperitoneal rather than intravenous administration, found a TLR4 dependence of thrombocytopenia and platelet sequestration in the lung [5, 8]. Higher concentrations of LPS can induce alternative and lectin-mediated activation of the complement cascade. Therefore, it is likely that acute intravenous exposure to the high concentrations of LPS (those >20 ng/g body weight) activated platelets in WT and dTLR4 by promoting complement activation in plasma or secondarily to activation of neutrophils by LPS and not to direct activation of TLR4 on platelets alone [5, 19, 20]. Nonetheless, lower doses of LPS accelerated platelet turnover without measurable changes in platelet count. Together with the finding that TLR4-dependent increases in basal P-selectin positive platelets 24 hours after the single intravenous LPS dose, and a corresponding increase in reticulated platelets two days later, indicate that the lower doses of LPS induce a sustained reactive platelet response. This response occurs without measurable changes in serum TNFα, so if this and other cytokines are involved they act locally in concentrations too low to be detected in the circulating concentrations.
Platelet activation and expression of P-selectin is accompanied by increased thrombin generating capacity of platelets [13]. Expression of platelet surface α-granule P-selectin also is necessary for platelets to interact with neutrophils, monocytes, T cells and endothelium [21–23]. Neutrophils stimulated with LPS, on the other hand, shed microvesicles having potent platelet activating activity [24]. Thus, these TLR4-dependent processes may promote thrombotic risk and processes associated with development of atherosclerosis [10, 25]. This expectation is based in part on the unique finding of a shortened platelet lifespan in asymptomatic relatives of individuals with early onset coronary artery disease [14]. Increased direct contact of platelets with other blood elements and products [24, 26] may explain, in part, the shortening of the platelet life span. The life-span of platelets determined by biotin-label in C57BL10 mice injected with saline was 3.5 days, which correlates well with the life-span determined with 35sulfur, 51chromium and biotin in other strains of mice [27–30]. Therefore, changes in platelet life span following injection with LPS are most likely reflective of the LPS and not the technique. The linear relationship between platelet clearance and reticulated platelets reflecting newly formed platelets and therefore, an indirect measurement of platelet production most likely accounts for the observation that total platelet count was not affected in WT mice exposed to low concentrations of LPS.
In conclusion, platelets adopt a pro-thrombotic phenotype in response to very low concentrations of LPS. Platelet responsiveness is mediated by TLR4 at concentrations of LPS which are 1/4 – 1/10 those required to stimulate thrombocytopenia and transient increases in serum TNFα. Therefore, even acute exposure to low doses of LPS could affect procoagulant activity of the blood and contribute to increased risk of thrombosis with incidental bacteremia, which can occur with processes as commonplace as toothbrushing [4]. These results may also provide insight into the relationship between LPS (or infection-associated) platelet activation and atherosclerosis in humans, as Asp299Gly polymorphism in TLR4 is associated with a lower risk of carotid atherosclerosis and less intima-media thickness [25, 31]. However, platelet phenotypes in individuals with this polymorphism remain to be defined.
Summary
Effects of in vivo stimulation of toll-like receptor 4 (TLR4) with LPS were evaluated on murine platelets. A single intravenous LPS injection, in amounts below those stimulating increases in serum TNFα, induced platelet activation and increased platelet clearance and production of reticulated (young and reactive) platelets. These changes did not occur in TLR4 deficient mice. At higher LPS doses, the dependence of the platelet changes on TLR4 was lost, and correlated with serum TNFα in WT mice. Thus, low levels of LPS increase platelet activation and shorten platelet life-span, which could increase the thrombotic and atherosclerotic risk of commensal or pre-clinical infection.
Acknowledgments
This work was supported by National Institute of Health grant HL78638, American Heart Association, AHA30503Z and Mayo Foundation.
Abbreviations
- TLR4
toll-like receptor 4
- dTLR4
deletion of TLR4
- LPS
lipopolysaccharide
- TNFα
tumor necrosis factor
- WT
wild type mice
- glycoprotein IIIa
CD61
- PE
Phycoerythrin
- FITC
fluorescein isothiocynate
- SA-PE
streptavidin-PE
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Epstein SE, Zhu J, Burnett MS, Zhou YF, Vercellotti GM, Hajjar D. Infection and atherosclerosis: Potential roles of pathogen burden and molecular mimicry. Arterioscler Thromb Vasc Biol. 2000;20:1417–20. doi: 10.1161/01.atv.20.6.1417. [DOI] [PubMed] [Google Scholar]
- 2.Zhu J, Nieto FJ, Horne BD, Anderson JL, Muhlestein JB, Epstein SE. Prospective study of pathogen burden and risk of myocardial infarction or death. Circulation. 2001;103:45–51. doi: 10.1161/01.cir.103.1.45. [DOI] [PubMed] [Google Scholar]
- 3.Stoll LL, Denning GM, Weintraub NL. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2227–36. doi: 10.1161/01.ATV.0000147534.69062.dc. [DOI] [PubMed] [Google Scholar]
- 4.Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008 Jun 17;117(24):3118–25. doi: 10.1161/CIRCULATIONAHA.107.758524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andonegui G, Kerfoot SM, McNagny K, Ebbert KVJ, Patel KD, Kubes P. Platelets express functional toll-like receptor-4 (TLR4) Blood. 2005;106:2417–23. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 6.Fritz JH, Le Bourhis L, Magalhaes JG, Philpott DJ. Innate immune recognition at the epithelial barrier drives adaptive immunity: APCs take the back seat. Trends Immunol. 2008 Jan;29(1):41–9. doi: 10.1016/j.it.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Liu F, Morris S, Epps J, Carrol R. Demonstration of an activation regulated NF-kappaB/1-kappaBalpha complex in human platelets. Thrombosis Research. 2002;106(4–5):199–203. doi: 10.1016/s0049-3848(02)00130-5. [DOI] [PubMed] [Google Scholar]
- 8.Aslam R, Speck ER, Kim M, Crow AR, Bang KA, Nestel FP, et al. Platelet toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-α production in vivo. Blood. 2006;107:637–41. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 9.Hoshi K, Ejiri S, Ozawa H. Ultrastructural, cytochemical, and biophysical aspects of mechanisms of bone matrix calcification. Acta Anat Nipppon. 2000;75:457–65. [PubMed] [Google Scholar]
- 10.Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med. 2002;162:1028–32. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- 11.Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 12.McBane RD, 2nd, Karnicki K, Tahirkheli N, Miller RS, Owen WG. Platelet characteristics associated with coronary artery disease. J Thromb Haemost. 2003 Jun;1(6):1296–303. doi: 10.1046/j.1538-7836.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 13.Merten M, Thiagarajan P. P-selectin in arterial thrombosis. Z Kardiol. 2004 Nov;93(11):855–63. doi: 10.1007/s00392-004-0146-5. [DOI] [PubMed] [Google Scholar]
- 14.Murphy EA, Mustard JF. Coagulation tests and platelet economy in atherosclerotic and control subjects. Circulation. 1962;25:114–25. doi: 10.1161/01.cir.25.1.114. [DOI] [PubMed] [Google Scholar]
- 15.Poltorak A, Merlin T, Nielsen PJ, Sandra O, Smirnova I, Schupp I, et al. A point mutation in the IL-12Rbeta2 gene underlies the IL-12 unresponsiveness of LPS-defective C57BL/10ScCr mice. J Immunol. 2001;167:2106–11. doi: 10.4049/jimmunol.167.4.2106. [DOI] [PubMed] [Google Scholar]
- 16.Jayachandran M, Brunn GJ, Karnicki K, RSM, Owen WG, Miller VM. In vivo effects of lipopolysaccharide and TLR4 on platelet production and activity: Implications for thrombotic risk. J Appl Physiol. 2007;102(1):429–33. doi: 10.1152/japplphysiol.01576.2005. [DOI] [PubMed] [Google Scholar]
- 17.Jayachandran M, Karnicki K, Miller RS, Owen WG, Korach KS, Miller VM. Platelet characteristics change with aging: Role of estrogen receptor β. J Gerontol: Biol Sci. 2005;60:815–9. doi: 10.1093/gerona/60.7.815. [DOI] [PubMed] [Google Scholar]
- 18.Jayachandran M, Okano H, Chatrath R, Owen WG, McConnell JP, Miller VM. Sex-specific changes in platelet aggregation and secretion with sexual maturity in pigs. J Appl Physiol. 2004;97:1445–52. doi: 10.1152/japplphysiol.01074.2003. [DOI] [PubMed] [Google Scholar]
- 19.Hellerud BC, Stenvik J, Espevik T, Lambris JD, Mollnes TE, Brandtzaeg P. Stages of meningococcal sepsis simulated in vitro, with emphasis on complement and Toll-like receptor activation. Infect Immun. 2008 Sep;76(9):4183–9. doi: 10.1128/IAI.00195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perlik V, Li Z, Goorha S, Ballou LR, Blatteis CM. LPS-activated complement, not LPS per se, triggers the early release of PGE2 by Kupffer cells. Am J Physiol Regul Integr Comp Physiol. 2005 Aug;289(2):R332–R9. doi: 10.1152/ajpregu.00567.2004. [DOI] [PubMed] [Google Scholar]
- 21.Mine S, Fujisaki T, Suematsu M, Tanaka Y. Activated platelets and endothelial cell interaction with neutrophils under flow conditions. Intern Med. 2001;40:1085–92. doi: 10.2169/internalmedicine.40.1085. [DOI] [PubMed] [Google Scholar]
- 22.Moore KL, Thompson LF. P-selectin (CD62) binds to subpopulations of human memory T lymphocytes and natural killer cells. Biochem Biophys Res Commun. 1992;186:173–81. doi: 10.1016/s0006-291x(05)80790-9. [DOI] [PubMed] [Google Scholar]
- 23.von Hundelshausen P, Weber KS, Huo Y, Proudfoot AE, Nelson PJ, Ley K, et al. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001 Apr 3;103(13):1772–7. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
- 24.Pluskota E, Woody NM, Szpak D, Ballantyne CM, Soloviev DA, Simon DI, et al. Expression, activation, and function of integrin alphaMbeta2 (Mac-1) on neutrophil-derived microparticles. Blood. 2008 Sep 15;112(6):2327–35. doi: 10.1182/blood-2007-12-127183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, et al. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–92. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 26.Montrucchio G, Bosco O, Del Sorbo L, Fascio Pecetto P, Lupia E, Goffi A, et al. Mechanisms of the priming effect of low doses of lipopolysaccharides on leukocyte-dependent platelet aggregation in whole blood. Thromb Haemost. 2003;90:872–81. doi: 10.1160/TH03-02-0085. [DOI] [PubMed] [Google Scholar]
- 27.Odell TT, Jr, Mc DT. Life span of mouse blood platelets. Proc Soc Exp Biol Med. 1961 Jan;106:107–8. doi: 10.3181/00379727-106-26252. [DOI] [PubMed] [Google Scholar]
- 28.Jackson CW, Krance RA, Edwards CC, Whidden MA, Gauthier PA. Shortened platelet survival as a cause of thrombocytopenia in mice with L1210 leukemia. Cancer Res. 1980 Mar;40(3):667–70. [PubMed] [Google Scholar]
- 29.Manning KL, Novinger S, Sullivan PS, McDonald TP. Successful determination of platelet lifespan in C3H mice by in vivo biotinylation. Lab Anim Sci. 1996;46:545–8. [PubMed] [Google Scholar]
- 30.Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007 Mar 23;128(6):1173–86. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 31.Pasterkamp G, van Keulen JK, de Kleijn DPV. Role of toll-like receptor 4 in the initiation and progression of atherosclerotic disease. Eur J Clin Invest. 2004;34:328–34. doi: 10.1111/j.1365-2362.2004.01338.x. [DOI] [PubMed] [Google Scholar]