Abstract
Enhancement of peripheral opioid analgesia following tissue injury or inflammation in animal models is well-documented, but clinical results of peripheral opioid therapy remain inconsistent. Previous studies in the central nervous system have shown that co-administration of μ- and δ-opioid receptor agonists can enhance analgesic outcomes; however, less is known about the functional consequences of opioid receptor interactions in the periphery. The present study examines the effects of intraplantar injection of the μ- and δ-opioid receptor agonists, morphine and deltorphin, alone and in combination on behavioral tests of nociception in naïve rats and on potassium-evoked release of CGRP from sciatic nerves of naïve rats. Neither drug alone affected nociceptive behaviors or CGRP release. Two separate measures of mechanical nociceptive sensitivity remained unchanged after co-administration of the two drugs. In contrast, when deltorphin was co-injected with morphine, dose-dependent and peripherally-restricted increases in paw withdrawal latencies to radiant heat were observed. Similarly, concentration-dependent inhibition of CGRP release was observed when deltorphin and morphine were administered in sequence prior to potassium stimulation. However, no inhibition was observed when morphine was administered prior to deltorphin. All combined opioid effects were blocked by co-application of antagonists. Deltorphin exposure also enhanced the in vivo and in vitro effects of another μ-opioid receptor agonist, DAMGO. Together, these results suggest that under normal conditions, δ-opioid receptor agonists enhance the effect of μ-opioid receptor agonists in the periphery, and local co-administration of δ- and μ-opioid receptor agonists may improve results of peripheral opioid therapy for the treatment of pain.
Keywords: delta opioid receptor, mu opioid receptor, calcitonin gene-related peptide, peripheral opioid analgesia, superadditivity
1. Introduction
Systemic and central administration of opioids for the treatment of pain is sometimes accompanied by serious adverse side effects, and tolerance often occurs with prolonged use. The abuse potential and legal restrictions of this drug class can also limit their clinical usage. The targeting of peripheral opioid receptors may provide pain relief while reducing many of these limitations. Peripherally applied opioids have little or no analgesic effect under normal conditions, but it is well-established that peripheral opioid receptor function is enhanced after tissue injury or inflammation [23,37,40,44]. Several mechanisms appear to contribute to improved peripherally-mediated opioid analgesia during inflammation, including increased synthesis and transport of opioid receptors to the periphery [17,21], enhanced G-protein coupling [47], and increased permeability of the perineurium [2] (for review, see[38]).
Recent studies have indicated that the efficacy of peripherally applied opioids can be improved in the absence of a complex or time-consuming inflammatory response. Peripheral μ- and δ-opioid receptors have both been shown to undergo functional upregulation following relatively brief exposure to a single inflammatory mediator such as bradykinin or a protease activated receptor-2 agonist. This rapid process of enhancing peripheral opioid receptor function has been referred to as priming [4,5,26,27,33]. The priming requirement for functional competence has also been demonstrated for the δ-opioid receptor in vivo [33].
Despite overwhelming evidence from animal studies suggesting that the efficacy of peripherally restricted opioids should be high after tissue insult, clinical results of peripheral opioid therapy remain inconsistent. For example, topical morphine has been shown to reduce nociceptive behaviors in rats and dogs [39,45] as well as pain scores in humans [10] following corneal injury. In contrast, many studies examining the efficacy of local morphine administration after arthroscopic knee surgery have failed to observe significant pain relief. Clinical reviews have concluded that this strategy provides little to no analgesic effect [15,32], and similar conclusions have been drawn for other opioids and procedures [28]. One strategy that has proven to be effective in improving pain relief clinically, however, is polyanalgesic therapy. For example, the α2-adrenergic receptor agonist clonidine enhances post-operative morphine analgesia without augmenting side effects [8]. Previous results from animal studies have indicated that δ- and μ-opioid receptor interactions also have important functional consequences in the central nervous system (CNS), including synergistic analgesic outcomes [1,13,18,19,24,42]. However, much less is known about how these receptors interact in the periphery.
Given the evidence of greater-than-additive analgesic effects resulting from combining δ- and μ-opioid receptor ligands in the CNS, we hypothesized that combining agonists for these two receptors would unmask or enhance the function of opioid receptors in the peripheral nervous system. To test this hypothesis, we examined (1) the effect of intraplantar injection of δ- and μ-opioid receptor agonists alone and in combination on measures of both mechanical and thermal nociception in naïve animals, (2) the effect of δ- and μ-opioid receptor agonists alone and together on neuroactive peptide release from normal peripheral tissue, and (3) whether combined opioid effects are dependent on a specific agonist sequence or combination.
2. Methods
2.1 Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Minnesota and were conducted according to the International Association for the Study of Pain [46] and federal guidelines. Male Sprague-Dawley (Harlan, Indianapolis, IN) rats weighing 250-325 g were used in this study. Animals were housed in a temperature-controlled room on a 12 hour light/dark schedule and had access to food and water ad libitum.
2.2 Compounds
Naltrindole hydrochloride and naloxone hydrochloride were purchased from Tocris (Ellisville, MO). [D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2] (CTAP) was purchased from American Peptide Company (Sunnyvale, CA). [D-ala]2-Deltorphin II (deltorphin) was purchased from Bachem (Torrance, CA). [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO) and morphine sulfate were purchased from Sigma-Aldrich (St. Louis, MO). For behavioral studies, drugs were diluted alone or in combination in sterile saline and administered via a single subcutaneous intraplantar injection in a final volume of 50 μL. The doses for each drug used in the current study were chosen based on those previously reported to produce significant, non-systemic antinociceptive effects when given alone in models of inflammatory or neuropathic pain [9,22,33]. For CGRP release studies, stock solutions were prepared in sterile saline (morphine sulfate) or water (all other drugs) and diluted in modified Hank’s buffer (Invitrogen, San Diego, CA; prepared as described below) on the day of experimental use.
2.3 Behavioral testing
For all behavioral tests, animals were handled by the experimenter and allowed to habituate to the testing room and procedures for at least three days before evaluation of drug effects. On the day of experimentation, animals were acclimated to the testing apparatus prior to testing. Measurements were then taken for both hind paws before and at 15, 30, 45, and 60 min after intraplantar injection of drug or vehicle (saline) in non-anesthetized rats. The same experimenter handled and tested each group of animals and was blinded to drug treatment groups.
2.3.1 Thermal nociceptive threshold testing
Paw withdrawal latency (PWL) to noxious heat was determined using methods similar to those previously described [16]. Briefly, rats were placed in individual plastic compartments on a glass table and allowed to acclimate for at least 20 min. A radiant heat stimulus was then applied to the plantar surface of the hind paw through the glass floor. PWLs were measured automatically to the nearest 0.1 s using a photoelectric cell. The light intensity was adjusted so that baseline withdrawal latencies were approximately 10 s. A cut-off time of 20 s was used to minimize tissue damage. Measurements were performed in duplicate at least 30 s apart, and the average of the two readings was used for statistical analysis.
2.3.2 Mechanical nociceptive testing
For mechanical nociceptive tests, rats were placed on an elevated wire mesh platform in individual plastic compartments and allowed to acclimate for at least 20 min. Mechanical withdrawal thresholds were determined using an electronic von Frey apparatus. A mechanical stimulus was applied to the middle of the plantar surface using a rigid filament with a tip diameter of 0.8 mm coupled with a force transducer (model 2390, IITC Inc. Woodland Hills, CA). Measurements were performed in triplicate at least 30 s apart, and the average of three measurements was used for statistical analysis.
Percent paw withdrawal to mechanical stimulation was determined using a 60 g Semmes–Weinstein von Frey monofilament (Stoelting, Wood Dale, IL). The filament was applied to the plantar surface of each hind paw ten times for 1–2 s each time with an interstimulus interval of at least 5 s. Percent withdrawal was then calculated as the number of paw withdrawals elicited/10 trials x 100.
2.4 Immunoreactive calcitonin gene related peptide (CGRP) release procedures
Procedures were slightly modified from those described previously [12]. Briefly, animals were sacrificed, and sciatic nerves were cleared of adjacent tissue and removed from just distal to the lumbar plexus and following the tibial branch to just above the ankle. Nerves were rinsed with room temperature modified Hank’s buffer ((Invitrogen, San Diego, CA) supplemented with 10 mM D-glucose, 10.9 mM HEPES, 4.2 mM NaHCO3, and 0.1% bovine serum albumin, pH 7.4) during dissection then placed intact into mesh-bottomed baskets to protect them from mechanical damage during experimental procedures. Nerves were rinsed and acclimated in oxygenated buffer for 30 min in a 37°C water bath before each experiment.
Each experimental sequence consisted of a set of five-min incubation steps. Nerves were incubated in glass test tubes containing 500 μL of warmed (37°C) modified Hank’s buffer with or without test compounds and were gently transferred via mesh-bottomed baskets directly from one test tube to the next. Each sequence began with measurement of basal release followed by two vehicle or drug treatment incubations, with the final five-min incubation step being a depolarizing stimulation with isotonic high potassium buffer (containing, in mM, 1.26 CaCl2, 0.5 MgCl2, 0.4 MgSO4, 50 KCl, 0.4 KH2PO4, 88 NaCl, 0.34 Na2HPO4, 15.6 D-glucose, 10.9 HEPES, 4.2 NaHCO3, 0.1% bovine serum albumin, pH 7.4). Nerves were weighed at the end of each experiment and were used for only one experimental sequence. Procedures for measurement of CGRP release from hind paw were modified from [34]. Briefly, the skin of the plantar surface of the hind paw was removed, taking care to keep nerves intact. The subsequent experimental methods were essentially the same as for nerves, except that hind paws were incubated in a 24-well plate at 37°C. Skin samples were gently transferred between wells containing buffer with or without compounds, and each incubation step was 20 minutes. As with nerve experiments, the final incubation step was a depolarizing stimulus of high potassium buffer. For both methods, incubation fluid samples (300 μL) were collected after each step and stored at −20°C until ready for analysis of CGRP content via enzyme-immuno assay (EIA). Samples were processed as directed using a commercially available rat CGRP kit (Cayman Chemicals, Ann Arbor, MI).
2.5 Data analysis
Prism software version 4 (GraphPad, San Diego, CA, USA) was used for statistical analysis. All data are expressed as means ± SEM. Behavioral time course data was analyzed using a two-way ANOVA, and corresponding area under the curve data was analyzed using a one-way ANOVA. CGRP data is expressed as % of potassium-evoked release (± SEM), which is defined as the difference between baseline CGRP release and that measured following exposure to isotonic high potassium buffer. Differences between pairs of groups were analyzed using a two-tailed Student’s t test, and multiple group data was compared using ANOVA. All one- and two-way ANOVAs were followed by Bonferroni post-hoc tests. The significance of all tests was set at p < 0.05.
3. Results
3.1 Combined peripheral opioid administration increases thermal nociceptive thresholds in naïve rats in a dose dependent manner
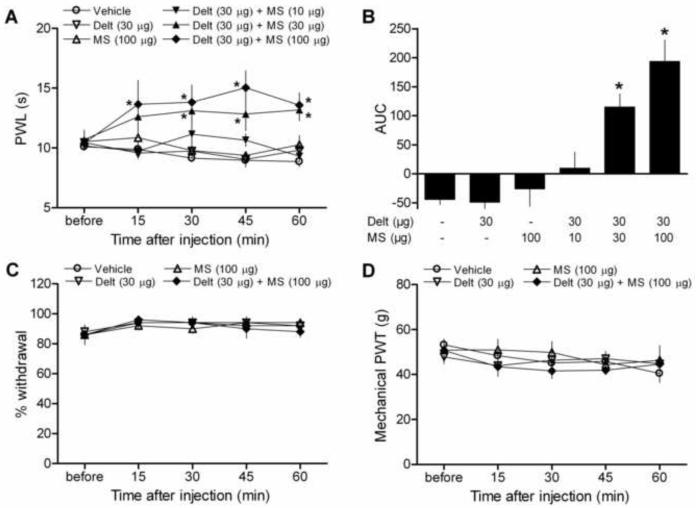
In order to determine whether combining δ- and μ-opioid receptor selective agonists could enhance peripherally-mediated analgesia in naïve animals, we first tested the ability of deltorphin and morphine alone and in combination to increase paw withdrawal latency (PWL) to noxious heat stimulation. Neither deltorphin (30 μg) nor morphine (100 μg) affected nociceptive thresholds when administered alone via intraplantar injection (Fig. 1A-B). In contrast, PWL values in response to a heat stimulus were significantly increased after combined deltorphin and morphine administration, indicative of thermal analgesia. The increase in PWL was significant as early as 15 min after injection, peaked at 45 min, and remained elevated above vehicle-injected values for the duration of the experiment (60 minutes). (Fig. 1A, Drug p < 0.0001, F = 16.29; Time p = 0.6349, F = 0.6396; Interaction p = 0.231, F = 1.233; two-way ANOVA). Combined opioid values were also significantly higher than deltorphin alone values at each time point, morphine alone at 30 and 45 min, and the lowest dose combination (Delt + 10 μg MS) at 15, 45, and 60 min (p < 0.05, Bonferroni post-hoc tests).
Figure 1.
Effects of intraplantar injection of deltorphin (Delt, 30 μg) and morphine (MS, 100 μg) alone and in combination on thermal and mechanical sensitivity in naïve animals. (A-D) Neither drug alone significantly affected any of the behavioral measurements compared to vehicle (saline) injection. (A-B) In contrast, combined deltorphin (30 μg) and morphine (10-100 μg) dose-dependently elevated paw withdrawal latencies (PWL) to thermal stimulation throughout the duration of testing. (A) Time course. *p < 0.05 vs. vehicle. (B) Area under the curve (AUC). *p < 0.05 vs. vehicle, deltorphin alone, morphine alone, and Delt + 10 μg MS groups. (C) Percent withdrawal to a noxious mechanical stimulus and (D) mechanical paw withdrawal threshold (PWT) values were unaffected by combined opioid administration. N = 5-9/group.
The increase in PWL observed was dependent on the dose of morphine accompanying deltorphin (Fig. 1B, P < 0.0001, F = 14.76; one-way ANOVA). It is of note that although 100 μg of morphine alone was unable to alter PWL values, significant thermal analgesia was observed at the 30, 45, and 60 min time points when a lower dose of morphine (30 μg) was combined with deltorphin. This dose combination was also significantly elevated above deltorphin alone values at the 30, 45, and 60 min time points, morphine alone values at 30 and 45 min, and Delt + 10 μg MS at the 60 min time point (Fig. 1A, p < 0.05, Bonferroni post-hoc tests).
3.2 Combined peripheral opioid administration has no effect on measures of mechanical nociception in naïve rats
To determine if combined opioid administration could influence responsiveness to mechanical stimulation, we examined the effect of intraplantar injection of deltorphin and morphine alone and in combination in two different tests of mechanical sensitivity in naïve rats. Neither drug alone affected mechanical paw withdrawal thresholds (PWT) or percent withdrawal to a noxious mechanical stimulus. In contrast to the results obtained in the test of thermal sensitivity, both mechanical behavioral measures remained unaffected after co-administration of deltorphin (30 μg) and morphine (100 μg), indicating that at these doses, combined opioid administration affects thermal, but not mechanical nociception (Fig. 1C and 1D).
3.3 Combined opioid effects on thermal nociception are mediated by peripheral δ- and μ -opioid receptors
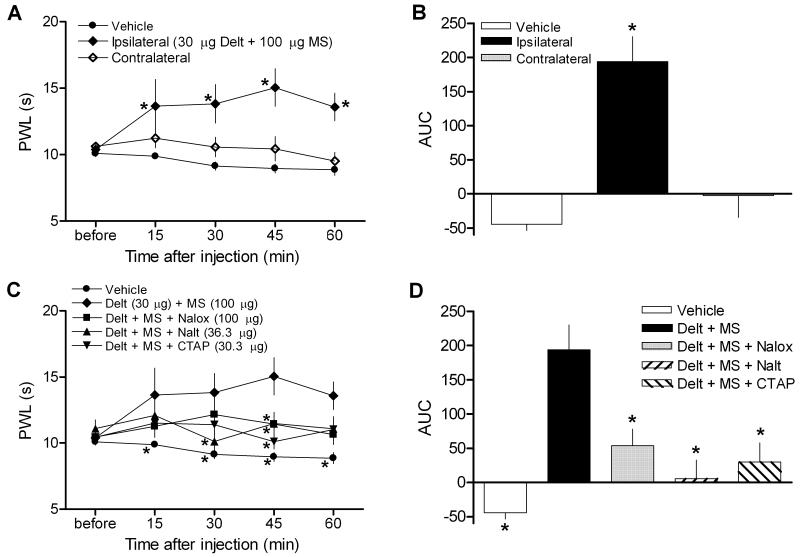
To confirm that the increase in PWL produced by combined deltorphin and morphine was due to a local, peripherally-mediated effect rather than a systemic one, thermal nociceptive thresholds were measured for the hind paws contralateral to drug injection before and after drug administration. PWL values from the drug injected side were confirmed to be significantly elevated compared to contralateral as well as vehicle-injected values (Fig 2A, Side p < 0.0001, F = 24.68; Time p = 0.41, F = 1.003; Interaction p = 0.1012, F = 1.734; two-way ANOVA, p < 0.05 contralateral vs. ipsilateral at 45 and 60 min, Bonferroni post-hoc tests). Even at the highest dose, combined deltorphin and morphine injection did not alter PWL readings from the contralateral hind paw at any time points (Fig. 2A, contralateral vs. vehicle p > 0.05 at each time point, Bonferroni post-hoc tests), indicating that the analgesic effect produced by the drug combination was restricted to the periphery. Area under the curve (AUC) values confirm a significant elevation in PWL for the ipsilateral, but not contralateral, side over the 60-minute testing period (Fig. 2B, p < 0.0001, F = 19.81; one-way ANOVA).
Figure 2.
Effects of combined opioid administration on thermal sensitivity are restricted to the periphery and blocked by antagonists. (A-B) While PWL measurements taken from hind paws injected with combined deltorphin (Delt, 30 μg) and morphine (MS, 100 μg) were elevated throughout the testing period, measurements from the contralateral paw did not vary compared to vehicle at any time point. (A) Time course. *p < 0.05 vs. vehicle. (B) Area under the curve (AUC). *p < 0.05 vs. vehicle and contralateral groups. (C-D) Co-administration of naloxone (Nalox, 100 μg), naltrindole (Nalt, 36.3 μg), or CTAP (30.3 μg) significantly reversed the effect of combined deltorphin and morphine administration. (C) Time course. (D) Area under the curve (AUC). Combined opioid data is repeated from a subset of fig. 1 for comparison. *p < 0.05 vs. Delt + MS group. N = 6-8/group.
In order to test whether the analgesia to thermal stimulation observed with combined deltorphin and morphine was mediated via opioid receptors, naloxone (100 μg), a non-selective opioid receptor antagonist, was co-injected with the combination of drugs. Naloxone significantly reversed the increase in thermal nociceptive thresholds produced by combined deltorphin and morphine (Fig. 2C-D). In addition, the selective δ-opioid receptor antagonist naltrindole (36.3 μg) or the selective μ-opioid receptor antagonist CTAP (30.3 μg) were co-administered in a 2:1 molar ratio with deltorphin and morphine, respectively, to examine whether activation of both receptors is required for combined opioid analgesia. Co-administration of either selective antagonist significantly blocked the increase in PWL observed with combined deltorphin and morphine (Fig 2C, Drug p < 0.0001, F = 12.82; Time p = 0.2567, F = 1.342; Interaction p = 0.2536, F = 1.226; two-way ANOVA). AUC values confirmed that co-application of naloxone, naltrindole, or CTAP significantly blocked the effects of combined opioid administration examined over the duration of testing (Fig 2D, p < 0.0001, F = 10.72; one-way ANOVA, p > 0.05 for vehicle vs. all antagonist groups, Bonferroni post-hoc tests).
3.4 Combined opioid pretreatment inhibits CGRP release from peripheral nerves in a concentration dependent manner
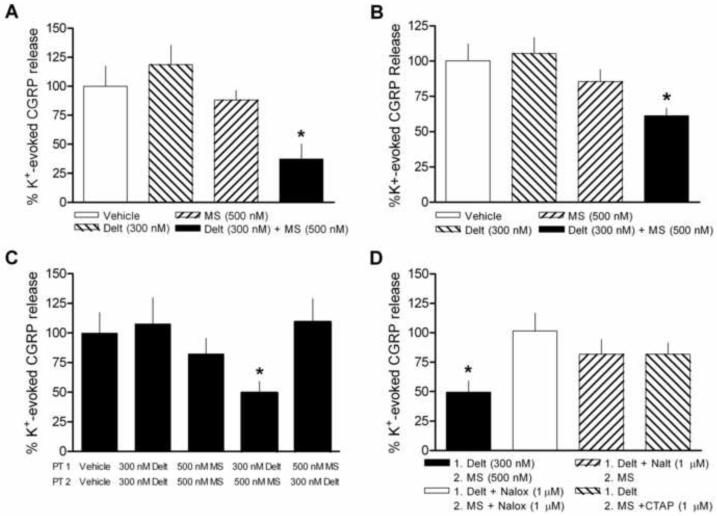
In order to investigate whether the effects of combined opioid administration on thermal sensitivity are reflected in the efferent action of peripheral nerves, we tested whether pretreatment with deltorphin, morphine, or the combination could alter evoked CGRP release from sciatic nerves of naïve rats. Incubation in high potassium buffer (50 mM) caused a significant increase in CGRP release compared to basal release levels from isolated rat nerves (32.17 ± 5.06 vs. 3.19 ± 0.16 pg/ml, p <0.0001, data not shown). Evoked release was considered to be the amount of increase over basal release caused by exposure to high extracellular potassium.
In accordance with behavioral results, neither morphine (100 nM to 1 μM) nor deltorphin (100 nM to 500 nM) pretreatment alone significantly attenuated potassium-evoked CGRP release from naïve peripheral nerves (Fig. 3A and Fig 4A-B). In fact, deltorphin exposure resulted in a consistent, though non-significant, trend towards potentiation at all concentrations. In contrast, when nerves were exposed to deltorphin (300 nM) and morphine (500 nM) concurrently, a significant inhibition of evoked release was observed (Fig. 3A, p < 0.01, F = 4.715; one-way ANOVA). In order to ensure that opioid effects on CGRP release from the nerve were congruent with modulation at the peripheral terminals themselves, we also tested the effects of deltorphin (300 nM) and morphine (500 nM) alone and in combination on CGRP release from hind paw skin. CGRP release from the hind paw was considerably less robust overall, but the opioid effects were, indeed, consistent with those observed in the nerve (Figure 3B, p < 0.01, F = 4.788; one-way ANOVA).
Figure 3.
Effect of deltorphin (300 nM) and morphine (500 nM) pretreatment alone and in combination on potassium (K+)-evoked CGRP release from sciatic nerves or hind paws of naïve animals. (A) Neither drug alone significantly altered evoked release from nerves compared to vehicle pretreatment. When drugs were administered together concurrently, K+-evoked release was inhibited. (B) The effects of drugs alone and in combination on release from hind paw are similar to those observed from nerve; only combined opioid administration significantly inhibited K+-evoked release. (C) K+-evoked release was inhibited when nerves were exposed to deltorphin (Delt) then morphine (MS) in sequence immediately prior to stimulation by high K+. No inhibition was observed when the order of drug pretreatment was reversed (MS then Delt) or when nerves were exposed to two separate pretreatments of either deltorphin or morphine in sequence. (D) The inhibitory effect of deltorphin and morphine sequential pretreatment was significantly reversed by including naloxone (Nalox, 1 μM) throughout pretreatment or by including the selective antagonists naltrindole (Nalt, 1 μM) or CTAP (1 μM) with deltorphin or morphine pretreatment, respectively. *p < 0.05 vs. vehicle pretreatment. N = 4-10/group.
Figure 4.
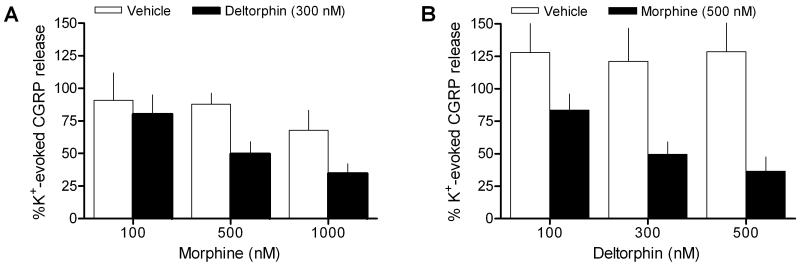
Combined deltorphin and morphine inhibition of potassium (K+)-evoked CGRP release is dependent on the concentration of both drugs. (A-B) Neither drug alone significantly altered evoked release compared to vehicle pretreatment at any concentration (open bars). (A) Concentration-response relationships are depicted for a consistent concentration (300 nM) of deltorphin followed by increasing concentrations of morphine and (B) increasing concentrations of deltorphin followed by a consistent concentration (500 nM) of morphine (closed bars). N = 4-8/group.
We then examined whether the order of drug exposure would affect the inhibition observed with combined deltorphin and morphine. To control for the extended duration of opioid exposure, nerves were first exposed to two separate deltorphin or morphine incubations prior to stimulation. Neither of these sequences significantly altered evoked CGRP release (Fig 3C). In contrast, when nerves were exposed to deltorphin then morphine pretreatments in sequence, a significant inhibition of evoked release was observed. However, when nerves were exposed to the two drugs in reverse order (morphine then deltorphin) prior to potassium-evoked stimulation, no inhibition of CGRP was observed, suggesting that deltorphin exposure is able to enhance subsequent morphine-induced inhibition, but not vice versa (Fig. 3C, p < 0.05, F = 2.839; one-way ANOVA).
The inhibition of evoked CGRP release resulting from sequential deltorphin and morphine (300 nM and 500 nM, respectively) was completely blocked by co-administration of 1 μM naloxone, indicating that the inhibition was mediated via opioid receptors (Fig. 3D). Combined opioid inhibition was also significantly reversed by co-administration of the δ-opioid receptor selective antagonist naltrindole (1 μM) or the μ-opioid receptor selective antagonist CTAP (1 μM) with deltorphin or morphine, respectively, further demonstrating the requirement for activation of both receptor types in sequence (Fig. 3D, p < 0.05, F = 3.82; one-way ANOVA).
Combined opioid inhibition of evoked CGRP was dependent on the concentration of both drugs. When nerves were exposed to a single concentration of deltorphin (300 nM) prior to morphine pretreatment (100 nM to 1 μM), concentration-dependent inhibition was observed (Fig. 4A). Similarly, the inhibitory effect resulting from a constant concentration of morphine (500 nM) was dependent on the preceding concentration of deltorphin given (100 nM to 500 nM) (Fig. 4B).
3.5 Deltorphin enhancing effects are not dependent on the μ-opioid receptor agonist used
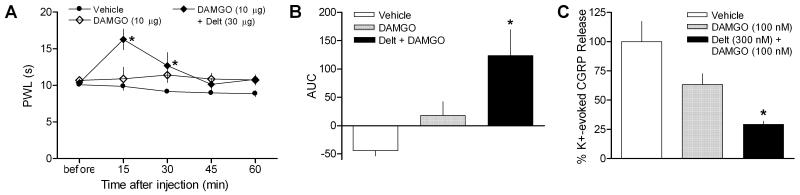
In order to determine whether deltorphin enhancement of μ-opioid receptor function was restricted to morphine, the in vivo and in vitro effects of a more selective μ-opioid receptor agonist, DAMGO, were tested alone and in combination with deltorphin. Similar to the results observed with morphine, intraplantar injection of DAMGO (10 μg) alone did not affect thermal sensitivity. In contrast, PWLs were elevated after co-administration of DAMGO with deltorphin (30 μg), although the duration of the analgesic effect was shorter than that observed with morphine (Fig. 5A, Drug p < 0.0001, F = 11.77; Time p < 0.01, F = 3.775; Interaction p < 0.05, F = 2.665; two-way ANOVA). AUC values confirmed that DAMGO alone did not alter thermal sensitivity when compared to the vehicle-injected group over the 60-min time course. Co-administration of DAMGO and deltorphin resulted in an increase in PWL that was significantly above either of the other two groups over the course of testing (Fig. 5B, p < 0.01, F = 8.607; one-way ANOVA).
Figure 5.
The enhancement of deltorphin exposure on μ-opioid receptor agonist effectiveness does not depend on the agonist used. (A-B) DAMGO (10 μg) alone did not affect PWLs to heat stimulation compared to vehicle-injected values. In contrast, co-administration of deltorphin (Delt, 30 μg) with DAMGO resulted in significantly elevated PWL measurements. (A) Time course. *p < 0.05 vs. vehicle. (B) Area under the curve (AUC). *p < 0.05 vs. vehicle and DAMGO alone groups. (C) DAMGO alone did not significantly inhibit K+-evoked CGRP release compared to vehicle pretreatment. When nerves were exposed to deltorphin prior to DAMGO pretreatment, robust inhibition was observed. *p < 0.05 vs. vehicle pretreatment. N = 4-7/group
The behavioral effects of combined deltorphin and DAMGO were reflected in the effects of the two drugs on CGRP release. Incubation with DAMGO (100 nM) did not result in significant inhibition of potassium-evoked CGRP release when preceded by vehicle exposure (Fig. 5C, p > 0.05 vehicle vs. DAMGO, Bonferroni post-hoc test). In contrast, deltorphin (300 nM) exposure prior to DAMGO pretreatment resulted in robust inhibition of evoked release, similar to results observed with morphine (Fig. 5C, p < 0.05, F = 4.833; one-way ANOVA).
4. Discussion
We hypothesized that δ- and μ-opioid receptor agonists, when delivered together, would unmask peripheral opioid receptor function in the absence of injury or inflammation. Our results support this hypothesis and add to previous findings demonstrating that co-administration of agonists for the two receptor types in the CNS can improve analgesic outcomes.
The primary finding of the present study is that co-administration of δ- and μ-opioid receptor agonists promotes peripheral opioid receptor function in naïve animals in vivo. In accordance with previous studies, the δ-opioid receptor agonist deltorphin as well as the μ-opioid receptor agonists morphine and DAMGO were all without effect on measures of behavioral nociception when given alone. We demonstrated here that when combined, δ- and μ- opioid receptor agonists are able to produce significant analgesia to noxious heat in naïve animals within minutes of intraplantar injection. The analgesic effect resulting from combined deltorphin and morphine was confirmed to be peripherally-restricted and was blocked by naloxone and the selective antagonists naltrindole and CTAP, indicating that it was mediated by peripheral δ- and μ-opioid receptors.
Previous research has established that co-administration of δ- and μ-opioid receptor agonists can have important functional consequences in the CNS, including the production of synergistic analgesic outcomes. Modulation of opioid receptor-mediated analgesia has been observed following intrathecal, intracerebroventricular, and systemic co-administration of δ- and μ-opioid receptor agonists [1,18,19,24,29,42]. The nature of the interaction between the agonists for the two receptor types appears to depend on the ratio of agonists given. For example, in a study of fixed-ratio combinations of morphine and sub-analgesic doses of the δ-opioid receptor agonist DPDPE, a 20% DPDPE combination was synergistic, while results from a 40% DPDPE combination did not differ from predicted additive values [1]. In the present study, co-administration of δ- and μ-opioid receptor agonists produced only enhanced effects on paw withdrawal latencies and CGRP release and did so at a variety of ratio combinations. It is possible that increased doses or concentrations could produce sub-additive interactions. However, given that none of the drugs were with significant effect on their own at the concentrations and doses used here, it would be somewhat surprising if such effects had been observed. Accordingly, the current results add to the accumulating evidence suggesting a role for δ- and μ-opioid receptor interactions in improving analgesic outcomes. Moreover, they demonstrate that such interactions can improve peripherally-mediated opioid analgesia specifically.
We observed combined opioid analgesia in tests of thermal, but not mechanical nociceptive sensitivity, suggesting that the effects were mediated by inhibition of a heat sensitive, TRPV1-positive subset of primary afferent sensory neurons. Because the TRPV1 expressing population of nociceptive primary afferent neurons shows extensive overlap with the peptidergic (Substance P and CGRP expressing) population of nociceptive primary afferent neurons [41], we hypothesized that combined δ- and μ-opioid receptor effects would be reflected in vitro using CGRP release from peripheral nerves as a functional assay. We employed methods similar to those that have previously been used to examine G protein-coupled receptor-mediated modulation of peptide release in sciatic nerves [11], thereby minimizing confounding interaction with other cell types such as keratinocytes, which have been shown to express opioid receptors and release neuroactive peptides [3,6,7]. As expected, while none of the opioid receptor agonists alone were able to significantly affect potassium-evoked CGRP release, pretreatment with δ- and μ-opioid receptor agonists together produced inhibition that was dependent on the concentration of both drugs and was blocked by the non-selective opioid receptor antagonist naloxone as well as by selective antagonists for either receptor.
We further extended the CGRP studies to show that the order of agonist pretreatment determines whether inhibition is produced. Specifically, when deltorphin was administered prior to morphine, opioid receptor-mediated inhibition was unmasked, but evoked release remained unchanged when the agonists were given in the reverse order. Furthermore, deltorphin enhanced the inhibition produced by another μ-opioid receptor agonist, DAMGO. These data suggest that activation of δ-opioid receptors can rapidly enhance μ-opioid receptor-mediated effects, but that the reverse does not occur.
Although deltorphin pretreatment alone modestly enhanced potassium-evoked CGRP release, this effect was not significant, consistent with results reported by Patwardhan et al [26]. Furthermore, no sensitization to thermal stimulation was observed following deltorphin treatment over the course of behavioral testing. This is an important finding because it demonstrates that peripheral opioid receptor function can be promoted in naïve animals or tissue without first producing allodynia or significant enhancement of functional measures that accompany other methods. For example, peripheral δ-opioid receptors show significant functional upregulation following 15 min of pre-exposure to bradykinin or arachidonic acid in vivo, but both agents themselves produce significant allodynia during that time [33]. It is possible that deltorphin produced a transitory allodynic effect that was missed during the time between injection of the drug and our first time point (15 min); however, we do not know of any report suggesting this would be the case.
A recent study by Scherrer et al. [36] using DOReGFP reporter mice suggests that μ-, but not δ-opioid receptors are present on the heat-sensitive, TRPV1-expressing, peptidergic subpopulation of primary afferent neurons. Rather, the δ-opioid receptor was found to be present only on myelinated fibers and the complimentary, lectin IB4-binding population of unmyelinated fibers. Accordingly, activation of the δ-opioid receptor affected mechanical, but not thermal sensitivity [36]. The interpretation that the function of μ-opioid receptors in particular was promoted in the current study is thus supported by the previous report, since combined opioid administration affected sensitivity only to heat, but not mechanical stimulation. If δ- and μ-opioid receptors do, indeed, have complementary expression patterns and roles in nociceptive behavior, then the enhancement of μ-opioid receptor function by δ-opioid receptor agonist exposure must be due to an indirect interaction between the two receptor types on separate cells.
Nonetheless, the present data do not rule out a contribution of the δ-opioid receptor in mediating the observed analgesic or inhibitory effects, nor do they exclude the possibility of δ-opioid receptor activation promoting μ-opioid receptor function via a direct mechanism. It is unclear if the thermal and mechanical modal separation found in mice also occurs in rats. The peptidergic and TRPV1-expressing neuronal populations themselves are substantially less distinct from the IB4-binding population in rats [14,30]. As such, δ-opioid receptors may be more poised to alter peptide release or thermal nociception in rats than in mice. Indeed, previous studies have indicated a role for peripheral δ-opioid receptors in the modulation of neuropeptide release [26,27] as well as thermal analgesia in rats [33], even in the absence of injury or prolonged inflammation, which can alter opioid receptor expression patterns.
In addition, in contrast to the results reported by Scherrer et al., a recent study provided evidence to suggest that μ- and δ-opioid receptors do, indeed, colocalize in mouse sensory neurons [43]. Wang et al. found that the δ-opioid receptor is expressed in the peptidergic as well as the IB4-binding population of primary afferent neurons using RT-PCR. Also, colocalization of δ- and μ-opioid receptors was observed using in situ hybridization and immunostaining methods, and agonists for both receptors were able produce inhibitory effects in the same dorsal root ganglion (DRG) neuron [43]. Previous studies have suggested that δ-opioid receptors are expressed in peptidergic, heat-sensitive sensory neurons in mice as well. For example, δ-opioid receptor activation has been reported to modulate CGRP release from mouse DRG neurons [4], and the potency of intrathecal deltorphin to produce thermal analgesia in the tail flick test has been shown to be unaltered in μ-opioid receptor knockout mice [20]. Furthermore, thermal analgesia and inhibition of neuropeptide release by deltorphin has been observed at the level of the central terminal of primary afferent neurons in studies using both mice and rats [25,31]. Other studies have supported a more direct mechanism of modulation between δ- and μ-opioid receptor function, including the formation of heterodimeric complexes, as well [13,35]. Therefore, further investigation is needed to elucidate the mechanism by which co-administration of δ- and μ-opioid receptor agonists promotes peripheral opioid receptor function and whether this occurs via an indirect effect or between receptors present on the same cell.
Summary
We found that combining δ- and μ-opioid receptor agonists promotes peripheral opioid receptor function under naïve conditions, while local administration of agonists for either receptor alone produces no significant effect. Our results suggest that, as in the CNS, opioid receptor interactions in the periphery may be useful targets for future analgesic therapies. Peripheral delivery of exogenous opioids may provide a means to avoid the centrally-mediated side effects and abuse potential associated with systemic opioid administration. Currently, the use of peripherally restricted opioids in pain treatment is limited by inconsistent and sometimes modest results. Increased understanding of the mechanisms by which peripheral opioid receptors gain functional competence may provide insight into the reasons for inconsistencies in the clinical efficacy of peripherally delivered opioids. Extension of our findings suggests a potential application for combined peripheral opioid administration under conditions wherein local delivery of a single opioid has been shown to be insufficient to reliably provide meaningful pain relief.
Acknowledgements
This work was supported by National Institute of Health grants DA009641 (C.N.H. and C.S.) and 2T32 DA007097 (C.S.) and the Minnesota Medical Foundation. The authors have no conflicts of interest. We thank Aaron Overland and George Wilcox for technical assistance and critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Adams JU, Tallarida RJ, Geller EB, Adler MW. Isobolographic superadditivity between delta and mu opioid agonists in the rat depends on the ratio of compounds, the mu agonist and the analgesic assay used. J Pharmacol Exp Ther. 1993;266:1261–7. [PubMed] [Google Scholar]
- [2].Antonijevic I, Mousa SA, Schafer M, Stein C. Perineurial defect and peripheral opioid analgesia in inflammation. J Neurosci. 1995;15:165–72. doi: 10.1523/JNEUROSCI.15-01-00165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bae S, Matsunaga Y, Tanaka Y, Katayama I. Autocrine induction of substance P mRNA and peptide in cultured normal human keratinocytes. Biochem Biophys Res Commun. 1999;263:327–33. doi: 10.1006/bbrc.1999.1285. [DOI] [PubMed] [Google Scholar]
- [4].Bao L, Jin SX, Zhang C, Wang LH, Xu ZZ, Zhang FX, Wang LC, Ning FS, Cai HJ, Guan JS, Xiao HS, Xu ZQ, He C, Hokfelt T, Zhou Z, Zhang X. Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron. 2003;37:121–33. doi: 10.1016/s0896-6273(02)01103-0. [DOI] [PubMed] [Google Scholar]
- [5].Berg KA, Patwardhan AM, Sanchez TA, Silva YM, Hargreaves KM, Clarke WP. Rapid modulation of micro-opioid receptor signaling in primary sensory neurons. J Pharmacol Exp Ther. 2007;321:839–47. doi: 10.1124/jpet.106.116681. [DOI] [PubMed] [Google Scholar]
- [6].Bigliardi-Qi M, Sumanovski LT, Buchner S, Rufli T, Bigliardi PL. Mu-opiate receptor and Beta-endorphin expression in nerve endings and keratinocytes in human skin. Dermatology. 2004;209:183–9. doi: 10.1159/000079887. [DOI] [PubMed] [Google Scholar]
- [7].Bigliardi PL, Bigliardi-Qi M, Buechner S, Rufli T. Expression of mu-opiate receptor in human epidermis and keratinocytes. J Invest Dermatol. 1998;111:297–301. doi: 10.1046/j.1523-1747.1998.00259.x. [DOI] [PubMed] [Google Scholar]
- [8].De Kock MF, Pichon G, Scholtes JL. Intraoperative clonidine enhances postoperative morphine patient-controlled analgesia. Can J Anaesth. 1992;39:537–44. doi: 10.1007/BF03008314. [DOI] [PubMed] [Google Scholar]
- [9].Endres-Becker J, Heppenstall PA, Mousa SA, Labuz D, Oksche A, Schafer M, Stein C, Zollner C. Mu-opioid receptor activation modulates transient receptor potential vanilloid 1 (TRPV1) currents in sensory neurons in a model of inflammatory pain. Mol Pharmacol. 2007;71:12–8. doi: 10.1124/mol.106.026740. [DOI] [PubMed] [Google Scholar]
- [10].Faktorovich EG, Basbaum AI. Effect of Topical 0.5% Morphine on Postoperative Pain After Photorefractive Keratectomy. J Refract Surg. 2010 doi: 10.3928/1081597X-20100212-06. doi: 10.3928/1081597X- 20100212-06. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [11].Fischer MJ, Reeh PW. Sensitization to heat through G-protein-coupled receptor pathways in the isolated sciatic mouse nerve. Eur J Neurosci. 2007;25:3570–5. doi: 10.1111/j.1460-9568.2007.05582.x. [DOI] [PubMed] [Google Scholar]
- [12].Fischer MJ, Reeh PW, Sauer SK. Proton-induced calcitonin gene-related peptide release from rat sciatic nerve axons, in vitro, involving TRPV1. Eur J Neurosci. 2003;18:803–10. doi: 10.1046/j.1460-9568.2003.02811.x. [DOI] [PubMed] [Google Scholar]
- [13].Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–58. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- [15].Gupta A, Bodin L, Holmstrom B, Berggren L. A systematic review of the peripheral analgesic effects of intraarticular morphine. Anesth Analg. 2001;93:761–70. doi: 10.1097/00000539-200109000-00042. [DOI] [PubMed] [Google Scholar]
- [16].Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- [17].Hassan AH, Ableitner A, Stein C, Herz A. Inflammation of the rat paw enhances axonal transport of opioid receptors in the sciatic nerve and increases their density in the inflamed tissue. Neuroscience. 1993;55:185–95. doi: 10.1016/0306-4522(93)90465-r. [DOI] [PubMed] [Google Scholar]
- [18].He L, Lee NM. Delta opioid receptor enhancement of mu opioid receptor-induced antinociception in spinal cord. J Pharmacol Exp Ther. 1998;285:1181–6. [PubMed] [Google Scholar]
- [19].Horan P, Tallarida RJ, Haaseth RC, Matsunaga TO, Hruby VJ, Porreca F. Antinociceptive interactions of opioid delta receptor agonists with morphine in mice: supra- and sub-additivity. Life Sci. 1992;50:1535–41. doi: 10.1016/0024-3205(92)90144-e. [DOI] [PubMed] [Google Scholar]
- [20].Hosohata Y, Vanderah TW, Burkey TH, Ossipov MH, Kovelowski CJ, Sora I, Uhl GR, Zhang X, Rice KC, Roeske WR, Hruby VJ, Yamamura HI, Lai J, Porreca F. delta-Opioid receptor agonists produce antinociception and [35S] GTPgammaS binding in mu receptor knockout mice. Eur J Pharmacol. 2000;388:241–8. doi: 10.1016/s0014-2999(99)00897-3. [DOI] [PubMed] [Google Scholar]
- [21].Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hokfelt T. Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci. 1995;15:8156–66. doi: 10.1523/JNEUROSCI.15-12-08156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kabli N, Cahill CM. Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain. 2007;127:84–93. doi: 10.1016/j.pain.2006.08.003. [DOI] [PubMed] [Google Scholar]
- [23].Levine JD, Taiwo YO. Involvement of the mu-opiate receptor in peripheral analgesia. Neuroscience. 1989;32:571–5. doi: 10.1016/0306-4522(89)90279-0. [DOI] [PubMed] [Google Scholar]
- [24].Malmberg AB, Yaksh TL. Isobolographic and dose-response analyses of the interaction between intrathecal mu and delta agonists: effects of naltrindole and its benzofuran analog (NTB) J Pharmacol Exp Ther. 1992;263:264–75. [PubMed] [Google Scholar]
- [25].Overland AC, Kitto KF, Chabot-Dore AJ, Rothwell PE, Fairbanks CA, Stone LS, Wilcox GL. Protein Kinase C Mediates the Synergistic Interaction Between Agonists Acting at {alpha}2-Adrenergic and Delta-Opioid Receptors in Spinal Cord. J Neurosci. 2009;29:13264–73. doi: 10.1523/JNEUROSCI.1907-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM. Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci. 2005;25:8825–32. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Patwardhan AM, Diogenes A, Berg KA, Fehrenbacher JC, Clarke WP, Akopian AN, Hargreaves KM. PAR-2 agonists activate trigeminal nociceptors and induce functional competence in the delta opioid receptor. Pain. 2006;125:114–24. doi: 10.1016/j.pain.2006.05.007. [DOI] [PubMed] [Google Scholar]
- [28].Picard PR, Tramer MR, McQuay HJ, Moore RA. Analgesic efficacy of peripheral opioids (all except intra-articular): a qualitative systematic review of randomised controlled trials. Pain. 1997;72:309–18. doi: 10.1016/s0304-3959(97)00040-7. [DOI] [PubMed] [Google Scholar]
- [29].Porreca F, Takemori AE, Sultana M, Portoghese PS, Bowen WD, Mosberg HI. Modulation of mu-mediated antinociception in the mouse involves opioid delta-2 receptors. J Pharmacol Exp Ther. 1992;263:147–52. [PubMed] [Google Scholar]
- [30].Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8:263–72. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Riedl MS, Schnell SA, Overland AC, Chabot-Dore AJ, Taylor AM, Ribeiro-da- Silva A, Elde RP, Wilcox GL, Stone LS. Coexpression of alpha 2A-adrenergic and delta-opioid receptors in substance P-containing terminals in rat dorsal horn. J Comp Neurol. 2009;513:385–98. doi: 10.1002/cne.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rosseland LA. No evidence for analgesic effect of intra-articular morphine after knee arthroscopy: a qualitative systematic review. Reg Anesth Pain Med. 2005;30:83–98. doi: 10.1016/j.rapm.2004.08.022. [DOI] [PubMed] [Google Scholar]
- [33].Rowan MP, Ruparel NB, Patwardhan AM, Berg KA, Clarke WP, Hargreaves KM. Peripheral delta opioid receptors require priming for functional competence in vivo. Eur J Pharmacol. 2009;602:283–7. doi: 10.1016/j.ejphar.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain. 2008;135:271–9. doi: 10.1016/j.pain.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rutherford JM, Wang J, Xu H, Dersch CM, Partilla JS, Rice KC, Rothman RB. Evidence for a mu-delta opioid receptor complex in CHO cells co-expressing mu and delta opioid peptide receptors. Peptides. 2008;29:1424–31. doi: 10.1016/j.peptides.2008.03.019. [DOI] [PubMed] [Google Scholar]
- [36].Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–59. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J Pharmacol Exp Ther. 1989;248:1269–75. [PubMed] [Google Scholar]
- [38].Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat Med. 2003;9:1003–8. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- [39].Stiles J, Honda CN, Krohne SG, Kazacos EA. Effect of topical administration of 1% morphine sulfate solution on signs of pain and corneal wound healing in dogs. Am J Vet Res. 2003;64:813–8. doi: 10.2460/ajvr.2003.64.813. [DOI] [PubMed] [Google Scholar]
- [40].Taiwo YO, Levine JD. Kappa- and delta-opioids block sympathetically dependent hyperalgesia. J Neurosci. 1991;11:928–32. doi: 10.1523/JNEUROSCI.11-04-00928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–43. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- [42].Vaught JL, Takemori AE. Differential effects of leucine and methionine enkephalin on morphine-induced analgesia, acute tolerance and dependence. J Pharmacol Exp Ther. 1979;208:86–90. [PubMed] [Google Scholar]
- [43].Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, Wu SX, Hokfelt TG, Bao L, Zhang X. Coexpression of {delta}- and {micro}-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A. 2010;107:13117–22. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wenk HN, Brederson JD, Honda CN. Morphine directly inhibits nociceptors in inflamed skin. J Neurophysiol. 2006;95:2083–97. doi: 10.1152/jn.00394.2005. [DOI] [PubMed] [Google Scholar]
- [45].Wenk HN, Nannenga MN, Honda CN. Effect of morphine sulphate eye drops on hyperalgesia in the rat cornea. Pain. 2003;105:455–65. doi: 10.1016/S0304-3959(03)00260-4. [DOI] [PubMed] [Google Scholar]
- [46].Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- [47].Zollner C, Shaqura MA, Bopaiah CP, Mousa S, Stein C, Schafer M. Painful inflammation-induced increase in mu-opioid receptor binding and G-protein coupling in primary afferent neurons. Mol Pharmacol. 2003;64:202–10. doi: 10.1124/mol.64.2.202. [DOI] [PubMed] [Google Scholar]