Abstract
Malignant diseases of the upper gastrointestinal tract are common and often diagnosed at a point when the opportunity for curative surgical resection has passed. Symptoms of luminal obstruction include nausea, vomiting, weight loss, pain, pruritis and jaundice. The median survival of patients who cannot be cured surgically is extremely short, with a duration of only a few months. Effective palliative techniques with a low morbidity and associated mortality are required. The length of hospital stay, rapid recovery and reduction in recurrent symptoms are important factors for patients and doctors to consider when planning treatment. Traditionally, surgical techniques were used, but in the last 20 years the availability of both endoscopic and interventional radiological procedures has increased. Furthermore, advances in technology such as the development of self-expanding metal stents and covered stent designs have provided more therapeutic options for the endoscopist and radiologist. Here we discuss the available treatments for the palliation of gastric outlet and biliary tract obstruction and the evidence for the respective approaches.
Keywords: Endoscopic stents, gastric outlet obstruction, gastrojejunostomy, pancreatic cancer
Statement of Search Strategies Used and Sources of Information
For the purposes of the review, a literature search was carried out using Medline and the Cochrane Library for studies between 1985 and 2010. The following search headings were used: ‘palliative duodenal stenting’, ‘palliative biliary stenting’, ‘surgical palliation of gastric outlet obstruction’, ‘surgical palliation of malignant biliary obstruction’, ‘surgery versus stenting in gastric outlet obstruction’, ‘surgery versus stenting in malignant biliary obstruction’ and ‘radiological stenting for biliary obstruction’. The ‘related articles’ function was used to broaden the search and all abstracts, studies and citations were reviewed. No language restrictions were imposed. The references from articles were also used. The date of the most recent search was July 2010.
Introduction
Malignant diseases of the upper gastrointestinal tract are common and often diagnosed at a point when the opportunity for curative surgical resection has passed. Because of their involvement of luminal structures, obstructive symptoms from disruption to the flow of gut contents or biliary tract secretions are frequent. As such, palliative procedures aim to improve quality of life and short-term survival when such complications occur. Over the last 20 years, options for palliation of upper gastrointestinal malignancies have increased. Whereas previously only the realm of open surgery, endoscopic, interventional radiological and laparoscopic surgical techniques have become commonplace. The development of flexible self-expanding metal stents (SEMS) has allowed accurate deployment across strictures. Advances in imaging technology have enabled better localisation and planning of palliative procedures.
However, although palliation for oesophageal cancer is now almost exclusively non-surgical, endoscopic and radiological techniques for the relief of gastric outlet and biliary obstruction are not the sole modes of palliation. Here we review the various approaches for the palliation of malignancies causing obstruction to the gastric outlet and biliary tree, with a particular emphasis on evidence from randomised controlled trials.
For the purposes of the review, a literature search was carried out using Medline and the Cochrane Library for studies between 1985 and 2010. The following search headings were used: ‘palliative duodenal stenting’, ‘palliative biliary stenting’, ‘surgical palliation of gastric outlet obstruction’, ‘surgical palliation of malignant biliary obstruction’, ‘surgery versus stenting in gastric outlet obstruction’, ‘surgery versus stenting in malignant biliary obstruction’ and ‘radiological stenting for biliary obstruction’. The ‘related articles’ function was used to broaden the search and all abstracts, studies and citations were reviewed. No language restrictions were imposed. The references from articles were also used. The date of the most recent search was July 2010.
Palliation of Gastric Outlet Obstruction
Gastric outlet obstruction (GOO) from malignant disease may occur as a consequence of intraluminal obstruction, tumour ingrowth from surrounding structures or extrinsic compression. Pancreatic cancer most commonly causes GOO with an incidence of 10–20% in published studies [1]. Gastric and biliary tract cancers, as well as metastatic deposits to locoregional lymph nodes, and peritoneal disease are other, less common, causes. Traditionally, surgical bypass with gastrojejunostomy, carried out via laparotomy or laparoscopically, was the only possible therapeutic option to re-establish enteral feeding in this group of patients. In the last few years there has been rapid development in endotherapy devices, more specifically SEMS, to re-establish luminal patency.
Surgical Bypass Procedures for Gastric Outlet Obstruction
GOO is a complication of advanced malignant disease in the distal stomach, duodenum and pancreas [2]. Clinical symptoms include nausea and vomiting (11–50% of patients with pancreatic cancer) eventually resulting in malnutrition and dehydration, most patients with GOO are in a poor clinical condition with a greatly reduced quality of life [3]. The origins of the symptoms of GOO are due to tumour infiltration of the coeliac nerve plexus or mechanical obstruction of the duodenum by direct tumour ingrowth or extrinsic compression of the duodenum by tumour [4]. At presentation, about 5% of patients with pancreatic tumours have mechanical obstruction and 3–20% of patients with advanced cancer will develop GOO. In cases of gastric cancer, the disease is unresectable in up to 40% of patients and GOO is a common occurrence in these patients [3].
The standard treatment for GOO in these patients has been open gastrojejunostomy. In recent years, laparoscopic gastrojejunostomy has been introduced as an alternative to open surgery. Metallic stent placement has also been shown to provide safe, minimally invasive and effective palliation for patients with GOO. There are, however, few randomised trials that have compared endoscopic stenting with surgical bypass in GOO and there are none that have included a large enough sample size to assess outcomes in the context of confounding factors. Many other studies have involved small numbers of patients with inconsistent outcome measures. The use of stents in GOO is discussed below.
Palliation of GOO will depend on symptoms, overall health status, estimated survival, procedure-related morbidity and mortality and local clinical and technical expertise.
Indications
Indications for palliative bypass include clinical evidence of GOO. These will include symptoms of GOO such as nausea, vomiting and an inability to have a normal food intake. The diagnosis of unresectable cancer may be made using computed tomography and endoscopy or endoluminal ultrasound and biopsy. About 10% of patients will be deemed to have unresectable disease at the time of surgical exploration (not identified on preoperative imaging) and undergo a prophylactic surgical bypass instead of resection. These patients form a subgroup who do not have symptomatic GOO at the time of surgery. A recent meta-analysis examined the role of prophylactic gastroenterostomy in patients with unresectable pancreatic cancer [2]. Three prospective studies were included that compared patients who had a prophylactic gastroenterostomy plus biliodigestive anastomosis with those who had no bypass or a biliodigestive anastomosis alone (218 patients in total). The likelihood of GOO during follow-up was significantly lower (odds ratio 0.06; 95% confidence interval 0.02–0.21; P < 0.001) after prophylactic gastrojejunostomy. The rates of postoperative delayed gastric emptying were similar in both groups (odds ratio 1.93; 95% confidence interval 0.57–6.53; P = 0.290), as were morbidity and mortality. The estimated duration of hospital stay after prophylactic gastroenterostomy was 3 days longer than for patients without bypass (weighted mean difference 3.1; 95% confidence interval 0.7–5.5; P = 0.010). Therefore, prophylactic gastrojejunostomy should be carried out in those patients who are found to have unresectable pancreatic cancer at the time of surgical staging.
Patient selection
The median survival for patients with unresectable pancreatic cancer is 3–6 months and for advanced gastric cancer is 6–10 months [5]. It is thus essential that palliation of symptoms is achieved quickly and there is an associated improvement in the patient's quality of life. Patients with large volume metastatic disease and consequent low survival times are more likely to undergo palliative stenting for GOO, depending on local expertise. Patients with locally advanced disease and GOO may undergo either open or laparoscopic bypass or palliative stenting [4].
Surgical procedures
Open gastrojejunostomy
The abdominal cavity is accessed through a midline or rooftop incision. A side to side ante-colic or retrocolic gastrojejunostomy is fashioned using a jejunal loop 40–60 cm distal to the ligament of Treitz. The anastomosis may be hand sewn (using an absorbable continuous running suture) or stapled to either the anterior or posterior surface of the stomach. A choledochojejunostomy or hepaticojejunostomy may be carried out at the same time in the case of jaundiced patients (see below) [6,7].
Laparoscopic gastrojejunostomy
A CO2 pneumoperitoneum is established and a 30° laparoscope is used. Following a staging laparoscopy two trocars are placed to allow triangulation during surgery. The ligament of Treitz is identified and a jejunal loop 40–60 cm distal to this is used for the antecolic gastrojejunostomy. The jejunum is then anastomosed to either the anterior or posterior surface of the stomach using a linear stapler fired through openings in the stomach and jejunum. The enterostomy is then closed using absorbable sutures [8].
Outcome
Surgical bypass is almost always technically successful. It is rare that a gastroenterostomy cannot be carried out, due usually to widespread peritoneal metastases. The time taken to tolerate solid food varies, but can take 4–10 days [6,9]. However, there is a high rate of complication — up to 60% in some series [10]. Laparoscopic gastroenterostomy was developed as a minimally invasive approach to surgical bypass [11]. There is some evidence of a quicker recovery rate, but it can still be associated with a high rate of complication. The mortality rate associated with surgical bypass is high and remains so even after the introduction of laparoscopic gastroenterostomy [12–14]. This is due in part to the physical and nutritional state of the patient. The median length of stay may be over 2 weeks, but there is a low rate of late complications and re-intervention [1].
Complications
Complications after surgical bypass include anastomotic leak at the gastroenterostomy site. If the patient is stable, conservative management can be undertaken. Intraabdominal collections can be drained percutaneously. If the patient is unstable and there are signs of peritonitis, then laparotomy will be required. Delayed gastric emptying can follow surgical bypass. Further complications include sepsis, abdominal wound dehiscence, subphrenic abscess, pneumonia, bleeding anastomotic ulcer, perforation of anastomotic ulcer, deep venous thrombosis and, rarely, port site metastases for laparoscopic approaches.
Endoscopic Stenting
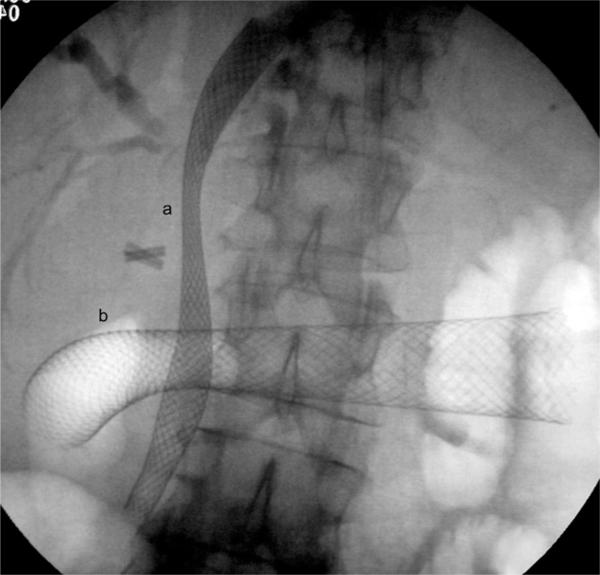
The use of SEMS in GOO is a relatively recent development using stent assemblies that can be passed down the channel of a therapeutic upper gastrointestinal endoscope. The procedure is usually carried out under conscious sedation in an endoscopy room with screening facilities. Briefly, a standard upper gastrointestinal endoscopy is carried out to assess the stricture location. Usually, the diameter of the therapeutic endoscope (~13 mm) is too large to allow safe passage through the stricture so the stricture is delineated with the introduction of a radio-opaque contrast agent, and negotiated with a guide wire under endoscopic and fluoroscopic control. After stent deployment over the guide wire, the position and patency is checked using endoscopy and fluoroscopy (Fig. 1).
Fig. 1.
Screening image showing (a) biliary and (b) duodenal self-expanding metal mesh stents (SEMS) in a patient with locally advanced hilar cholangiocarcinoma with gastric outflow obstruction. Laparoscopic cholecystectomy clips are also noted.
There have been a large number of studies reporting on the safety and efficacy of endoscopic duodenal stenting using SEMS. However, most of these are limited by retrospective data collection and the lack of a randomised control group. A 2004 meta-analysis pooled data from a total of 32 case series (10 prospectively collected) including 606 patients who had attempted duodenal stenting [15]. Most studies were carried out using the WallStent (Boston Scientific, MA, USA) system. Of all studies, technical success was achieved in 589 patients (97%). Clinical success, which was defined as relief of symptoms and/or improvement in food intake, was achieved in 526 patients (89% of those in whom technical success was achieved; 87% of all patients). Data on oral intake after stenting were available for 401 of 526 clinical successes. Eighty-seven per cent of these patients tolerated a full (48%) or soft diet (39%), with 13% managing liquids only. There was secondary stent obstruction in 104 patients (17%), due mostly to local disease progression, i.e. tumour ingrowth or overgrowth. There was a 5% rate of stent migration. Perforation or bleeding occurred in seven patients (1.2%). There were no reports of procedure-related mortality.
Since this meta-analysis, two studies have assessed the efficacy of a new generation uncovered duodenal stent (Duodenal WallFlex, Boston Scientific) [16,17]. Levels of technical success (95–98%) and clinical success (75–84%) were similar to the meta-analysis data. Complications of stent occlusion (9–12%) and stent migration (0–2%) seemed lower than with older generation stents, although the total numbers were small (43 and 51 patients, respectively) and no comparative studies of these stents were carried out.
Surgery versus Duodenal Stenting for Gastric Outlet Obstruction
There have been four randomised controlled trials comparing gastrojejunostomy with SEMS in malignant GOO (Table 1). As part of a study of laparoscopic staging of pancreatic cancer, 27 patients who were found to have unresectable disease at the time of laparoscopic staging were randomised to either surgical (13 patients) or endoscopic (14 patients) palliation [18]. The mean survival in the surgical group was 192 days, with a hospital-free survival of 164 days. In the endoscopic group, these figures fell to 116 days and 94 days, respectively (P = 0.05). Morbidity (8% bypass, 7% stenting group), complication rates during follow-up (32%, 43%) and mortality (0% in both groups) were similar between the two groups.
Table 1.
Summary data of randomised studies of surgery versus endoscopic stenting of malignant gastric outlet obstruction
| Reference | Number of patients in each group | Days until restoration of oral intake | Length of hospitalisation (days) | Complication rates | Median survival (days) | |
|---|---|---|---|---|---|---|
| [18] | Surgery | 13 | NR | 12 | 32% | 192 |
| Endoscopic | 14 | NR | 3 | 43% | 116 | |
| (P = 0.05) | ||||||
| [19] | Surgery | 9 | 6.3 | 10 | 11% | NR |
| Endoscopic | 9 | 2.1 | 3.1 | 11% | NR | |
| [10] | Surgery | 14 | NR | 11.4 | 57% | NR |
| Endoscopic | 13 | NR | 5.2 | 0% | NR | |
| (P = 0.02) | ||||||
| [20] | Surgery | 18 | 8 | 15 | 33% | 78 |
| Endoscopic | 21 | 5 | 7 | 48% | 56 | |
| (P < 0.01) | (P = 0.04) | |||||
In a study of 18 patients randomised to either gastroenterostomy or endoscopic stenting (nine patients in each group), the times to restoration of oral intake and hospital discharge were significantly shorter in the stenting group (2.1 versus 6.3 days; 3.1 versus 10 days) [19]. No statistically significant differences were seen in the incidence of delayed gastric emptying (100% satisfactory after 3 months), clinical outcomes at the 3 month follow-up, complications (11.1% both groups) or mortality (0% both groups).
Mehta et al. [10] randomised 27 patients to laparoscopic gastrojejunostomy (14 patients) or radiological stenting (13 patients). In the surgical group, there was a higher number of patients with complications (8 versus 0 patients) and length of hospital stay after intervention (11.4 versus 5.2 days; P = 0.02). The post-procedure visual analogue pain score was also significantly higher in the surgical group (P = 0.05). Mortality was similar between the two groups.
Jeurnink et al. [1] randomised 39 patients to gastrojejunostomy (16 open and two laparoscopic) or endoscopic stenting (n = 21). Patients who underwent endoscopic stenting were able to recommence oral intake earlier than the surgical group (median of 5 days versus 8 days; P < 0.01). However, at 60 days significantly more patients in the surgical group maintained oral intake (P = 0.05), and recurrent obstructive symptoms were higher in the stenting group (P = 0.02). Survival was similar between the groups (median 78 days versus 56 days; P = 0.19). A subsequent cost comparison showed that overall costs were higher in the surgical group than the endoscopic group, although this difference was comparatively small (€12 433 versus €8819 per patient; P = 0.049) [20].
Malignant Biliary Obstruction
Biliary obstruction occurs frequently in patients with pancreatic adenocarcinoma and is the presenting symptom in 37% of patients with disease affecting the pancreatic head [21]. Cholangiocarcinoma (including gallbladder cancer) and metastatic disease affecting the hilar lymph nodes are other causes of malignant obstruction. Approaches for the treatment of biliary obstruction are surgical bypass, endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic drainage.
Surgical Bypass for Biliary Obstruction
In patients with cancer of the periampullary region who present with obstructive jaundice, palliative drainage, either endoscopically, percutaneously or surgically, is essential to relieve the jaundice and improve symptoms and quality of life. Although initial results with surgical bypass showed low rates of recurrent jaundice (2–5%), the surgery itself carries an appreciable risk of postoperative morbidity and mortality, up to 24% in some trials [22]. Laparoscopic biliary bypass has been developed as a minimally invasive approach [23]. Stenting techniques are effective in the short-term, but may require further intervention and can be associated with recurrent attacks of cholangitis [24]. The site of obstruction is a key factor when considering which approach to take. Distal structures may be approached surgically, endoscopically or percutaneously. Proximal strictures are usually complex and often require a combination of endoscopic and percutaneous approaches to achieve adequate palliation [25]. Surgical drainage of proximal strictures is a major undertaking, but, as outlined below, is an option in highly selected patients.
Peripancreatic and distal extrahepatic biliary obstruction
The abdominal cavity is accessed through a midline or rooftop incision. A side to side hepaticojejunostomy (between the hepatic duct and the jejunum, leaving the hepatic duct in continuity) is fashioned using a jejunal loop 40–60 cm distal to the ligament of Treitz. The anastomosis may be hand sewn using interrupted absorbable suture. This anastomosis may be antecolic or retrocolic depending on the tumour and access. A roux-en-y reconstruction may be carried out. This may be in conjunction with a gastrojejunostomy, either as a prophylactic procedure or for established GOO [2,24].
Laparoscopically, either a cholecystojejunostomy or a hepaticojejunostomy are fashioned. A cholecystojejunostomy is considered if the cystic duct insertion is above the distal biliary stricture (although this is considered an inferior anastomosis to the hepaticojejunostomy). The cholecystojejunostomy is fashioned (stapled sutured technique) using an antecolic loop of proximal jejunum, although in some patients a totally sutured (double posterior layer and single anterior layer of continuous absorbable suture) anastomosis can be fashioned. A side to side hepaticojejunostomy can be constructed using a roux-en-y antecolic configuration and a stapled sutured technique [23].
Hilar obstuction — hepaticojejunostomy
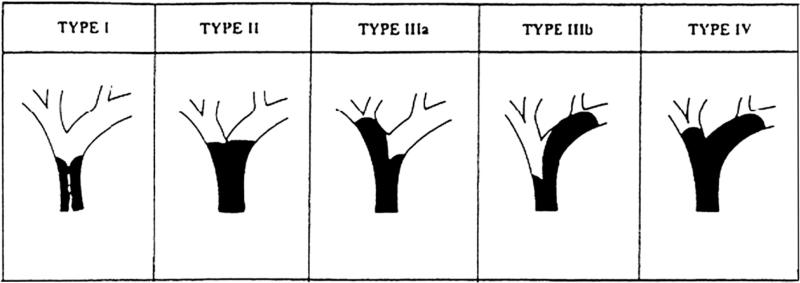
The degree of obstruction is commonly defined by the Bismuth–Corlette classification (Fig. 2). For these proximal strictures, an intrahepatic biliary-enteric bypass may be performed. This may be a segment III and or right sectoral hepatic duct bypass. The segment III duct is exposed and access can be enhanced by making a hepatotomy to the left of the falciform ligament. The duct is incised over 1 cm. To expose the right sectoral ducts, the main right portal pedicle is controlled through hepatotomies at the base of the gall-bladder fossa and caudate lobe. The overlying parenchyma is divided and the duct is identified and opened. A side to side biliary-enteric anastomosis is then performed using a loop of jejunum and a roux-en-y reconstruction is fashioned. This may be retrocolic or antecolic [25].
Fig. 2.
Bismuth–Corlette classification of hilar biliary strictures. Type I: subhilar tumours involving the common hepatic duct; type II: involvement of the common duct and hilar bifurcation; type IIIa: involvement of the bifurcation and right hepatic duct; type IIIb: involvement of the bifurcation and left hepatic duct; type IV: bilateral hepatic duct involvement.
Outcome data
A large retrospective study looked at short- and long-term outcome after palliative surgical drainage in 269 patients with malignant biliary obstruction. The majority of patients (n = 234) underwent a combined hepaticojejunostomy and gastrojejunostomy, whereas 35 patients underwent hepaticojejunostomy alone [26]. The complication rate was 28% and the mortality rate was 3%. Another study reported mortality and morbidity rates of 16 and 48%, respectively, in 102 patients who underwent planned biliary and gastric bypass for palliation [24]. A small laparoscopic study of 21 patients showed mortality and morbidity rates of 5 and 14%, respectively [23]. In a study of 58 patients with proximal biliary strictures, bypass surgery was associated with a mortality rate of 11% and a morbidity rate of 48% [25].
Complications
Complications include anastomotic leakage, haemorrhage, abdominal abscess, wound infection, delayed gastric emptying, renal failure, pancreatitis, enterocutaneous fistula [25,26].
Endoscopic and Percutaneous Biliary Drainage
With advances in both endoscopic and radiological technology, non-surgical biliary drainage procedures are an accepted initial approach in patients with obstructive jaundice in many centres. Both ERCP and percutaneous transhepatic drainage are highly efficacious in expert hands and are often complementary, particularly in patients with complex stricturing lesions of the liver hilum.
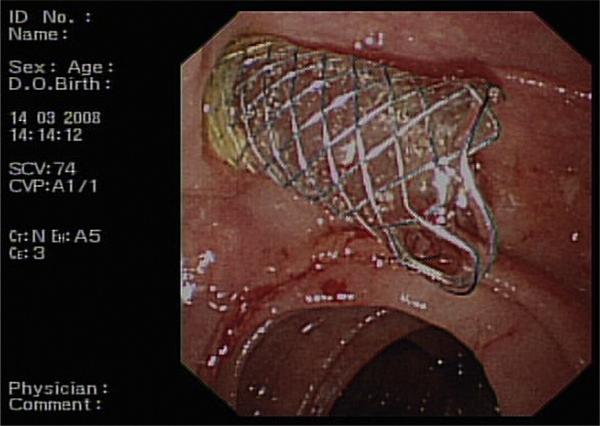
ERCP allows for histopathological correlation with the ability to take biopsy samples and biliary brushings. It also enables direct visualisation of the stomach, duodenum and ampulla, which may be subject to direct infiltration by the tumour. In the case of malignant biliary obstruction, stenting is usually carried out and this can be performed using a plastic stent or a SEMS (Fig. 3). Limitations include incomplete assessment of the anatomy of complex strictures and also the chance of failure in establishing biliary drainage. Potential complications are bleeding, visceral perforation and pancreatitis.
Fig. 3.
A biliary Wallstent projecting from the ampulla.
Percutaneous transhepatic drainage and stenting are usually carried out when the initial ERCP is unsuccessful, or when further treatment of undrained hepatic segments is required after endoscopic stenting. It is usually carried out under local anaesthesia and intravenous sedation, and may be performed as a two-stage procedure with initial external access and drainage, followed by later stenting. With prior imaging it allows a targeted approach to multiple obstructed hepatic segments, which may not all be accessible from the ampulla. Because more than one session is often needed, and due to the discomfort of hepatic capsular puncture, the recovery time and the length of hospital admission can be longer than via an endoscopic approach. Complications primarily include cholangitis and bleeding from the liver parenchyma.
Choice of stents in malignant biliary obstruction
It is usual in cases of biliary obstruction to obtain a confirmatory diagnosis of malignancy before metal stent insertion. As such, most patients are initially managed with a plastic stent at first presentation and the decision on subsequent metal or plastic stenting is made once further stenting is required.
A meta-analysis of seven studies (1992–2006) compared plastic and metal stenting for malignant distal biliary obstruction [22]. The relative risk of recurrent biliary obstruction was significantly lower in the metal stent group at 4 months (relative risk 0.44; 95% confidence interval 0.3–0.63), and prior to death/end of study (relative risk 0.52; 95% confidence interval 0.39–0.69). No differences were seen in technical success, therapeutic success or complication rates (relative risk 1.01; 95% confidence interval 1.0–1.34).
A 2008 study retrospectively compared plastic and metal stent insertion in patients with advanced pancreatic carcinoma [27]. Patients were excluded if the stents were inserted percutaneously, and those in the SEMS group were excluded if they had had prior treatment with a plastic stent. In total, 154 patients (91 metal stents, 63 plastic stents) were included. Stent occlusion was significantly more likely in the plastic stent group than in the SEMS group (33% versus 19%; P = 0.023). Cumulative days spent in hospital were also higher in the plastic stent group (16.5 days versus 7 days; P < 0.001). There was no difference in median survival between the plastic and metal stent groups (4.4 and 5.9 months, respectively; P = 0.074).
A prospective multicentre study, which was non-randomised, compared plastic (28 patients) with metal (34 patients) stenting in patients with hilar cholangiocarcinomas [28]. Despite the metal stent group having more advanced Bismuth stage disease, comorbidity scores and metastatic disease, rates of adverse outcomes (cholangitis, stent occlusion, migration, perforation and repeat intervention) were lower in the SEMS group (11.8% versus 39.3%; P = 0.017).
Three randomised controlled trials have compared uncovered and covered SEMS insertion in distal biliary obstruction [29–31]. Covered stents feature the addition of an artificial polymeric covering across their surface, which aims to prevent tumour ingrowth and improve the functioning life of the stent.
Krokidis et al. [29] included procedures carried out solely through the transhepatic route in patients with extrahepatic cholangiocarcinoma (60 patients; 30 in each group). The rate of stent dysfunction was significantly lower in the covered stent group (13.3% versus 30%; P = 0.046) and the median survival was also significantly longer with a covered stent (243.5 versus 180.5 days; P < 0.05). No difference in complications was seen, with rates of 10 and 13.3% in the covered and uncovered groups, respectively.
Isayama et al. [30] used ERCP as the intended stent insertion technique, only using transhepatic puncture if this failed (112 patients; 57 covered, 55 uncovered). Stent dysfunction rates were significantly lower in the covered stent group (38% versus 14%; P = 0.0032), but survival was similar (255 and 237 days). Non-occlusive complication rates were 14% in the covered stent group and 5.5% in the uncovered group, but analysis of individual complications (pancreatitis, cholecystitis, haemorrhage and migration) showed no difference between the groups.
In a preliminary report that compared covered and uncovered stenting in 400 patients, there was no difference in stent patency (first quartile stent patency time: 132 and 165 days in the covered and uncovered groups, respectively), failure (23 and 22%) or median survival (106 and 159 days) [31]. Rates of pancreatitis and cholecystitis were also similar. Seven patients in the covered stent group (1.8%) compared with none in the uncovered group developed stent migration (P < 0.01).
Endoscopic versus percutaneous approaches
A multicentre retrospective study compared endoscopic and transhepatic biliary metal stenting, specifically in patients with advanced Bismuth III or IV hilar cholangiocarcinoma [32]. Eighty-five patients (44 had endoscopic SEMS; 41 received percutaneous SEMS) were assessed, having excluded patients who had surgery, chemotherapy or radiotherapy. The success rate of palliation of cholestasis was significantly higher in the percutaneous group than in the endoscopic group (92.7% versus 77.3%; P = 0.049). Once successful biliary decompression was achieved, the overall complication rate was similar between the two procedures, but the length of hospital stay was lower in the endoscopic group (16.1 versus 29.9 days; P = 0.019). No significant differences were found in the median duration of stent patency (9.8 months in the endoscopic group, 11 months in the percutaneous group).
Endoscopic biliary stenting versus surgical bypass
In a 2007 meta-analysis of biliary stenting, 24 studies were included [22]. All patients had malignant biliary obstruction and were deemed unsuitable for curative surgical treatment. Patients who had percutaneous procedures were excluded. Three studies (total 306 patients; Table 2) were specifically found to compare surgical bypass with (plastic) biliary stenting [33–35]. There was no difference found in the rates of technical or therapeutic success (relative risk 1.01 and 1.00; 95% confidence interval 0.95–1.07 and 0.93–1.08). The relative risk of all complications was significantly reduced in the stenting group when compared with the surgery group (relative risk 0.60; 95% confidence interval 0.45–0.81; P = 0.0007). The 30-day mortality showed a non-significant trend to improvement in the stenting group (relative risk 0.58; P = 0.07). In the two trials that were suitable for inclusion there was a highly significant reduction in recurrent biliary obstruction in the surgical group (relative risk 18.59; 95% confidence interval 5.33–64.86; P < 0.00001). One limitation of this meta-analysis is that all three studies were relatively old, with published dates between 1988 and 1994, and so may not reflect recent advances in surgery and endoscopic therapy. Two previous meta-analyses had been published using the same data and reached similar conclusions [36,37].
Table 2.
Summary data from randomised studies of surgery versus endoscopic stenting of malignant biliary obstruction
| Reference | Number of patients in each group | Therapeutic success | Complications | 30 day mortality | Recurrent obstruction | Median length of hospital stay (days) | ||
|---|---|---|---|---|---|---|---|---|
| [33] | Surgery | 101 | 92% | Major | 29% | 15% | 2% | 26 |
| Endoscopic | 100 | 92% | 11% | 8% | 36% | 9 | ||
| (P = 0.02) | (NS) | (NS) | (NS) | |||||
| Minor | 29% | |||||||
| 18% | ||||||||
| (NS) | ||||||||
| [34] | Surgery | 25 | 88% | 20% | 24% | NR | 27 | |
| Endoscopic | 25 | 96% | 36% | 20% | NR | 26 | ||
| (NS) | (NS) | (NS) | ||||||
| [35] | Surgery | 25 | 92% | 56% | 20% | 0% | 13 | |
| Endoscopic | 23 | 91% | 30% | 9% | 43% | 5 | ||
| (NS) | (NS) | (P < 0.002) | ||||||
One retrospective study compared biliary bypass (41 patients, with or without gastrojejunostomy) with SEMS (19 patients) in unresectable pancreatic carcinoma [38]. The SEMS group included eight patients who had SEMS insertion via a transhepatic route, the remaining 11 patients had stents inserted endoscopically. There was no significant difference in procedure success rate between the surgical and SEMS groups (100 and 95%, respectively; P = 0.15). The difference in rate of early complications was not statistically significant between the two groups (22 and 5%; P = 0.10), although the length of hospitalisation was longer in the surgery group (mean 32 versus 12 days; P = 0.002). The prevalence of late complications (mainly due to recurrent biliary obstruction) was significantly lower in the surgery group than in the stenting group (10% versus 42%; P = 0.04). No difference was found in the survival statistics.
Preoperative Stenting
Van der Gaag et al. [39] randomised 202 jaundiced patients with resectable pancreatic adenocarcinoma to either preoperative biliary drainage followed by surgery 4–6 weeks later (106 patients) or early surgery within 1 week of diagnosis (96 patients). Biliary drainage was carried out via ERCP initially (75% successful), but in patients who did not achieve adequate drainage, repeat ERCP or percutaneous drainage was carried out. At 120 days after randomisation, 74% of patients in the preoperative drainage group had suffered a serious complication, compared with 39% of patients in the early surgery group (P < 0.001). Mortality and length of hospital stay did not vary significantly between the groups.
Conclusions
This review has explored the evidence base for the palliation of malignant gastric and biliary obstruction. Although there have been several randomised controlled trials in this area, with advances in endoscopic and surgical techniques further good-quality clinical outcome and quality of life studies are clearly needed. In the palliation of GOO, levels of technical and therapeutic success with both surgical and endoscopic stenting techniques are high. The morbidity and mortality rates associated with surgical gastrojejunostomy, including laparoscopic approaches, are significant and the length of hospital stay may be prolonged. The duration of luminal patency may be longer in patients treated surgically than in those who are stented endoscopically, but there are no large randomised comparative studies to guide management. In general, patients with locally advanced disease and a good performance status are candidates for surgical bypass, whereas patients who are generally more frail with widespread metastatic disease may be best palliated with endoscopic stenting to minimise short-term complications, time to restoration of oral intake and hospital stay.
Endoscopic stenting is currently the accepted initial treatment for the palliation of jaundiced patients, with a lower complication rate than surgery, although there is a risk of stent occlusion and the need for repeat procedures. Exceptions include patients undergoing laparotomy for an attempted curative resection who are probably best served with concomitant bypass surgery, and those with distal biliary obstruction due to resectable disease, in whom preoperative endoscopic intervention may increase the complication rate.
Acknowledgement
S.P. Pereira is supported by grant NIH P01 CA084203.
References
- 1.Jeurnink SM, Steyerberg EW, van Hooft JE, et al. Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71:490–499. doi: 10.1016/j.gie.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 2.Hüser N, Michalski CW, Schuster T, Friess H, Kleeff J. Systematic review and meta-analysis of prophylactic gastroenterostomy for unresectable advanced pancreatic cancer. Br J Surg. 2009;96:711–719. doi: 10.1002/bjs.6629. [DOI] [PubMed] [Google Scholar]
- 3.Ly J, O'Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc. 2010;24:290–297. doi: 10.1007/s00464-009-0577-1. [DOI] [PubMed] [Google Scholar]
- 4.Gouma DJ, Busch ORC, van Gulik TM. Pancreatic carcinoma: palliative surgical and endoscopic treatment. HPB. 2006;8:369–376. doi: 10.1080/13651820600804021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut. 2007;56:1134–1152. doi: 10.1136/gut.2006.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeurnink SM, Steyerberg EW, Hof G, van Eijck CH, Kuipers EJ, Siersema PD. Gastrojejunostomy versus stent placement in patients with malignant gastric outlet obstruction: a comparison in 95 patients. J Surg Oncol. 2007;96:389–396. doi: 10.1002/jso.20828. [DOI] [PubMed] [Google Scholar]
- 7.Johnsson E, Thune A, Liedman B. Palliation of malignant gastroduodenal obstruction with open surgical bypass or endoscopic stenting: clinical outcome and health economic evaluation. World J Surg. 2004;28:812–817. doi: 10.1007/s00268-004-7329-0. [DOI] [PubMed] [Google Scholar]
- 8.Navarra G, Musolino C, Venneri A, De Marco ML, Bartolotta M. Palliative antecolic isoperistaltic gastrojejunostomy: a randomized controlled trial comparing open and laparoscopic approaches. Surg Endosc. 2006;20:1831–1834. doi: 10.1007/s00464-005-0454-5. [DOI] [PubMed] [Google Scholar]
- 9.Mittal A, Windsor J, Woodfield J, Casey P, Lane M. Matched study of three methods for palliation of malignant pyloroduodenal obstruction. Br J Surg. 2004;91:205–209. doi: 10.1002/bjs.4396. [DOI] [PubMed] [Google Scholar]
- 10.Mehta S, Hindmarsh A, Cheong E, et al. Prospective randomized trial of laparoscopic gastrojejunostomy versus duodenal stenting for malignant gastric outflow obstruction. Surg Endosc. 2006;20:239–242. doi: 10.1007/s00464-005-0130-9. [DOI] [PubMed] [Google Scholar]
- 11.Denley SM, Moug SJ, Carter CR, McKay CJ. The outcome of laparoscopic gastrojejunostomy in malignant gastric outlet obstruction. Int J Gastrointest Cancer. 2005;35:165–169. doi: 10.1385/IJGC:35:3:165. [DOI] [PubMed] [Google Scholar]
- 12.Espinel J, Sanz O, Vivas S, et al. Malignant gastrointestinal obstruction: endoscopic stenting versus surgical palliation. Surg Endosc. 2006;20:1083–1087. doi: 10.1007/s00464-005-0354-8. [DOI] [PubMed] [Google Scholar]
- 13.Artifon EL, Sakai P, Cunha JE, et al. Surgery or endoscopy for palliation of biliary obstruction due to metastatic pancreatic cancer. Am J Gastroenterol. 2006;101:2031–2037. doi: 10.1111/j.1572-0241.2006.00764.x. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt C, Gerdes H, Hawkins W, et al. A prospective observational study examining quality of life in patients with malignant gastric outlet obstruction. Am J Surg. 2009;198:92–99. doi: 10.1016/j.amjsurg.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 15.Dormann A, Meisner S, Verin N, Wenk Lang A. Gastroduodenal stenting. Endoscopy. 2004;36:543–550. doi: 10.1055/s-2004-814434. [DOI] [PubMed] [Google Scholar]
- 16.Piesman M, Kozarek RA, Brandabur JJ, et al. Improved oral intake after palliative duodenal stenting for malignant obstruction: a prospective multicenter clinical trial. Am J Gastroenterol. 2009;104:2404–2411. doi: 10.1038/ajg.2009.409. [DOI] [PubMed] [Google Scholar]
- 17.van Hooft JE, Uitdehaag MJ, Bruno MJ, et al. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): a prospective multicenter study. Gastrointestinal Endosc. 2009;69:1059–1066. doi: 10.1016/j.gie.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Nieveen van Dijkum EJM, Romijn MG, Terwee CB, et al. Laparoscopic staging and subsequent palliation in patients with peripancreatic carcinoma. Ann Surg. 2003;237:66–73. doi: 10.1097/00000658-200301000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiori E, Lamazza A, Volpino P, et al. Palliative management of malignant antro-pyloric strictures. Gastroenterostomy vs. endoscopic stenting. A randomized prospective trial. Anticancer Res. 2004;24:269–271. [PubMed] [Google Scholar]
- 20.Jeurnink SM, Polinder S, Steyerberg EW, Kuipers EJ, Siersema PD. Cost comparison of gastrojejunostomy versus duodenal stent placement for malignant gastric outlet obstruction. J Gastroenterol. 2010;45:537–543. doi: 10.1007/s00535-009-0181-0. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe I, Sasaki S, Konishi M, et al. Onset symptoms and tumor locations as prognostic factors of pancreatic cancer. Pancreas. 2004;28:160–165. doi: 10.1097/00006676-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Moss AC, Morris E, Leyden J, MacMathuna P. Malignant distal biliary obstruction: a systematic review and meta-analysis of endoscopic and surgical bypass results. Cancer Treat Rev. 2007;33:213–221. doi: 10.1016/j.ctrv.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Hamade AM, Al-Bahrani AZ, Owera AM, et al. Therapeutic, prophylactic, and preresection applications of laparoscopic gastric and biliary bypass for patients with periampullary malignancy. Surg Endosc. 2005;19:1333–1340. doi: 10.1007/s00464-004-2282-4. [DOI] [PubMed] [Google Scholar]
- 24.Mann CD, Thomasset SC, Johnson NA, et al. Combined biliary and gastric bypass procedures as effective palliation for unresectable malignant disease. Aust N Z J Surg. 2009;79:471–475. doi: 10.1111/j.1445-2197.2008.04798.x. [DOI] [PubMed] [Google Scholar]
- 25.Jarnagin WR, Burke E, Powers C, Fong Y, Blumgart LH. Intrahepatic biliary enteric bypass provides effective palliation in selected patients with malignant obstruction at the hepatic duct confluence. Am J Surg. 1998;175:453–460. doi: 10.1016/s0002-9610(98)00084-1. [DOI] [PubMed] [Google Scholar]
- 26.Kuhlmann KF, van Poll D, de Castro SM, et al. Initial and long-term outcome after palliative surgical drainage of 269 patients with malignant biliary obstruction. Eur J Surg Oncol. 2007;33:757–762. doi: 10.1016/j.ejso.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Weber A, Mittermeyer T, Wagenpfeil S, Schmid RM, Prinz C. Self-expanding metal stents versus polyethylene stents for palliative treatment in patients with advanced pancreatic cancer. Pancreas. 2009;38:e7–12. doi: 10.1097/MPA.0b013e3181870ab8. [DOI] [PubMed] [Google Scholar]
- 28.Perdue DG, Freeman ML, DiSario JA, et al. Plastic versus self-expanding metallic stents for malignant hilar biliary obstruction. J Clin Gastroenterol. 2008;42:1040–1046. doi: 10.1097/MCG.0b013e31815853e0. [DOI] [PubMed] [Google Scholar]
- 29.Krokidis M, Fanelli F, Orgera G, Bezzi M, Passariello R, Hatzidakis A. Percutaneous treatment of malignant jaundice due to extrahepatic cholangiocarcinoma: covered Viabil stent versus uncovered Wallstents. Cardiovasc Intervent Radiol. 2010;33:97–106. doi: 10.1007/s00270-009-9604-9. [DOI] [PubMed] [Google Scholar]
- 30.Isayama H, Komatsu Y, Tsujino T, et al. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729–734. doi: 10.1136/gut.2003.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kullman E, Söderlund C, Linder S, et al. Covered (cSEMS) versus uncovered (uSEMS) nitinol self-expandable metal stent in the palliative treatment of malignant distal biliary obstruction: preliminary results from a randomized controlled multicenter trial. Gastrointestinal Endoscopy. 2009:69. doi: 10.1016/j.gie.2010.07.036. AB132, abstract 933. [DOI] [PubMed] [Google Scholar]
- 32.Paik WH, Park YS, Hwang J-H, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointestinal Endosc. 2009;69:55–62. doi: 10.1016/j.gie.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Smith AC, Dowsett JF, Russell RCG, Hatfield ARW, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bile duct obstruction. Lancet. 1994;344:1655–1660. doi: 10.1016/s0140-6736(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 34.Anderson JR, Sorensen SM, Kruse A, Rokkjier M, Matzen P. Randomised trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundice. Gut. 1989;30:1132–1135. doi: 10.1136/gut.30.8.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shepherd HA, Royle G, Ross APR, Diba A, Arthur M, Colin-Jones D. Endoscopic biliary endoprosthesis in the palliation of malignant obstruction of the distal common bile duct: a randomized trial. Br J Surg. 1988;75:1166–1168. doi: 10.1002/bjs.1800751207. [DOI] [PubMed] [Google Scholar]
- 36.Flamm CR, Mark DH, Aronson N. Evidence-based assessment of ERCP approaches to managing pancreaticobiliary malignancies. Gastrointestinal Endosc. 2002;56:S218–S225. doi: 10.1067/mge.2002.129027. [DOI] [PubMed] [Google Scholar]
- 37.Taylor MC, McLeod RS, Langer B. Biliary stenting versus bypass surgery for the palliation of malignant distal bile duct obstruction: a meta-analysis. Liver Transplantation. 2000;6:302–308. doi: 10.1053/lv.2000.5196. [DOI] [PubMed] [Google Scholar]
- 38.Maosheng D, Ohtsuka T, Ohuchida J, et al. Surgical bypass versus metallic stent for unresectable pancreatic cancer. J Hepatobiliary Pancreat Surg. 2001;8:367–373. doi: 10.1007/s005340170010. [DOI] [PubMed] [Google Scholar]
- 39.van der Gaag NA, Rauws EAJ, van Eijck CHJ, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129–137. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]