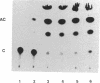
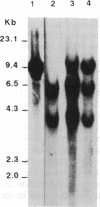
Abstract
Human immunodeficiency virus (HIV) type 1, isolated from diverse sources, exhibits genomic diversity. The mechanisms by which the genomic diversity takes place in individuals exposed to multiple virus isolates is yet to be elucidated. Genetic variation, in general, might result from mutagenic events such as point mutations, rearrangements (insertions and deletions), and recombination. In an attempt to evaluate the process of genetic diversity, we designed experiments to analyze recombination between HIV DNAs by using DNA transfection in cell cultures. Here we report the successful recombination between truncated HIV proviral DNAs with an overlap homology of 53 base pairs that leads to the formation of viable hybrid virus. Recombination was also seen between exogenous DNA introduced into cells and homologous HIV sequences resident in the cells. These results indicate that recombination among various HIV isolates may play a significant role in the generation of genetic diversity of HIV. Further, the method used here enables the construction of hybrid HIV genomes to identify the viral determinants responsible for tropism, replication, and cytopathic effects.
Full text
PDF
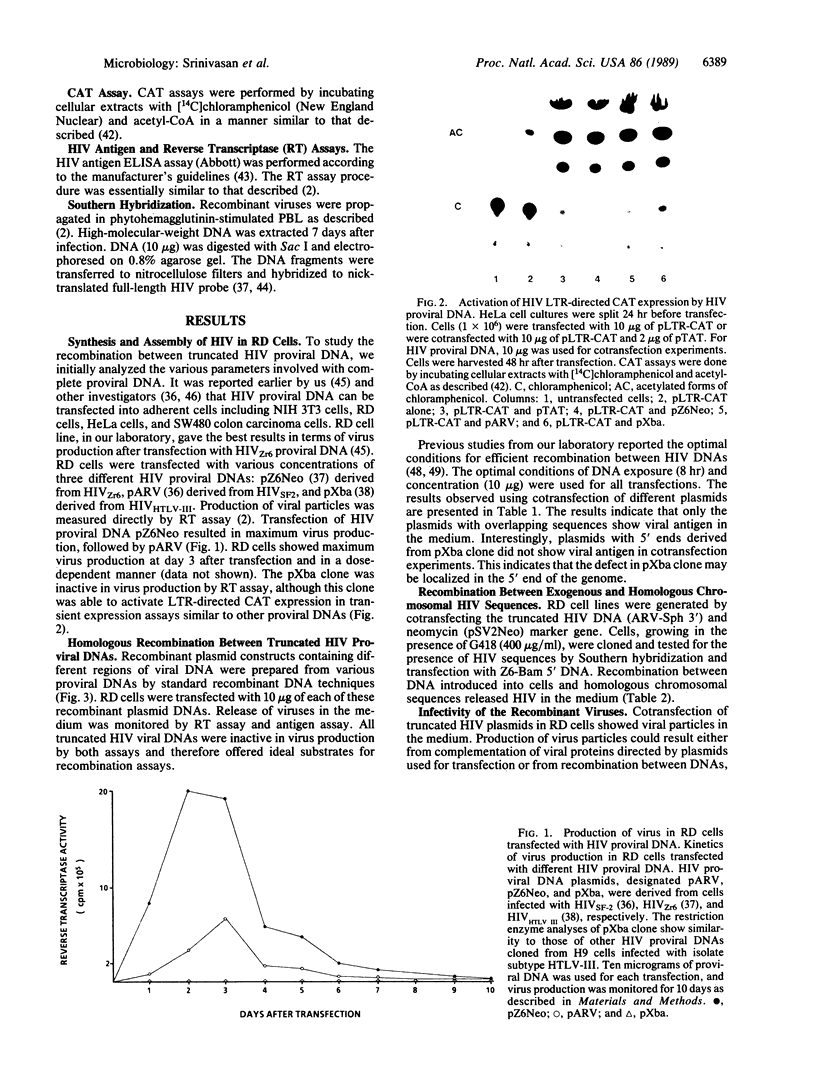
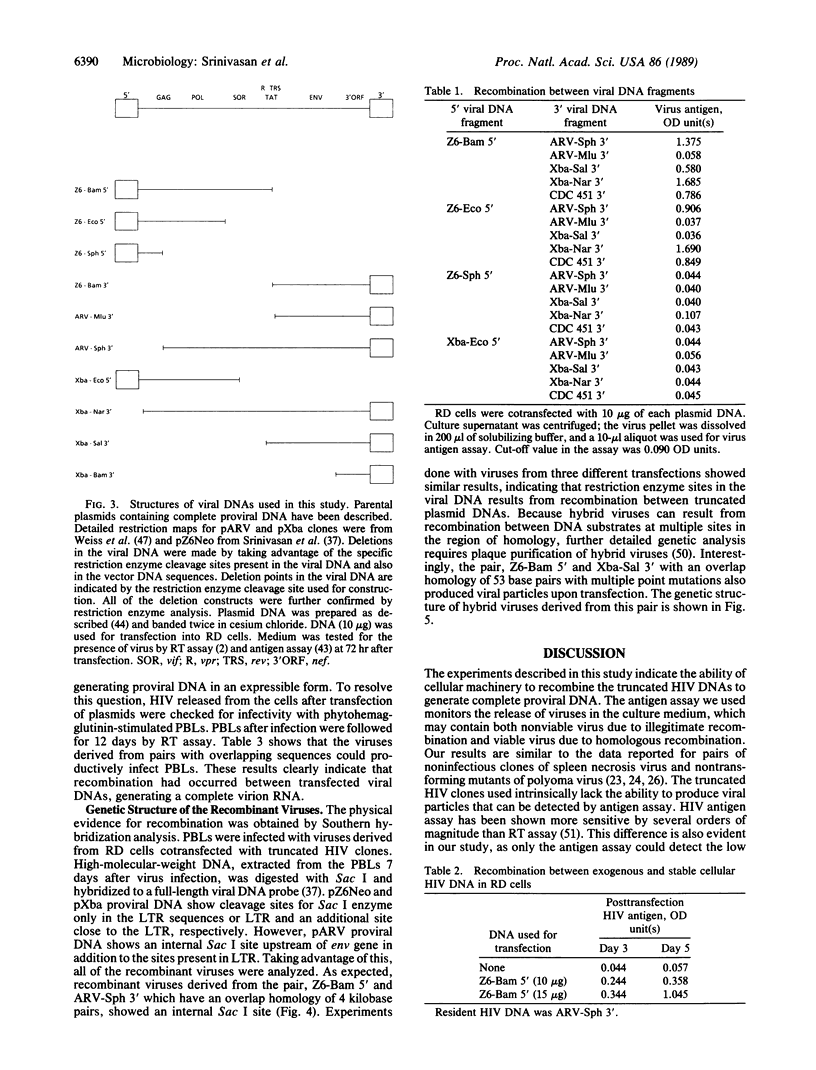

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R., Siegal F., Reed C., Cheung T., Forlenza S., Moore J. Non-cytocidal natural variants of human immunodeficiency virus isolated from AIDS patients with neurological disorders. Lancet. 1987 Aug 1;2(8553):234–238. doi: 10.1016/s0140-6736(87)90826-9. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Asjö B., Morfeldt-Månson L., Albert J., Biberfeld G., Karlsson A., Lidman K., Fenyö E. M. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986 Sep 20;2(8508):660–662. [PubMed] [Google Scholar]
- Ayares D., Spencer J., Schwartz F., Morse B., Kucherlapati R. Homologous recombination between autonomously replicating plasmids in mammalian cells. Genetics. 1985 Oct;111(2):375–388. doi: 10.1093/genetics/111.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay P. K., Watanabe S., Temin H. M. Recombination of transfected DNAs in vertebrate cells in culture. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3476–3480. doi: 10.1073/pnas.81.11.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Rutka J. T., Rosenblum M. L., McHugh T., Stites D. P., Levy J. A. Human immunodeficiency virus can productively infect cultured human glial cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3526–3530. doi: 10.1073/pnas.84.10.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnadurai G., Chinnadurai S., Brusca J. Physical mapping of a large-plaque mutation of adenovirus type 2. J Virol. 1979 Nov;32(2):623–628. doi: 10.1128/jvi.32.2.623-628.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M. Genetic variation in AIDS viruses. Cell. 1986 Jul 4;46(1):1–4. doi: 10.1016/0092-8674(86)90851-2. [DOI] [PubMed] [Google Scholar]
- Curran J. W., Morgan W. M., Hardy A. M., Jaffe H. W., Darrow W. W., Dowdle W. R. The epidemiology of AIDS: current status and future prospects. Science. 1985 Sep 27;229(4720):1352–1357. doi: 10.1126/science.2994217. [DOI] [PubMed] [Google Scholar]
- Dahl K., Martin K., Miller G. Differences among human immunodeficiency virus strains in their capacities to induce cytolysis or persistent infection of a lymphoblastoid cell line immortalized by Epstein-Barr virus. J Virol. 1987 May;61(5):1602–1608. doi: 10.1128/jvi.61.5.1602-1608.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai S. M., Kalyanaraman V. S., Casey J. M., Srinivasan A., Andersen P. R., Devare S. G. Molecular cloning and primary nucleotide sequence analysis of a distinct human immunodeficiency virus isolate reveal significant divergence in its genomic sequences. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8380–8384. doi: 10.1073/pnas.83.21.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devare S. G., Srinivasan A., Bohan C. A., Spira T. J., Curran J. W., Kalyanaraman V. S. Genomic diversity of the acquired immunodeficiency syndrome retroviruses is reflected in alteration of its translational products. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5718–5722. doi: 10.1073/pnas.83.15.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Ensoli B., Looney D., Rose A., Gallo R. C., Saag M. S., Shaw G. M., Hahn B. H., Wong-Staal F. Biologically diverse molecular variants within a single HIV-1 isolate. Nature. 1988 Aug 4;334(6181):444–447. doi: 10.1038/334444a0. [DOI] [PubMed] [Google Scholar]
- Folger K. R., Wong E. A., Wahl G., Capecchi M. R. Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol. 1982 Nov;2(11):1372–1387. doi: 10.1128/mcb.2.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Gallo R. C. Human tumor and immunodeficiency viruses. AIDS Res Hum Retroviruses. 1987;3 (Suppl 1):187–196. doi: 10.1089/aid.1987.3.187. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gallo R., Wong-Staal F., Montagnier L., Haseltine W. A., Yoshida M. HIV/HTLV gene nomenclature. Nature. 1988 Jun 9;333(6173):504–504. doi: 10.1038/333504a0. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit J., de Wolf F., Paul D. A., Epstein L. G., Lange J. M., Krone W. J., Speelman H., Wolters E. C., Van der Noordaa J., Oleske J. M. Expression of human immunodeficiency virus antigen (HIV-Ag) in serum and cerebrospinal fluid during acute and chronic infection. Lancet. 1986 Jul 26;2(8500):177–180. doi: 10.1016/s0140-6736(86)92485-2. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Taylor M. E., Redfield R. R., Markham P. D., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Parks E. S., Parks W. P. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science. 1986 Jun 20;232(4757):1548–1553. doi: 10.1126/science.3012778. [DOI] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Schooley R. T., Kaplan J. C., Allan J. D., Groopman J. E., Resnick L., Felsenstein D., Andrews C. A., Hirsch M. S. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N Engl J Med. 1985 Dec 12;313(24):1493–1497. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman S., Jannoun-Nasr R., York D., Luciw P. A., Robinson R., Srinivasan A. Homologous recombination between human immunodeficiency viral DNAs in cultured human cells: analysis of the factors influencing recombination. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1051–1060. doi: 10.1016/s0006-291x(88)80981-1. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Kucherlapati R. S., Eves E. M., Song K. Y., Morse B. S., Smithies O. Homologous recombination between plasmids in mammalian cells can be enhanced by treatment of input DNA. Proc Natl Acad Sci U S A. 1984 May;81(10):3153–3157. doi: 10.1073/pnas.81.10.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Cheng-Mayer C., Dina D., Luciw P. A. AIDS retrovirus (ARV-2) clone replicates in transfected human and animal fibroblasts. Science. 1986 May 23;232(4753):998–1001. doi: 10.1126/science.3010461. [DOI] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Model for homologous recombination during transfer of DNA into mouse L cells: role for DNA ends in the recombination process. Mol Cell Biol. 1984 Jun;4(6):1020–1034. doi: 10.1128/mcb.4.6.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. K., Temin H. M. High-efficiency ligation and recombination of DNA fragments by vertebrate cells. Science. 1983 May 6;220(4597):606–609. doi: 10.1126/science.6301012. [DOI] [PubMed] [Google Scholar]
- Newell A. L., Malkovsky M., Orr D., Taylor-Robinson D., Dalgleish A. G. Antigen test versus reverse transcriptase assay for detecting HIV. Lancet. 1987 Nov 14;2(8568):1146–1147. doi: 10.1016/s0140-6736(87)91570-4. [DOI] [PubMed] [Google Scholar]
- Pomerantz B. J., Naujokas M., Hassell J. A. Homologous recombination between transfected DNAs. Mol Cell Biol. 1983 Sep;3(9):1680–1685. doi: 10.1128/mcb.3.9.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Quinn T. C., Mann J. M., Curran J. W., Piot P. AIDS in Africa: an epidemiologic paradigm. Science. 1986 Nov 21;234(4779):955–963. doi: 10.1126/science.3022379. [DOI] [PubMed] [Google Scholar]
- Rabson A. B., Martin M. A. Molecular organization of the AIDS retrovirus. Cell. 1985 Mar;40(3):477–480. doi: 10.1016/0092-8674(85)90189-8. [DOI] [PubMed] [Google Scholar]
- Rando R. F., Pellett P. E., Luciw P. A., Bohan C. A., Srinivasan A. Transactivation of human immunodeficiency virus by herpesviruses. Oncogene. 1987 Mar;1(1):13–18. [PubMed] [Google Scholar]
- Rubnitz J., Subramani S. The minimum amount of homology required for homologous recombination in mammalian cells. Mol Cell Biol. 1984 Nov;4(11):2253–2258. doi: 10.1128/mcb.4.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag M. S., Hahn B. H., Gibbons J., Li Y., Parks E. S., Parks W. P., Shaw G. M. Extensive variation of human immunodeficiency virus type-1 in vivo. Nature. 1988 Aug 4;334(6181):440–444. doi: 10.1038/334440a0. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Popovic M., Sarngadharan M. G., Orndorff S., Fladagar A., Patel A., Gold J., Gallo R. C. Isolation of infectious human T-cell leukemia/lymphotropic virus type III (HTLV-III) from patients with acquired immunodeficiency syndrome (AIDS) or AIDS-related complex (ARC) and from healthy carriers: a study of risk groups and tissue sources. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5530–5534. doi: 10.1073/pnas.82.16.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G. M., Hahn B. H., Arya S. K., Groopman J. E., Gallo R. C., Wong-Staal F. Molecular characterization of human T-cell leukemia (lymphotropic) virus type III in the acquired immune deficiency syndrome. Science. 1984 Dec 7;226(4679):1165–1171. doi: 10.1126/science.6095449. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Harper M. E., Hahn B. H., Epstein L. G., Gajdusek D. C., Price R. W., Navia B. A., Petito C. K., O'Hara C. J., Groopman J. E. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science. 1985 Jan 11;227(4683):177–182. doi: 10.1126/science.2981429. [DOI] [PubMed] [Google Scholar]
- Small J., Scangos G. Recombination during gene transfer into mouse cells can restore the function of deleted genes. Science. 1983 Jan 14;219(4581):174–176. doi: 10.1126/science.6294829. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Dayton A., Terwilliger E., Haseltine W. A second post-transcriptional trans-activator gene required for HTLV-III replication. Nature. 1986 May 22;321(6068):412–417. doi: 10.1038/321412a0. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Rosen C., Wong-Staal F., Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985 Jul 5;229(4708):74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- Srinivasan A., Anand R., York D., Ranganathan P., Feorino P., Schochetman G., Curran J., Kalyanaraman V. S., Luciw P. A., Sanchez-Pescador R. Molecular characterization of human immunodeficiency virus from Zaire: nucleotide sequence analysis identifies conserved and variable domains in the envelope gene. Gene. 1987;52(1):71–82. doi: 10.1016/0378-1119(87)90396-9. [DOI] [PubMed] [Google Scholar]
- Srinivasan A., York D., Anand R., Goldsmith C. S., Bohan C., Kalyanaraman V. S. Rodent and primate monolayer cells support the expression and assembly of viral particles directed by human immunodeficiency virus (HIV) proviral DNA. Microbios. 1987;50(203):81–90. [PubMed] [Google Scholar]
- Srinivasan A., York D., Butler D., Jr, Jannoun-Nasr R., Getchell J., McCormick J., Ou C. Y., Myers G., Smith T., Chen E. Molecular characterization of HIV-1 isolated from a serum collected in 1976: nucleotide sequence comparison to recent isolates and generation of hybrid HIV. AIDS Res Hum Retroviruses. 1989 Apr;5(2):121–129. doi: 10.1089/aid.1989.5.121. [DOI] [PubMed] [Google Scholar]
- Srinivasan A., York D., Ranganathan P., Ferguson R., Butler D., Jr, Feorino P., Kalyanaraman V., Jaffe H., Curran J., Anand R. Transfusion-associated AIDS: donor-recipient human immunodeficiency virus exhibits genetic heterogeneity. Blood. 1987 Jun;69(6):1766–1770. [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- Upcroft P., Carter B., Kidson C. Mammalian cell function mediating recombination of genetic elements. Nucleic Acids Res. 1980 Dec 11;8(23):5835–5844. doi: 10.1093/nar/8.23.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake C. T., Gudewicz T., Porter T., White A., Wilson J. H. How damaged is the biologically active subpopulation of transfected DNA? Mol Cell Biol. 1984 Mar;4(3):387–398. doi: 10.1128/mcb.4.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake C. T., Vernaleone F., Wilson J. H. Topological requirements for homologous recombination among DNA molecules transfected into mammalian cells. Mol Cell Biol. 1985 Aug;5(8):2080–2089. doi: 10.1128/mcb.5.8.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Saint Vincent B. R., Wahl G. M. Homologous recombination in mammalian cells mediates formation of a functional gene from two overlapping gene fragments. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2002–2006. doi: 10.1073/pnas.80.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]