Arsenic- and platinum-based drugs are highly potent but also toxic agents used in cancer therapy.[1, 2] Arsenic trioxide (Trisenox®, As2O3) is a front-line drug for treatment of acute promyelocytic leukemia[3] and is in clinical trials for treatment of other malignancies, including multiple myeloma.[4] However, clinical outcomes of As2O3 in solid tumors have been poor in many cases,[1, 5] mainly due to limited bioavailability of the drug in the tumor site. Clinical application to solid tumors is also impeded by toxicity including neutropenia, liver failure and cardiac toxicity[1, 6] at higher doses.[7] Cisplatin (cis-diamine dichloroplatinumII, cisPt, Figure 1a) is commonly used in the treatment of a variety of solid tumors, including lung, ovarian, bladder, and testicular cancer.[2] The active intracellular species appear to be the hydrolyzed monoaqua- and diaqua-cisplatin (aqua-cisPt, Figure 1a).[8] Broader therapeutic applications of cisPt are limited by serious systemic toxicities, development of drug resistance, and rapid inactivation of the drug due to complexation with plasma and tissue proteins.[2, 8] These problems can be reduced by using a drug delivery system that prevents drug deactivation, extends the circulation time of drug in blood and increases its accumulation at tumor sites.[9] Lipid-based carriers have been successfully applied in clinics for improving the therapeutic efficacy of numerous drugs, such as liposomal doxorubicin (Doxil®),[10] mainly via the enhanced permeability and retention (EPR) effects.[9]
Figure 1.
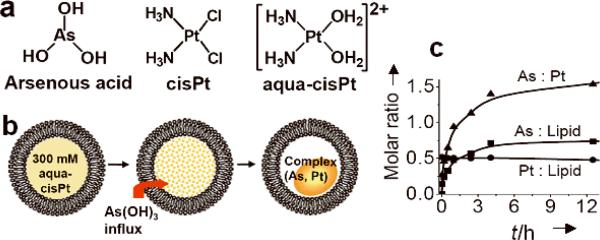
a) Structures of arsenic and platinum drugs related in this study. b) Scheme of arsenic loading into a liposome in response to a transmembrane gradient of aqua-cisPt acetate ([cis-(NH3)2Pt(OH2)2]2+). c) The kinetics of arsenic loading into liposomes (DPPC/DOPG/Chol = 51.4/3.6/45 mol%) using 300 mM aqua-cisPt acetate solution (pH 5.1) as intraliposomal medium with an initial As-to-lipid molar ratio of 4.0 at 50°C.
Several liposomal formulations of cisPt have been prepared, including STEALTH SPI-077,[11] and negative-lipid coated cisPt nanocapsules;[12] however, their clinical applications have been hindered by low encapsulation efficiencies (0.02 Pt-to-lipid molar ratio) which limits bioavailability,[13, 14] and poor serum stability (lifetime < 1 hour).[15] Preparations of liposomal As2O3 have also faced challenges because the neutral As(OH)3 species (which is predominant at pH < 9.0)[16] diffuses readily across lipid membranes,[17] thus making stable drug-encapsulation difficult.[18] Recently, the latter issues were overcome via development of an efficient system for loading high densities of As2O3 nanoparticles into liposomes (0.5 drug-to-lipid molar ratio) with excellent retention (shelf life > 6 months) and good serum stability.[17, 19] This system employs transmembrane gradients of transition metal ions to produce As2O3-nanoparticles within liposomes. These nanoparticulate forms of the drug encapsulated in liposomes (denoted as “nanobin” for short) exhibit enhanced anticancer efficacy relative to the parent drug in both breast cancer and lymphoma xenograft, as well as reduced systemic toxicity (R. W. Ahn, et al. we shall report on this in detail later). Here we report on expanding this strategy to the stable and efficient coencapsulation of both arsenic- and platinum-based drugs in a single vesicle. This approach ameliorates systemic toxicity of both drugs and allows for enhanced antitumor efficacy and specificity through targeted delivery of anticancer drug combinations to specific tumor cells.
We first prepared 300 mM aqua-cisPt acetate (pH 5.1, with cis-(NH3)2Pt(OH2)2]2+ species predominant[20]) as previously described.[21] The dry lipid film (dipalmitoylphosphatidylcholine(DPPC)/dioleoylphosphatidylglycerol(DOPG)/cholesterol(Chol) = 51.4/3.6/45 mol%) was hydrated in this solution and extruded to 100-nm diameter (Figures 1 and S1). The acetate solution of aqua-cisPt was chosen as the intraliposomal medium because we previously found that acetate anions in the liposomal nickel acetate were critical for arsenic loading.[17] Other platinum complexes, such as monoaqua-cisPt acetate and aqua-cisPt carbonate, have too low aqueous solubility (< 15 mM) to be used here. The extraliposomal platinum was then removed by gel filtration, forming a gradient of aqua-cisPt acetate from the internal to the external aqueous phase of the liposomes (Figure S1). Addition of concentrated As2O3 solution at pH 6.6, resulted in active loading of As(OH)3 into the liposome with t1/2 ≈ 40 min at 50°C, giving final molar ratios of As/lipid 0.63 ± 0.05 and Pt/lipid 0.48 ± 0.06 after 11 h (n = 7, the number of independent experiments). The nanoparticle formation mechanism appears to involve in the influx of As(OH)3 followed by complexation with aqua-cisPt species (Figure 1b), as occured in the arsenic loading process for liposomal nickel acetate.[17] [22] The extraliposomal pH dropped from 6.6 to 6.0 after 11 h, indicating proton release from liposomal cores that may be through deprotonation of aqua-cisPt/As(OH)3 upon coordination. Quantitative analysis indicate that one liposomal core (liposome mean size = 112 ± 9 nm) can be loaded with ~12 × 104 As atoms and ~9 × 104 Pt atoms, with intraliposomal concentratrions of ~390 mM arsenic and ~300 mM platinum via this method (see Supplementary Information). Large scale uniform batches of arsenic and platinum co-loaded liposomes, denoted as NB(As, Pt), were prepared at various drug payload levels depending on concentration of aqua-cisPt acetate (Figures S1–2). The aqua-cisPt loaded liposomes are denoted as NB(Pt).
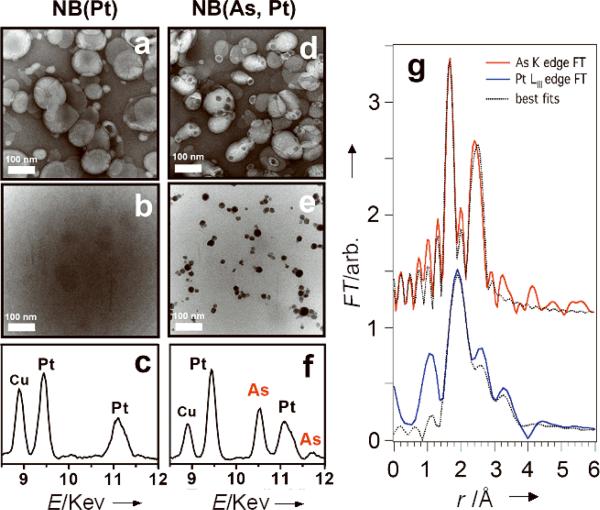
The transmission electron microscopy (TEM) and energy-dispersive X-ray analysis (EDX)[23] reveal the presence of As and Pt cores within NB(As, Pt) with high electronic density (Figure 2e). We isolated these inorganic nanoparticles (pale yellow, air-stable) from the lipid shell, allowing for analysis by X-ray photoelectron spectroscopy (XPS, Figure S10 and Table S3). The XPS As(3d) (44.9 ± 0.5 eV) and Pt(4f7/2) (73.5 ± 0.4 eV) chemical shifts indicated that the As and Pt species remained AsIII and PtII. The NB(As, Pt) in the aqueous buffer were also analyzed by X-ray absorption spectroscopy (XAS). The X-ray absorption edge energies corroborate the XPS oxidation state assignments of AsIII and PtII, and extended X-ray absorption fine structure (EXAFS) data provide direct evidence for an intimate interaction between As(OH)3 and aqua-cisPt within the liposome (Figures 2g and S11, best fit results summarized in Tables S4–5). The As K edge EXAFS is modeled by an As-O shell at 1.72 Å and an As-Pt shell at 2.33 Å. Because the As K edge overlaps the Pt LIII EXAFS and prevents the collection of Pt LIII data to high k, we also measured Pt LII EXAFS. Both the LIII and LII data are consistent with the As EXAFS; fits to the LIII data give a Pt-(N/O) shell at 2.00 Å and a Pt-As shell at 2.32 Å (Table S5). The fact that As-Pt and Pt-As both have apparent coordination numbers of ~1 indicates that most of the As and Pt atoms are in a dinuclear complex. The short As-Pt distances indicate As(OH)3 is coordinated to Pt through As where the AsIII donated its lone pair to form a Lewis adduct with PtII (Figure S11b). Similar coordinations of As(OH)3 as a lewis base to metal ions have been reported for other heterometallic complexes, including [Mo3PdAs(OH)3S4Cl3-(OH2)6],[24] [W3(NiAs(OH)3)S4(H2O)8Cl]3+,[25] and arsine complex [PtCl2[As(CH2CMe=CH2)3]2],[26] with the As-Pd, As-Ni and As-Pt distances of 2.23–2.37 Å. Formation of a Pt-As sigma bond upon As(OH)3 substitution for the OH2 of aqua-cisPt may partially account for the retention of AsIII within liposomes (Figure 3).
Figure 2.
TEM images and EDX spectra of NB(Pt) (a, b and c) and NB(As, Pt) (d, e and f). Samples of a and d were stained by 2% uranyl acetate; b and e are unstained, showing discrete electron-dense inorganic cores within liposomes; the single-particle EDX spectra c and f correspond to b and e, respectively, revealing Pt (c), Pt and As (f) cores. Cu peaks arise from the EM grid. (g) Phase-corrected EXAFS Fourier transforms for NB(As, Pt). DPPC/DOPG/Chol = 51.4/3.6/45 mol%.
Figure 3.
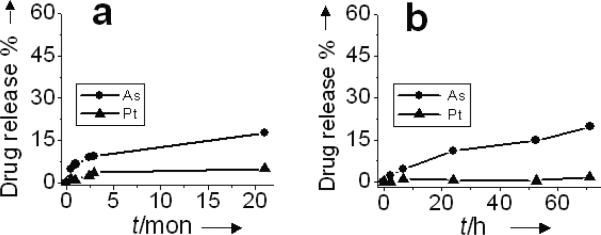
Drug release of NB(As, Pt) as the function of time under 4°C storage conditions (a), and at 37°C with 80% fetal bovine serum (FBS) (b). DPPC/DOPG/Chol = 51.4/3.6/45 mol%.
In the absence of arsenic, the aqueous solution of aqua-cisPt acetate alone was unstable with or without liposomes and changed into brown and then black after one month at 4°C, perhaps due either to photosensitivity[27] or to the formation of platinum carbonato complexes upon absorption of CO2 from air.[28] The co-encapsulation strategy described here provides a way of stably sequestering both arsenic and platinum drugs within liposomal cores: drug release studies reveal high stability of NB(As, Pt) under physiologically relevant conditions. Less than 17% of the drugs were released over a 21-month storage at 4°C (Figure 3a). When NB(As, Pt) were kept at 37°C, pH 7.4 for 3 days (Figure S3a), only 5% of the drugs were released. Although the solid complex(As, Pt) tends to hydrolyze when pH > 6.0 (Figure S9), it is less sensitive to extraliposomal pH once sequestered within the liposome.
In addition, the low drug release rate in serum suggest that NB(As, Pt) possess significant stability upon intervenous administration. The presence of a serum challenge (at pH 7.4) only slightly increased the drug release (20% drug release after 3 days at 37°C, Figure 3b, DPPC/DOPG/Chol = 51.4/3.6/45 mol%). Upon reducing membrane stability of NB(As, Pt) by lowering the cholesterol content[29] of the lipid bilayer (DPPC/DOPG/Chol mol%) from 51.4/3.6/45 to 86.4/3.6/10 and 96.4/3.6/0, we found the drug release in serum was 30% As and 13% Pt for the 86.4/3.6/10 composition (Figure S4b), and 41% As and 23% Pt for the 96.4/3.6/0 composition (Figure S4c), three times faster than those (12% As and 0.5% Pt release) of the 51.4/3.6/45 composition (Figure 3b) after 24 h at 37°C. The faster drug release led to higher cytotoxic effects of NB(As, Pt) against SU-DHL-4 human lymphoma and MDA-MB-231 human breast cancer cells (Figures S4d–e): the IC50 (As level) of the 96.4/3.6/0 composition was 4.0 μM for SU-DHL-4 and 10.5 μM for MDA-MB-231 cells, three times lower than those of the 51.4/3.6/45 composition (13.6 μM and 27.7 μM, respectively) after a 72 or 96 h treatment. The drug bio-availability appears to result from gradual dissociation of arsenic and platinum nanoparticulates into active As(OH)3 and [cis-(NH3)2Pt(OH2/OH)2]n+ species which diffuse from liposomes into cellular environments.
The activity of NB(As, Pt) with the 51.5/3.6/45 composition against a panel of human tumor cell lines of lymphoma SU-DHL-4 and IM-9, breast cancer MDA-MB-231, ovarian cancer OVCAR-3 and multiple myeloma MM.1S, was compared with those of NB(Pt), As2O3, aqua-cisPt, and cisPt under the same conditions (Tables 1 and S1, Figures S6–8). In general, both NB(As, Pt) and NB(Pt) show attenuated cytotoxicities relative to free As2O3, aqua-cisPt, and cisPt (Tables 1 and S1). This indicates that liposome coencapsulation lowers general toxicity of both arsenic and platinum drugs in vivo. Notably, NB(As, Pt) was more cytotoxic (3–7 fold) than NB(Pt), indicating the bioavailability of both arsenic and platinum species from NB(As, Pt). The cytotoxicity of NB(As, Pt) increased gradually over time, approaching those of free drugs at long incubation times (> 72 h, Figures S6–7), consistent with the gradual drug release over time at 37°C in serum (Figure 3b).
Table 1.
Cytotoxicity (IC50) of various platinum and arsenic formulations to human tumor cells
| IC50 (μM)[b] |
||||
|---|---|---|---|---|
| Cell lines[a] | NB(As, Pt)[c] | NB(Pt)[c] (Pt) | Aqua-cisPt (Pt) | As2O3 (As) |
| SU-DHL-4 | 20.6 ± 3.6 (As) 15.6 ± 2.8 (Pt) |
42.4 ± 15.0 | 5.5 ± 0.4 | 3.7 ± 0.2 |
| IM-9 | 5.0 ± 0.6 (As) 3.8 ± 0.4 (Pt) |
10.7± 1.9 | 1.0 ± 0.01 | 2.1 ± 0.03 |
| MDA-MB-231 | 35.0 ± 7.4 (As) 26.6 ± 5.6 (Pt) |
> 200 | 17.9 ± 0.1 | 10.0 ± 2.4 |
| OVCAR-3 | 8.8 ± 1.6 (As) 6.6 ± 1.2 (Pt) |
18.4 ± 0.6 | 2.6 ± 0.8 | 2.7 ± 1.1 |
48 h-drug treatment for SU-DHL-4 and IM-9, and 72 h-drug treatment for MDA-MB-231 and OVCAR-3 cells.
IC50 values (± SD) are based on As or Pt concentration (μM), from 2–5 independent experiments.
DPPC/DOPG/Chol = 51.4/3.6/45 mol%
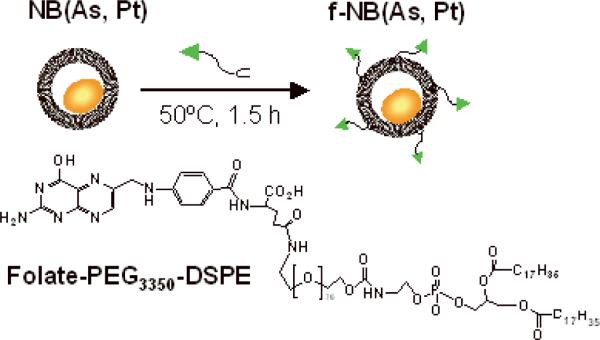
This system for co-loading arsenic and platinum drugs presents a robust platform for further optimization by conjugation with specific ligands or antibodies to target specific tumors. To examine the efficacy of a targeted version of this dual-acting agent, we used folate-PEG3350-DSPE (folic acid conjugated with polyethyleneglycol[MW3350]-derivatized distearoylphosphatidyl-ethanolamine) as a targeting ligand[30] (Figure 4). Folic acid and its derivatives have been used as effective targeting ligands for treatment of certain tumors because the folate receptor (FR) is often overexpressed in malignant tissues.[31] A small amount of folate-PEG3350-DSPE was mixed with the preformed NB(As, Pt) at an initial folate/lipid molar ratio of 0.7%, 50°C for 1.5 h (Figure 4). This allowed the two chains of the DSPE moiety to insert into the lipid shell with the Folate-PEG3350 moiety in the external leaflet of NB(As, Pt) to be available for targeting. Such a “post-insertion” process[32] caused little liposomal drug release (< 6%), and allowed for high incorporation efficiency (80%), giving a folate/lipid molar ratio of 0.56%. After post-insertion, the mean diameter (129 ± 4 nm) of folate-targeted arsenic and platinum liposomes (f-NB(As, Pt)) was incresed by 17 nm, relative to that of NB(As, Pt) (112 ± 9 nm).
Figure 4.
Preparation of folate-targeted arsenic and platinum liposomes by post-insertion of targeting ligands (Folate-PEG3350-DSPE) into NB(As, Pt). DPPC/DOPG/Chol = 51.4/3.6/45 mol%.
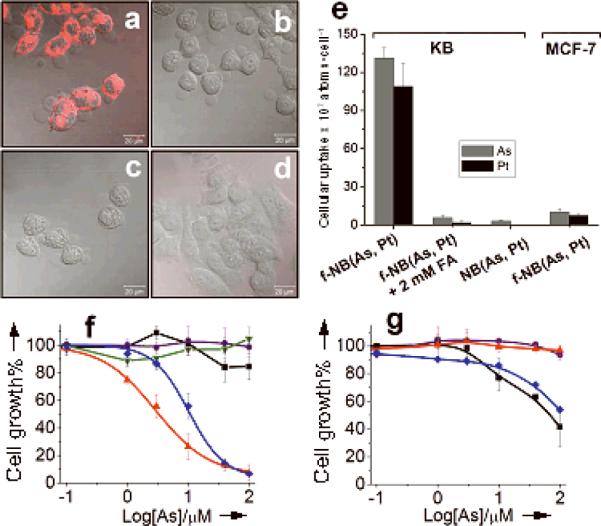
These folate-targeted f-NB(As, Pt) exhibit efficient cellular uptake and anti-cancer activities to FR-positive tumor cells. Confocal microscopy studies indicated significant uptake of f-NB(As, Pt) via FR-mediated endocytosis into FR+ KB (human nasopharyngeal), but not FR− MCF-7 (human breast) tumor cells[19, 33] after 3 h at 37°C (Figures 5a–d). Quantitative analysis also revealed substantial accumulation of f-NB(As, Pt) within KB cells (131 × 107 As atoms/cell, 109 × 107 Pt atoms/cell), 8–270 times higher than those of NB(As, Pt) (3 × 107 As atoms/cell, 0.3 × 107 Pt atoms/cell), As2O3 (17 × 107 As atoms/cell) and aqua-cisPt (0.4 × 107 Pt atoms/cell) (Figure 5e and Table S2). Accordingly, the cytoxic effect of f-NB(As, Pt) on KB cells was significantly enhanced relative to those of NB(As, Pt) (> 60 fold), As2O3 (> 60 fold), and aqua-cisPt (4 fold) at 3 h (Figure 5f). The potentiation of f-NB(As, Pt) versus NB(As, Pt) decreased at a 72-h drug treatment (Table 2), probaly due to non-specific cellular liposome association and/or passive drug release: both would contribute to the cytotoxicity of NB(As, Pt). The addition of 2 mM free folic acid (FA, as a competitive ligand for f-NB(As, Pt) targeting) resulted in a dramatic decrease of cytotoxic effects for f-NB(As, Pt) (Figure 5b, e, f), consistant with FR-mediated efficacy. In contrast, for FR− MCF-7 cells, f-NB(As, Pt) didn't show significantly increased anticancer efficacy relative to NB(As, Pt) and free drugs, consistent with its low cellular uptake (Figure 5e). As shown in Table 1, liposome encapsulation reduces general toxicity of arsenic and platinum drugs, and thus we anticipate few off-target interations and lower systemic toxicity. When such drug-loaded liposomes are further linked to a targeting ligand, such as folate, and delivered into tumor cells through receptor-mediated endocytosis (Figure 5a), an efficient cytosolic unloading of liposomal drug contents can be achieved through enzymatic degradation[34] and thus accelerate the biological actions of drugs. This may account for the significantly enhanced anti-cancer efficacy of f-NB(As, Pt) relative to free drugs.
Figure 5.
Comparison of cellular drug uptake and cytotoxicity of various drug formulations. Confocal micrograghs (merged with DIC images) showing cellular uptake of (a) f-NB(As, Pt), (b) f-NB(As, Pt) + 2 mM FA, (c) NB(As, Pt) by KB cells, and of (d) f-NB(As, Pt) by MCF-7 cells after 3 h at 37°C. Liposomes were labeled with rhodamine (Rh). Scale bar: 20 μm. e) KB and MCF-7 cellular arsenic and platinum uptake. Cytotoxic effects of f-NB(As, Pt) (▲), f-NB(As, Pt) + 2 mM FA (▼), NB(As, Pt) (●), As2O3 (■) and aqua-cisPt (◆) towards KB (f) and MCF-7 (g) cells. Cells exposed to drugs at 37°C for 3 h, washed by PBS and further incubated up to 72 h in drug-free medium.
Table 2.
Cytotoxicity (IC50) of folate-targeted arsenic and platinum liposomes to human tumor cells[a]
| Formulations | KB (FR+), IC50 (μM) | MCF-7 (FR−), IC50 (μM) | ||
|---|---|---|---|---|
| 3 h | 72 h | 3 h | 72 h | |
| As2O3 | > 200 | 5.2 ± 1.0 | 63.7 ± 22.3 | 6.9 ± 1.7 |
| NB(As, Pt) | > 200 | 20.1 ± 0.03 (As) 15.8 ± 0.02 (Pt) |
> 200 | 40.8 ± 1.3 (As) 32.1 ± 1.1 (Pt) |
| f-NB(As, Pt) | 3.3 ± 1.4 (As) 2.6 ± 1.1 (Pt) |
1.2 ± 0.01 (As) 1.0 ± 0.01 (Pt) |
> 200 | 34.8 ± 4.5 (As) 27.4 ± 3.5 (Pt) |
| f-NB(As, Pt) + 2 mM FA | > 200 | 13.1 ± 1.0 (As) 10.3 ± 0.7 (Pt) |
— | — |
| Aqua-cisPt | 9.7 ± 2.5 | 1.3 ± 0.2 | 88.9 ± 14.4 | 12.2 ± 0.4 |
Cells were incubated with drugs continuously for 72 h, or exposed to drugs for 3 h, then washed and further incubated up to 72 h at 37°C in drug-free medium before IC50 measurement. IC50 values (± SD) are based on As or Pt concentration (μM), from 2–3 independent experiments.
In summary, we have developed a strategy for efficiently co-encapsulating arsenic- and platinum-based drugs into 100–nm–scale liposomes by combining passive and active loading methods. The nanoparticulate form of two drugs within liposomes is stabilized by a new type of AsIII-PtII adduct. The resultant therapeutic agent shows a long shelf life (< 17% release over a 21-month period at 4°C) and is stable in serum (< 20% drug release after 72 h at 37 °C), suggesting a long circulation time in vivo. Coordinating the release rates of both drugs can be achieved by altering the lipid composition, which thus modulates the bioactivity of NB(As, Pt). These stably co-encapsulated arsenic and platinum drugs exhibit attenuated cellular toxicity towards both hematological cancer and solid tumors derived cells, relative to free As2O3, aqua-cisPt, and cisPt. Targeted delivery of co-encapsulated arsenic and platinum drugs by folate significantly enhanced the cellular uptake and thus anti-cancer efficacy against FR-overexpressing tumor cells. Such arsenic and platinum “nanobins” can be used for direct therapeutic trials and also for further surface modifications with other targeting agents that recognize specific types of cancer, and hold the promise of improving the therapeutic index and profile for both drugs. Further modification with a pH-sensitive polymer coating[35] presents an alternative approach for optimizing the bioactivity of co-encapsulated agents. The coencapsulation strategy developed in this report will be expanded to other conventional chemotherapeutic drugs and their synergistic agents.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (GM054111 and GM038047), the Center of Cancer Nanotechnology Excellence (U54CA119341), the CDMRP Breast Cancer Research Program (BC073413 and BC076723). Thanks to Prof. David H. Thompson (Purdue University) for providing folate-PEG3350-DSPE, Rebecca Marvin for help with ICP-MS, Prof. Robert C. MacDonald for critical reading, Sang-Min Lee and Dr. Mala Shanmugam for advice.
Footnotes
Supporting information for this article is available on http://www.angewandte.org or from the author.
References
- [1].Dilda PJ, Hogg PJ. Cancer Treat. Rev. 2007;33:542–564. doi: 10.1016/j.ctrv.2007.05.001. [DOI] [PubMed] [Google Scholar]
- [2].Kelland L. Nature Reviews Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- [3].Wang ZY, Chen Z. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- [4].Berenson R, Yeh HS. Clin. Lymphoma Myeloma. 2006;7:192–198. doi: 10.3816/CLM.2006.n.058. [DOI] [PubMed] [Google Scholar]
- [5].Chen Z, Chen GQ, Shen ZX, Sun GL, Tong JH, Wang ZY, Chen SJ. Semin. Hematol. 2002;39:22–26. doi: 10.1053/shem.2002.33611. [DOI] [PubMed] [Google Scholar]
- [6].Evens AM, Tallman MS, Gartenhaus RB. Leuk. Res. 2004;28:891–900. doi: 10.1016/j.leukres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- [7].Liu B, Pan S, Dong X, Qiao H, Jiang H, Krissansen GW, Sun X. Cancer Sci. 2006;97:675–681. doi: 10.1111/j.1349-7006.2006.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang D, Lippard SJ. Nat. Rev. Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- [9].Allen TM, Cullis PR. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- [10].Gabizon AA. Cancer Invest. 2001;19:424–436. doi: 10.1081/cnv-100103136. [DOI] [PubMed] [Google Scholar]
- [11].Peleg-Shulman T, Gibson D, Cohen R, Abra R, Barenholz Y. Biochim. Biophys. Acta. 2001;1510:278–291. doi: 10.1016/s0005-2736(00)00359-x. [DOI] [PubMed] [Google Scholar]
- [12].Burger Koert NJ, Staffhorst Rutger WHM, de Vijlder Hanke C, Velinova Maria J, Bomans Paul H, Frederik Peter M, de Kruijff B. Nat Med. 2002;8:81–84. doi: 10.1038/nm0102-81. [DOI] [PubMed] [Google Scholar]
- [13].Harrington KJ, Lewanski CR, Northcote AD, Whittaker J, Wellbank H, Vile RG, Peters AM, Stewart JS. Ann. Oncol. 2001;12:493–496. doi: 10.1023/a:1011199028318. [DOI] [PubMed] [Google Scholar]
- [14].Bandak S, Goren D, Horowitz A, Tzemach D, Gabizon A. Anti-Cancer Drugs. 1999;10:911–920. doi: 10.1097/00001813-199911000-00007. [DOI] [PubMed] [Google Scholar]
- [15].Velinova MJ, Staffhorst RWHM, Mulder WJM, Dries AS, Jansen BAJ, de Kruijff B, de Kroon AIPM. Biochimica et Biophysica Acta, Biomembranes. 2004;1663:135–142. doi: 10.1016/j.bbamem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- [16].Ni Dhubhghaill OM, Sadler PJ. Struct. Bonding (Berlin) 1991;78:129–190. [Google Scholar]
- [17].Chen H, MacDonald RC, Li S, Krett NL, Rosen ST, O'Halloran TV. Journal of the American Chemical Society. 2006;128:13348–13349. doi: 10.1021/ja064864h. [DOI] [PubMed] [Google Scholar]
- [18].Kallinteri P, Fatouros D, Klepetsanis P, Antimisiaris SG. J. Liposome Res. 2004;14:27–38. doi: 10.1081/lpr-120039661. [DOI] [PubMed] [Google Scholar]
- [19].Chen H, Ahn R, Bossche J. V. d., Thompson DH, O'Halloran TV. Mol. Cancer Ther. 2009;8(7) doi: 10.1158/1535-7163.MCT-09-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Berners-Price SJ, Frenkiel TA, Frey U, Ranford JD, Sadler PJ. J. Chem. Soc., Chem. Commun. 1992:789–791. [Google Scholar]
- [21].Appleton TG, Berry RD, Davis CA, Hall JR, Kimlin HA. Inorg. Chem. 1984;23:3514–3521. [Google Scholar]
- [22].Lide D. Handbook of Chemistry and Physics. 2002;83rd(8):46–48. [Google Scholar]
- [23].Meldrum FC, Wade VJ, Nimmo DL, Heywood BR, Mann S. Nature. 1991;349:684–687. [Google Scholar]
- [24].Sokolov MN, Virovets AV, Dybtsev DN, Chubarova EV, Fedin VP, Fenske D. Inorg. Chem. 2001;40:4816–4817. doi: 10.1021/ic015520p. [DOI] [PubMed] [Google Scholar]
- [25].Hernandez-Molina R, Sokolov MN, Clausen M, Clegg W. Inorg. Chem. 2006;45:10567–10575. doi: 10.1021/ic061146n. [DOI] [PubMed] [Google Scholar]
- [26].Phadnis PP, Jain VK, Klein A, Schurr T, Kaim W. New J. Chem. 2003;27:1584–1591. [Google Scholar]
- [27].Perumareddi JR, Adamson AW. J. Phys. Chem. 1968;72:414–420. [Google Scholar]
- [28].Lippert B, Lock CJL, Rosenberg B, Zvagulis M. Inorg. Chem. 1978;17:2971–2975. [Google Scholar]
- [29].New RRC. In: Rickwood D, editor. Oxford University Press; New York: 1990. pp. 19–22. [Google Scholar]
- [30].Rui Y, Wang S, Low PS, Thompson DH. J. Am. Chem. Soc. 1998;120:11213–11218. [Google Scholar]
- [31].Low PS, Henne WA, Doorneweerd DD. Acc. Chem. Res. 2008;41:120–129. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- [32].Allen TM, Sapra P, Moase E. Cell. Mol. Biol. Lett. 2002;7:889–894. [PubMed] [Google Scholar]
- [33].Sonvico F, Dubernet C, Marsaud V, Appel M, Chacun H, Stella B, Renoir M, Colombo P, Couvreur P. J. Drug Del. Sci. Tech. 2005;15:407–410. [Google Scholar]
- [34].Bareford M, Swaan PW. Adv. Drug. Deliv. Rev. 2007;59:748–758. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lee S-M, Chen H, Dettmer CM, O'Halloran TV, Nguyen ST. Journal of the American Chemical Society. 2007;129:15096–15097. doi: 10.1021/ja070748i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.