Abstract
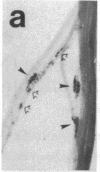
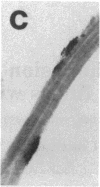
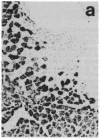
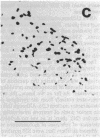
Many adult avian muscles contain two types of muscle fiber: those that receive innervation at single focal terminals and those with multiple terminals. The muscles of the syrinx, the vocal organ of birds, are such mixed muscles. To study this heterogeneity of fiber type and innervation, we combined immunocytochemistry to classify muscle fibers with techniques to visualize neuromuscular junctions. One monoclonal antibody, S58, directed against a slow class of myosin, labels only fibers that have multiple terminals. We also examined the distribution of immunoreactivity for neural cell adhesion molecule (N-CAM), which has been suggested to play a role in innervation of muscle and formation of neuromuscular junctions. S58-positive fibers have elevated N-CAM staining, indicating that multiple innervation of a fiber is correlated with the fiber's expression of high levels of N-CAM immunoreactivity. Most, and perhaps all, fibers that have multiple terminals also contain abundant N-CAM immunoreactivity. This suggests that N-CAM may play a role in the maintenance of multiterminal innervation in adult innervated muscle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore C. R., Doerr L. Postnatal development of fiber types in normal and dystrophic skeletal muscle of the chick. Exp Neurol. 1971 Mar;30(3):431–446. doi: 10.1016/0014-4886(71)90144-0. [DOI] [PubMed] [Google Scholar]
- Ashmore C. R., Kikuchi T., Doerr L. Some observations on the innervation patterns of different fiber types of chick muscle. Exp Neurol. 1978 Jan 15;58(2):272–284. doi: 10.1016/0014-4886(78)90140-1. [DOI] [PubMed] [Google Scholar]
- Barnard E. A., Lyles J. M., Pizzey J. A. Fibre types in chicken skeletal muscles and their changes in muscular dystrophy. J Physiol. 1982 Oct;331:333–354. doi: 10.1113/jphysiol.1982.sp014375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby J. L., Reichardt L. F. Effects of antibodies to neural cell adhesion molecule (N-CAM) on the differentiation of neuromuscular contacts between ciliary ganglion neurons and myotubes in vitro. Dev Biol. 1987 Feb;119(2):363–372. doi: 10.1016/0012-1606(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Covault J., Merlie J. P., Goridis C., Sanes J. R. Molecular forms of N-CAM and its RNA in developing and denervated skeletal muscle. J Cell Biol. 1986 Mar;102(3):731–739. doi: 10.1083/jcb.102.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J., Sanes J. R. Distribution of N-CAM in synaptic and extrasynaptic portions of developing and adult skeletal muscle. J Cell Biol. 1986 Mar;102(3):716–730. doi: 10.1083/jcb.102.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J., Sanes J. R. Neural cell adhesion molecule (N-CAM) accumulates in denervated and paralyzed skeletal muscles. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4544–4548. doi: 10.1073/pnas.82.13.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson G., Gower H. J., Barton C. H., Prentice H. M., Elsom V. L., Moore S. E., Cox R. D., Quinn C., Putt W., Walsh F. S. Human muscle neural cell adhesion molecule (N-CAM): identification of a muscle-specific sequence in the extracellular domain. Cell. 1987 Sep 25;50(7):1119–1130. doi: 10.1016/0092-8674(87)90178-4. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- Fedde M. R. Electrical properties and acetylcholine sensitivity of singly and multiply innervated avian muscle fibers. J Gen Physiol. 1969 May;53(5):624–637. doi: 10.1085/jgp.53.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBORG B. L. Some properties of avian skeletal muscle fibres with multiple neuromuscular junctions. J Physiol. 1960 Dec;154:581–598. doi: 10.1113/jphysiol.1960.sp006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A. Vertebrate slow muscle fibers. Physiol Rev. 1970 Jan;50(1):40–62. doi: 10.1152/physrev.1970.50.1.40. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J. THE LOCALIZATION OF CHOLINESTERASE ACTIVITY IN RAT CARDIAC MUSCLE BY ELECTRON MICROSCOPY. J Cell Biol. 1964 Nov;23:217–232. doi: 10.1083/jcb.23.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRUGER P., GUNTHER P. G. Innervation und pharmakologisches Verhalten des M. gastrocnemius und M. pectoralis maior der Vogel. Acta Anat (Basel) 1958;33(4):325–338. [PubMed] [Google Scholar]
- KUFFLER S. W., VAUGHAN WILLIAMS E. M. Small-nerve junctional potentials; the distribution of small motor nerves to frog skeletal muscle, and the membrane characteristics of the fibres they innervate. J Physiol. 1953 Aug;121(2):289–317. doi: 10.1113/jphysiol.1953.sp004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay B. K., Schwartz L. M., Rutishauser U., Qiu T. H., Peng H. B. Patterns of N-CAM expression during myogenesis in Xenopus laevis. Development. 1988 Jul;103(3):463–471. doi: 10.1242/dev.103.3.463. [DOI] [PubMed] [Google Scholar]
- Kopriwa B. M., Moss F. P. A radioautographic technique for whole mounts of muscle fibers. J Histochem Cytochem. 1971 Jan;19(1):51–55. doi: 10.1177/19.1.51. [DOI] [PubMed] [Google Scholar]
- Landmesser L., Dahm L., Schultz K., Rutishauser U. Distinct roles for adhesion molecules during innervation of embryonic chick muscle. Dev Biol. 1988 Dec;130(2):645–670. doi: 10.1016/0012-1606(88)90358-2. [DOI] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Further studies on the control of ACh sensitivity by muscle activity in the rat. J Physiol. 1975 Nov;252(3):603–626. doi: 10.1113/jphysiol.1975.sp011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrke G., Jockusch H., Schachner M. Development of mammalian nerve-muscle synapses in culture: lack of interference by antibodies to the neural cell adhesion molecule N-CAM and its L2/HNK-1 carbohydrate epitope. Neurosci Lett. 1987 Aug 5;78(3):247–252. doi: 10.1016/0304-3940(87)90368-5. [DOI] [PubMed] [Google Scholar]
- Miller J. B., Crow M. T., Stockdale F. E. Slow and fast myosin heavy chain content defines three types of myotubes in early muscle cell cultures. J Cell Biol. 1985 Nov;101(5 Pt 1):1643–1650. doi: 10.1083/jcb.101.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. E., Walsh F. S. Specific regulation of N-CAM/D2-CAM cell adhesion molecule during skeletal muscle development. EMBO J. 1985 Mar;4(3):623–630. doi: 10.1002/j.1460-2075.1985.tb03675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F., Stokes T. M., Leonard C. M. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976 Feb 15;165(4):457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Reinach F. C., Masaki T., Shafiq S., Obinata T., Fischman D. A. Isoforms of C-protein in adult chicken skeletal muscle: detection with monoclonal antibodies. J Cell Biol. 1982 Oct;95(1):78–84. doi: 10.1083/jcb.95.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger F., Grumet M., Edelman G. M. N-CAM at the vertebrate neuromuscular junction. J Cell Biol. 1985 Jul;101(1):285–293. doi: 10.1083/jcb.101.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger F., Nicolet M., Pinçon-Raymond M., Murawsky M., Levi G., Edelman G. M. Distribution and role in regeneration of N-CAM in the basal laminae of muscle and Schwann cells. J Cell Biol. 1988 Aug;107(2):707–719. doi: 10.1083/jcb.107.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins N., Olek A., Kelly S. S., Takach P., Christopher M. Quantitative study of motor endplates in muscle fibres dissociated by a simple procedure. Proc R Soc Lond B Biol Sci. 1980 Oct 15;209(1177):555–562. doi: 10.1098/rspb.1980.0112. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Acheson A., Hall A. K., Mann D. M., Sunshine J. The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science. 1988 Apr 1;240(4848):53–57. doi: 10.1126/science.3281256. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Grumet M., Edelman G. M. Neural cell adhesion molecule mediates initial interactions between spinal cord neurons and muscle cells in culture. J Cell Biol. 1983 Jul;97(1):145–152. doi: 10.1083/jcb.97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Schachner M., Covault J. Expression of several adhesive macromolecules (N-CAM, L1, J1, NILE, uvomorulin, laminin, fibronectin, and a heparan sulfate proteoglycan) in embryonic, adult, and denervated adult skeletal muscle. J Cell Biol. 1986 Feb;102(2):420–431. doi: 10.1083/jcb.102.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosney K. W., Watanabe M., Landmesser L., Rutishauser U. The distribution of NCAM in the chick hindlimb during axon outgrowth and synaptogenesis. Dev Biol. 1986 Apr;114(2):437–452. doi: 10.1016/0012-1606(86)90208-3. [DOI] [PubMed] [Google Scholar]
- Vicario D. S., Nottebohm F. Organization of the zebra finch song control system: I. Representation of syringeal muscles in the hypoglossal nucleus. J Comp Neurol. 1988 May 15;271(3):346–354. doi: 10.1002/cne.902710305. [DOI] [PubMed] [Google Scholar]