Abstract
Background
Health-care associated infections due to methicillin-resistant strains of Staphylococcus aureus (MRSA) are increasing worldwide despite current infection control measures. Novel methods for disinfection of MRSA would be useful.
Methods
We tested the effectiveness of atmospheric, non-thermal plasma discharge at killing S. aureus, including USA300 strains, and at disinfecting experimentally contaminated hospital pagers.
Results
Exposure of S. aureus to plasma at different concentrations and for varying lengths of time resulted in up to a 4–5 log10 kill on tryptic soy agar plates within 10 minutes and was not toxic to epithelial cells. USA300 strains of MRSA were more resistant to plasma-based killing than other tested strains. Disinfection of hospital pagers experimentally coated with clinically relevant amounts of MRSA could be achieved in as little as 30 seconds.
Conclusions
Generation of plasma is a promising method for disinfection of objects or surfaces that warrants further study in hospital settings. The USA300 strains of S. aureus may be more resistant to disinfection than other strains.
Keywords: MRSA, Staphylococcus aureus, infection control, plasma, disinfection
Staphylococcus aureus is the leading cause of healthcare-associated infections (HAI)1. Infections with methicillin-resistant forms of S. aureus (MRSA) increased about ten-fold in the decade between 1995 and 2005 such that an estimated 278,000 to 368,000 hospitalizations for treatment of this organism now occur annually1;2. This trend is linked to the emergence of the USA300 and USA400 clones of MRSA. Because the scope and seriousness of this problem have increased despite implementation of numerous infection control measures, there is interest in new approaches and technologies.
Infection control for prevention of MRSA HAIs is typically a layered approach encompassing surveillance, handwashing, barrier precautions, and disinfection of contaminated surfaces and objects3. Transmission of MRSA from health-care workers to patients is generally considered to occur via contamination of the hands; good hand hygiene practices are considered the cornerstone of any infection control program. Weaker evidence suggests that bacterial contamination of objects in the environment plays a role in transmission, probably through an indirect mechanism by contributing to colonization of health-care workers4. Disinfection of potentially contaminated surfaces and objects is recommended to reduce this potential risk5. Few studies have been done linking contamination of inanimate objects carried by or worn on the health-care worker to nosocomial transmission. Concern over the role of clothing including neck ties6 in nosocomial transmission has led to calls for changes in dress code for health-care workers7. The potential for involvement of other commonly carried items, such as hospital badges, beepers, and cell phones, has received relatively little attention8.
Most sterilization and disinfection techniques involve exposure to chemical compounds or intense heat for prolonged periods of time5. These treatments are not ideal for a number of potential vectors of transmission because of potential damage to the objects being treated (e.g., electronics, health-care workers’ hands) and are impractical for casual treatment of common items. Recently, increased interest in alternative methods of disinfection and sterilization has led to development of techniques using exposure to plasmas to kill bacteria, viruses and bacterial spores9. Plasmas are generated by inducing gases to enter an ionized state10;11. These plasmas contain short-lived active oxygen species such as ozone, hydroxyl, superoxide, and nitrogen oxides which can have antimicrobial effects. Because plasmas can be generated by application of an electric current at atmospheric pressure and at room temperature, they are being increasingly considered for disinfection or inactivation of bacteria in situations where typical sterilization techniques would cause damage such as with fresh produce12;13. This technique has been shown to be active against a number of gram-negative and gram-positive organisms including biofilm forming agents9;12–14. We conducted the current study to determine whether sterilization of S. aureus including clinically relevant MRSA strains was possible using an atmospheric non-thermal plasma discharge apparatus. Furthermore, we tested its efficacy at disinfecting common hospital items such as beepers which might become contaminated with MRSA.
Materials and Methods
Methods
Bacterial strains and growth conditions
S. aureus strains NRS193 (USA400), LAC (USA300), and Newman were obtained through the Network on Antimicrobial Resistance in S. aureus (NARSA, NIAID). S. aureus strain Le Bonheur (LB) was obtained from Dr. Steve Buckingham at the Le Bonheur Children Medical Center in Memphis, TN and is a USA300 type clinical isolate from a patient with necrotizing pneumonia. Escherichia coli strain DH5α was obtained from New England Biolabs, Inc. (Ipswich, MA). Cell cultures of S. aureus and E. coli cells were grown at 37°C on trypticsoy (TS) broth (to late-log phase) and agar plates.
Plasma discharge apparatus
The plasma discharge apparatus was built by author IA and generates a non equilibrium resistive barrier plasma discharge at atmospheric pressure9;10. The plasma is generated between two planar electrodes using a low frequency alternative current (120V, 60 HZ) fed through a step transformer (Output voltage-15KV across secondary terminals at 60 mA). The experimental design set up of the plasma reactor consists of two electrodes- a bottom and top electrode which rests in a highly resistive wetted unglazed ceramic barrier. The ceramic barrier is cooled and rendered conductive by using either water or hydrogen peroxide (30%), while ensuring no contact with the inoculated agar plates or test items. The working medium in the plasma reactor is air and traces of water vapor or hydrogen peroxide. The resistive barrier has a resistance of 1M-Ohm, which prevents the diffuse discharge from contracting into an arc. A non conducting frame separates the two electrodes creating an air gap of 0.25 inch. The electrode arrangement and rotary blower to circulate the generated ozone and other plasma produced species during plasma discharge are housed in the upper glass compartment. The high voltage circuitry and plasma reactor which generates up to 1800cc of air plasma is housed in the lower compartment.
Plasma discharge parameters and conditions
All bacteria samples were exposed to an atmospheric pressure plasma discharge in a gas medium of air and residue from water or hydrogen peroxide. The discharged products reached the surface of the specimen through diffusion from the region of discharge aided by a rotary fan which generated air currents across the top of the plates.
Plasma bactericidal activity
Inocula of E. coli and various S. aureus strains were prepared by adding 100ul of bacterial suspension (ranging from 6.0–6.3 × 108 to 6.0–6.3 × 101 CFU/ml) to tryptic soy agar plates prior to exposure to plasma discharge for times varying by 30 second intervals from 30 seconds to 10 minutes. The percentage ratio of viable bacterial colony forming units (CFU) from treated samples was compared to untreated samples after a 48 hour incubation at 37°C. Each data set represents the mean value plus standard deviation of at least three exposure experiments.
Plasma bacterial decontamination from objects
Inocula were prepared by adding 100ul of bacterial suspension (6.0–6.3 × 101 to 6.0–6.3 × 102 CFU/ml) to the surface of one way pagers and either exposed immediately (wet) or dried at 37°C (for ~20min) prior to exposure. Before and after treatment, surviving bacteria were isolated from different areas on the surface of the pager (~10% of the initial inoculum was recovered by this method at each timepoint) using a sterile swab inoculated with sterile saline solution and streaked on TS agar plates.
Toxicity tests
In vitro cytotoxic activity of plasma discharge sterilization was determined by viability as measured by the trypan blue dye exclusion assay. Briefly, a continuous cell line of Madin–Darby canine kidney (MDCK) cells was maintained as described15. Culture media overlaying 80% confluent cells in 6 well tissue culture plates was removed and cells were treated with plasma discharge for 0 or 10 minutes without liquid media present. After 10 minutes, fresh culture media was added and cells were incubated at 37°C in 5% CO2. The percentage of cytotoxicity was determined by trypan blue exclusion using the following equation: cytotoxicity (%) = 100 × (value experimental-value spontaneous)/(value maximum - value spontaneous) where the cell suspension of MDCK cells served as the control for spontaneous death.
Statistics
Comparison of bacterial titers between groups was done using Student’s t-test with Bonferroni correction for pairwise comparisons and analysis of variance (ANOVA) for multiple comparisons. A p-value of < 0.05 was considered significant for these comparisons. SigmaStat for Windows (SysStat Software, Inc., V 3.11) was utilized for all statistical analyses.
Results
Plasma can sterilize S. aureus
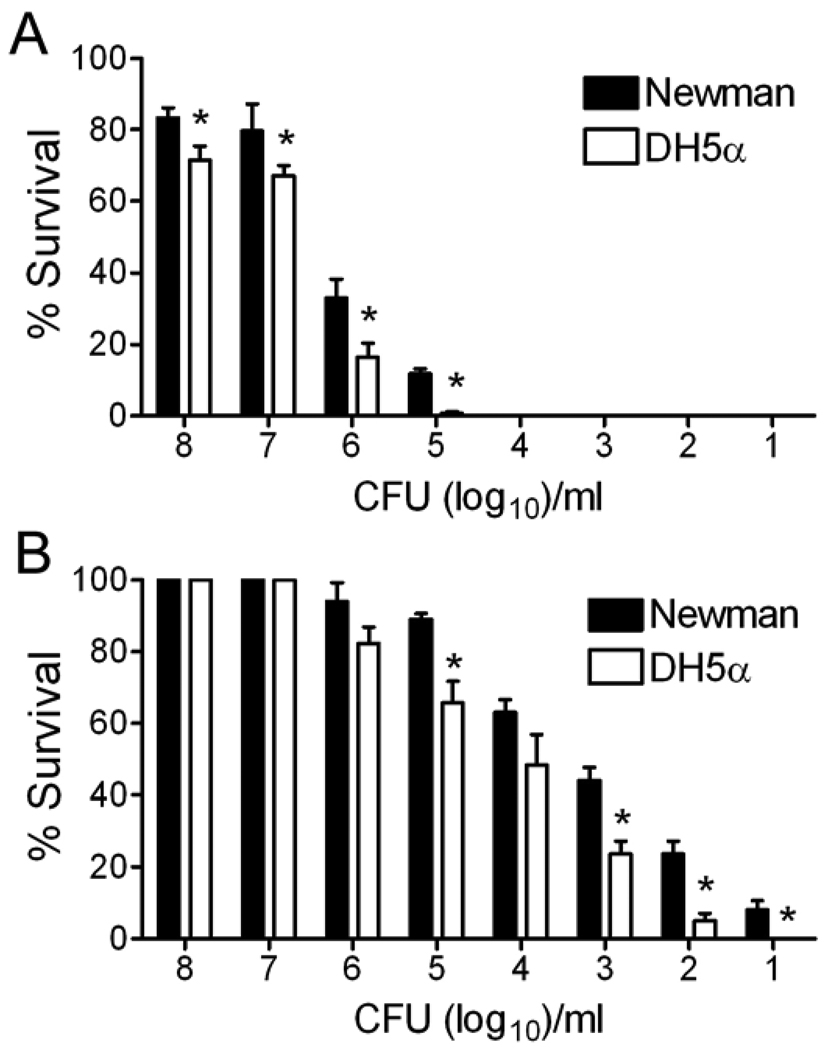
The purpose of this study was to evaluate the ability of atmospheric non-thermal plasmas to sterilize or achieve high-level disinfection of S. aureus. As an initial test, inocula of a commonly used laboratory strain of S. aureus were spread onto agar plates and exposed to plasma for intervals ranging from 30 seconds to 10 minutes followed by incubation at 37°C for 48 hours (data shown for only the 30s and 10m timepoints). Results for S. aureus were compared to those for E. coli which has previously been demonstrated to be highly sensitive to the effects of plasma9. A 10 minute exposure was able to disinfect up to 10,000 CFU of either S. aureus or E. coli (Figure 1A). Partial disinfection of higher bacterial loads was seen, with superior efficacy against E. coli compared to S. aureus (p < 0.05 by ANOVA). Noticeable disinfection could also be seen at even the briefest exposure time of 30 seconds (Figure 1B), with significantly better killing of E coli at most doses compared to S. aureus.
Figure 1. Plasma treatment is bactericidal for S. aureus.
Aliquots of 101 to 108 CFU of either the DH5α strain of E. coli or the Newman strain of S. aureus were exposed to plasma for A) 10 minutes or B) 30 seconds. An asterisk (*) indicates significantly better killing of E. coli compared to S. aureus at that inoculum (p < 0.05 by ANOVA).
USA300 strains of MRSA are relatively resistant to plasma sterilization
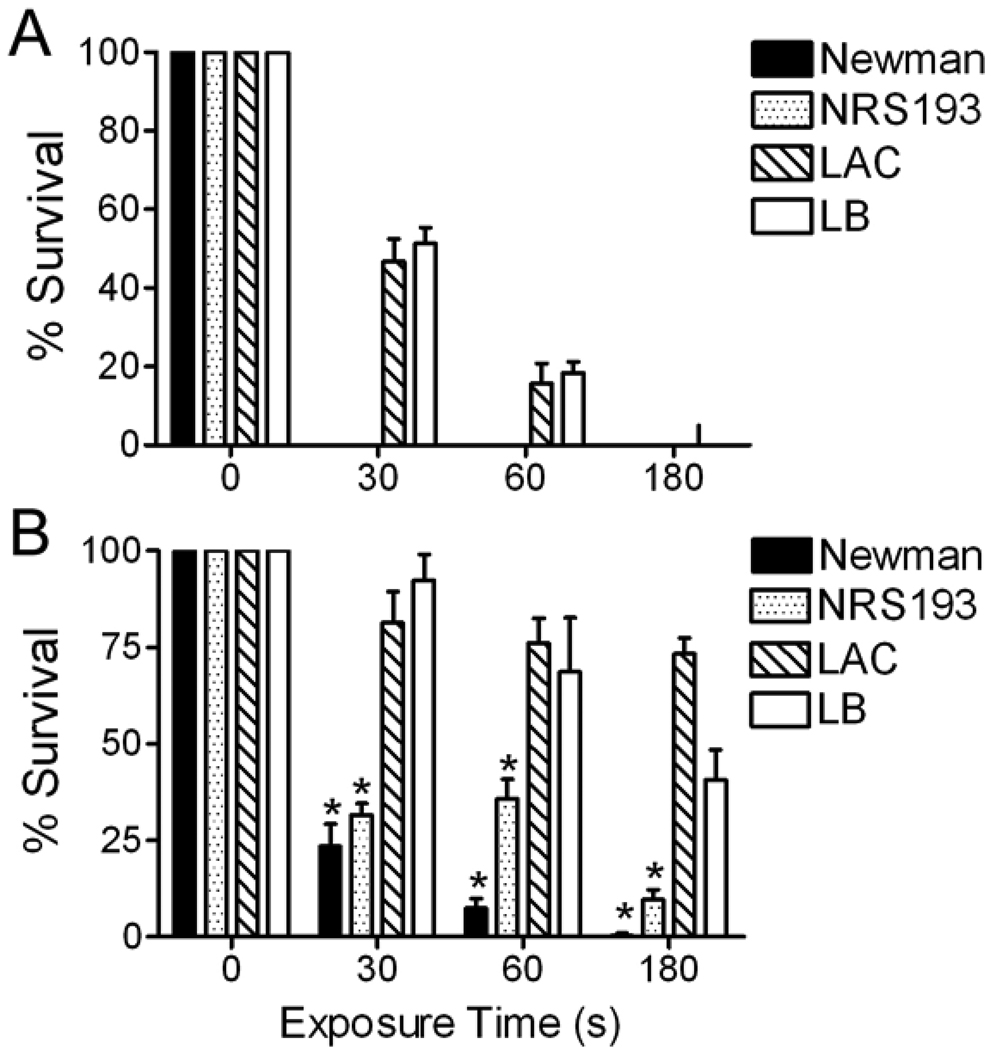
Most hospital-acquired infections are now caused by USA300 and USA400 strains of MRSA. We therefore compared plasma disinfection of the laboratory strain Newman of S. aureus to these more relevant clinical strains. At an initial inoculum of 100 CFU, the Newman and USA400 strains were killed within 30 seconds (Figure 2A). However, two USA300 clinical strains, one from NARSA and the other from a local children’s hospital, required a minimum of 3 minutes for complete sterilization. At a ten-fold higher dose of 1000 CFU, significant differences in the ability of plasma to disinfect USA300 strains were demonstrated compared to USA400 and Newman strains (Figure 2B, p < 0.05 by ANOVA). From these experiments it is clear that plasma can kill clinically relevant amounts and strains of S. aureus in a short period of time. However, there are differences between S. aureus strains in their sensitivity to the method, with the medically important USA300 strains requiring longer exposures for complete disinfection. To determine whether the H2O2 contributed to bacterial killing we repeated several disinfection experiments comparing killing of E. coli and 2 strains of S. aureus using either H2O2 or tap water as the conductant. Water was found to be an acceptable alternative to H2O2 for killing of both bacterial species since no differences were apparent using the different liquids (data not shown).
Figure 2. USA300 strains are relatively resistant to plasma treatment.
Aliquots of either A) 102 or B) 103 CFU of four strains of S. aureus were exposed to plasma for 30, 60, or 180 seconds. An asterisk (*) indicates significantly better killing of the Newman or USA400 (NRS193) strains compared to the two USA300 (LAC and LB) strains at the indicated timepoints and inocula (p < 0.05 by ANOVA).
Plasma can be utilized for disinfection of common objects in the hospital
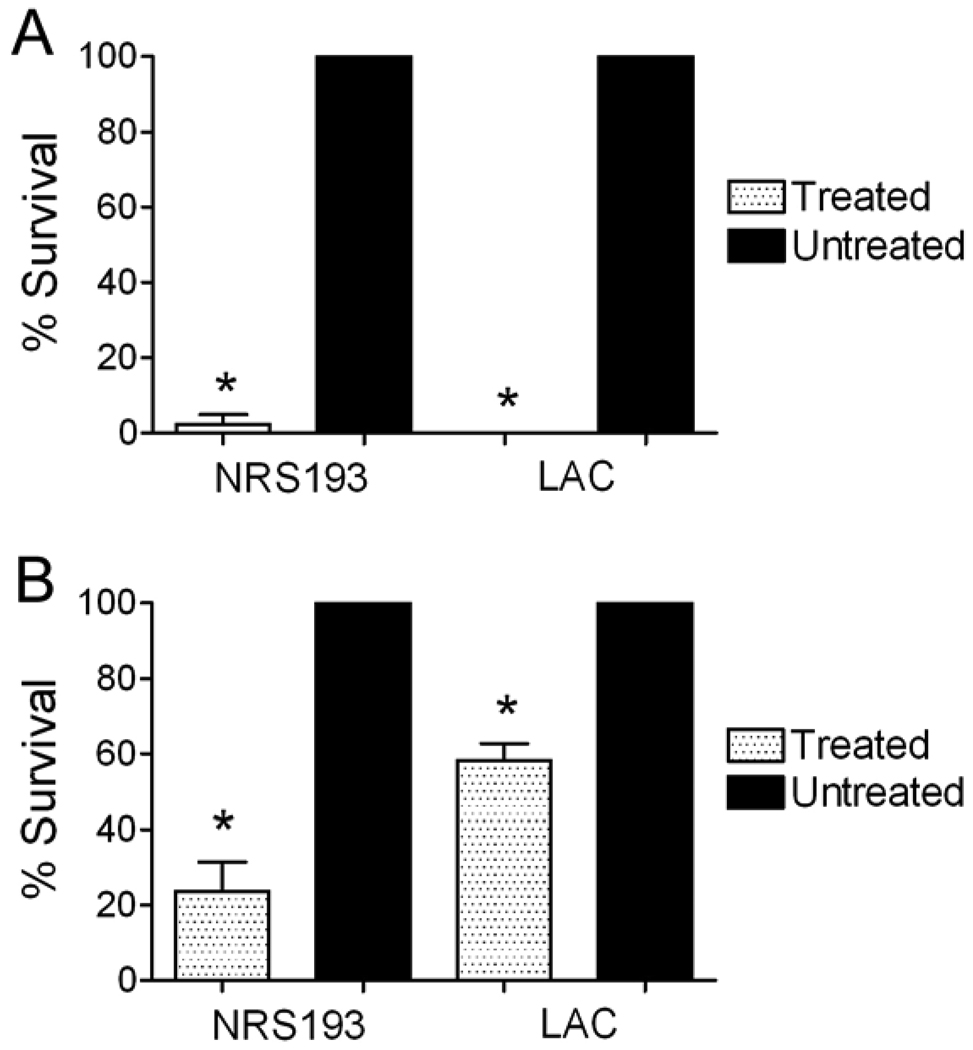
Most disinfection and sterilization methods require prolonged exposure to caustic chemical solutions and thus are inappropriate for routine use by healthcare workers for non-critical items including delicate electronics. We therefore sought to test the plasma disinfection technique using a relevant inoculum and relevant conditions on medical pagers, an electronic device carried by most hospital personnel from room to room and frequently in contact with the hands of health-care workers. Pagers were inoculated with 100 CFU of S. aureus in a 100 µl volume then exposed to a brief 30 second plasma discharge either while the inoculum remained wet or after allowing it to dry. Near complete disinfection was seen for both USA300 and USA400 strains against the dried inoculum (Figure 3A). An intermediate but significant (p < 0.05 by Student’s t-test) level of disinfection was demonstrated when the inocula remained wet, and the USA300 strain was more resistant to killing in this scenario then the USA400 strain (Figure 3B).
Figure 3. Hospital pagers can be disinfected by exposure to plasma.
Pagers were inoculated with 100 CFU of two strains of S aureus (USA 400 strain NRS193 and USA300 strain LAC) and exposed to plasma for 30 seconds either A) after drying or B) while still wet. An asterisk (*) indicates significantly better killing compared to the groups which were not treated (p < 0.05 by Student’s t-test).
To assess whether plasma treatment is non-toxic for living cells and thus might be potentially useful for disinfection of health-care worker’s hands, we exposed cultured epithelial cells to plasma for 10 minutes and assessed viability 24 and 48 hours later. Viability did not differ between exposed and unexposed cells at either timepoint (at 48 hours, 68.6 ± 4.2% of unexposed cells were viable, compared with 65.0 ± 3.0% of exposed cells; p > 0.1 by Student’s t-test).
Discussion
Methicillin-resistant strains of S. aureus are now the leading cause of HAIs and are a major concern for infection control programs. Novel means of disinfecting and sterilizing contaminated items are of interest. We elected to study a promising new technology, atmospheric non-thermal plasmas, with clinically relevant strains of MRSA. We demonstrate that a prototype plasma disinfection apparatus can kill S. aureus including medically relevant USA300 strains in a short period of time. The results presented here compare favorably to published results for commonly used disinfectants. The 4–5 log10 reduction in CFU for S. aureus is similar to the 2–8 log10 reduction reported with a variety of disinfectants mixed directly with S. aureus cultures16;17 and the 3–5 log10 reduction seen when disinfectants were applied to hard surfaces with a mop18. Furthermore, the process is non-toxic to epithelial cells in these preliminary experiments and requires only an electrical current and water to work. USA300 strains required longer exposures to kill than other strains of S. aureus, reinforcing the need to test disinfection or sterilization methods against clinically relevant strains instead of common laboratory strains.
Current guidelines for intermediate or low-level disinfection of semi-critical or non-critical items recommend only liquid solutions containing alcohols, chlorine, phenolics, iodophors, or quarternary ammonia compounds as options5. These are clearly not suitable for many objects in the healthcare environment, including clothing, paper products, electronics, and any other items that may be discolored or damaged by exposure to chemicals. While the question of whether there is a need or a benefit for disinfection of these objects as a part of infection control is not clear due to lack of study, the shortcomings of current methods are well appreciated arguing that alternative approaches should be explored. Plasma based disinfection has several potential advantages that make it an attractive technology. It is a gas, so can penetrate by diffusion into crevices that may be inaccessible to topical treatments and some liquids. It does not produce or require heat or pressure to have an effect, does not require chemicals, and is predicted to be non-toxic to skin, so is likely to be suitable for many different applications including delicate or complex objects and perhaps even usable in antisepsis of living tissue. In a previous study using skin explants, application of nonthermal plasma was able to kill S. aureus but did not damage skin19. The prototype device utilized in this study was inexpensive and assembled from common items available at a hardware store – it is likely that commercial development and improvements in the design will result in better antimicrobial effects. In previous studies it was demonstrated to be virucidal and sporicidal in addition to its bactericidal effects9, so further development and testing may indicate utility for sterilization of critical items or high-level disinfection.
In this study we have tested the ability of plasma to kill S. aureus and disinfect objects such as hospital pagers. This has been done using an apparatus that is essentially a box, with the idea that it could be useful for point of care disinfection applications by placing items inside and turning the device on for a certain amount of time. Other investigators have pursued different applications, however. Since the plasma is in a gaseous state, it can be forced through a nozzle13;14 and sprayed over items that are large, fixed in place, or otherwise unsuitable for how our experiment was designed. Thus, the method may have utility for processes such as terminal room cleaning after discharge of a patient infected with MRSA20, and may be safer and easier than proposed alternatives such as spraying with hydrogen peroxide vapor21.
In summary, we have demonstrated that an emerging technology, generation of atmospheric non-thermal plasmas, is bactericidal for MRSA including clinically relevant USA300 strains and can be used for disinfection of non-critical items. We suggest that further study of this technology and development of improved plasma devices will have broad applications in healthcare including control of infectious agents important in the development of HAIs.
Acknowledgments
Support: Dr. McCullers’ salary is partially supported by ALSAC and NIH grant AI-66349.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13(12):1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elixhauser A, Steiner C. Infections with methicillin-resistant Staphylococcus aureus (MRSA) in U.S. hospitals, 1993–2005. 2007 July 1; [HCUP Statistical Brief #35] Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb35.pdf. [PubMed]
- 3.Boyce JM, Havill NL, Kohan C, Dumigan DG, Ligi CE. Do infection control measures work for methicillin-resistant Staphylococcus aureus? Infect Control Hosp Epidemiol. 2004;25(5):395–401. doi: 10.1086/502412. [DOI] [PubMed] [Google Scholar]
- 4.Hota B. Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis. 2004;39(8):1182–1189. doi: 10.1086/424667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutala WA, Weber DJ the Healthcare Infection Control Practices Advisory Committee (HICPAC) Guildelines for disinfection and sterilization in healthcare facilities. 2008 Available at: http://www.cdc.gov/ncidod/dhqp/pdf/guidelines/Disinfection_Nov_2008 pdf.
- 6.Dixon M. Neck ties as vectors for nosocomial infection. Intensive Care Med. 2000;26(2):250. doi: 10.1007/s001340050056. [DOI] [PubMed] [Google Scholar]
- 7.Ditchburn I. Should doctors wear ties? J Hosp Infect. 2006;63(2):227–228. doi: 10.1016/j.jhin.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Kotsanas D, Scott C, Gillespie EE, Korman TM, Stuart RL. What's hanging around your neck? Pathogenic bacteria on identity badges and lanyards. Med J Aust. 2008;188(1):5–8. doi: 10.5694/j.1326-5377.2008.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 9.Alexeff I, Balasundaram A, Pradeep EP, Karnam N, Pulasani NR. Biological decontamination using an atmospheric pressure resistive barrier plasma discharge. In: Selcuk G, Alexeff I, editors. Plasma Assisted Decontamination of Biological and Chemical Agents. Dordrecht, The Netherlands: Springer Science and Business Media, B.V.; 2008. pp. 3–19. [Google Scholar]
- 10.Laroussi M, Alexeff I, Kang WL. Biological decontamination by nonthermal plasmas. IEEE Trans Plasma Sci. 2000;28(1):184–188. [Google Scholar]
- 11.Moisan M, Crevier M-C, Pelletier NPJ. Plasma sterilization. methods and mechanisms. Pure Appl Chem. 2002;74(3):349–358. [Google Scholar]
- 12.Critzer FJ, Kelly-Wintenberg K, South SL, Golden DA. Atmospheric plasma inactivation of foodborne pathogens on fresh produce surfaces. J Food Prot. 2007;70(10):2290–2296. doi: 10.4315/0362-028x-70.10.2290. [DOI] [PubMed] [Google Scholar]
- 13.Perni S, Shama G, Kong MG. Cold atmospheric plasma disinfection of cut fruit surfaces contaminated with migrating microorganisms. J Food Prot. 2008;71(8):1619–1625. doi: 10.4315/0362-028x-71.8.1619. [DOI] [PubMed] [Google Scholar]
- 14.Sladek RE, Filoche SK, Sissons CH, Stoffels E. Treatment of Streptococcus mutans biofilms with a nonthermal atmospheric plasma. Lett Appl Microbiol. 2007;45(3):318–323. doi: 10.1111/j.1472-765X.2007.02194.x. [DOI] [PubMed] [Google Scholar]
- 15.Peltola VT, Murti KG, McCullers JA. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J Infect Dis. 2005;192(2):249–257. doi: 10.1086/430954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutala WA, Barbee SL, Aguiar NC, Sobsey MD, Weber DJ. Antimicrobial activity of home disinfectants and natural products against potential human pathogens. Infect Control Hosp Epidemiol. 2000;21(1):33–38. doi: 10.1086/501694. [DOI] [PubMed] [Google Scholar]
- 17.Fisher RG, Chain RL, Hair PS, Cunnion KM. Hypochlorite killing of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2008;27(10):934–935. doi: 10.1097/INF.0b013e318175d871. [DOI] [PubMed] [Google Scholar]
- 18.Exner M, Vacata V, Hornei B, Dietlein E, Gebel J. Household cleaning and surface disinfection: new insights and strategies. J Hosp Infect. 2004;56 Suppl 2:S70–S75. doi: 10.1016/j.jhin.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 19.Watts AE, Fubini SL, Vernier-Singer M, Golkowski C, Shin S, Todhunter RJ. In vitro analysis of nonthermal plasma as a disinfecting agent. Am J Vet Res. 2006;67(12):2030–2035. doi: 10.2460/ajvr.67.12.2030. [DOI] [PubMed] [Google Scholar]
- 20.Boyce JM, Havill NL, Otter JA, Adams NM. Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect Control Hosp Epidemiol. 2007;28(10):1142–1147. doi: 10.1086/520737. [DOI] [PubMed] [Google Scholar]
- 21.Otter JA, Cummins M, Ahmad F, van TC, Drabu YJ. Assessing the biological efficacy and rate of recontamination following hydrogen peroxide vapour decontamination. J Hosp Infect. 2007;67(2):182–188. doi: 10.1016/j.jhin.2007.07.019. [DOI] [PubMed] [Google Scholar]