It has long been thought, on the basis of observations with vital microscopy, that a sudden decrease occurs in the blood flow of skin homografts at the time of their rejection.15,22 There is evidence that the same is true in homotransplanted whole organs. In 1953, Dempster2 demonstrated a striking loss of small branches of the arterial tree in renal homografts which were excised during their rejection and studied by means of angiography. More than 10 years later, Kountz and associates7 and Williams26 and their associates obtained serial flow measures in canine kidney homografts with a radioactive-hippuran technique. In untreated recipients, there was a decline in total renal flow which was most dramatic at the time of rejection.
Shortly after, it was reported that rejection after clinical renal homotransplantation was accompanied by changes which could be readily explained only by ischemia. These included a drop in urine sodium concentration, an increase in urine urea and creatinine concentration, oliguria, a reduction in creatinine clearance, and arterial hypertension.21 The findings, which simulate those which can be produced experimentally by partial occlusion of a renal artery, were in patients who had developed rejection while receiving azathioprine therapy. They were quickly reversed with the addition of prednisone. Subsequent studies in dogs have confirmed both that a reduction in blood flow is coincident with renal homograft rejection5, 6, 14, 16, 24 and that this change can be prevented or reversed with appropriate immunosuppressive therapy.6, 16
Such studies have raised the possibility that ischemia is an important general mechanism of rejection. In the present study this question has been examined in liver transplants by determining hepatic blood flow in both treated and untreated recipients of orthotopic homografts. In addition, a separate electron microscopic study was made with serial liver biopsies from untreated recipients, with the special objective of looking for ultrastructural abnormalities in either large or small blood vessels which could explain hemodynamic changes.
METHODS
Experimental groups
Mongrel dogs, with an average weight of 8 to 16 kilograms, were immunized against hepatitis and distemper and used as homograft recipients. Orthotopic hepatic transplants were performed, as previously described,20 with pentobarbital anesthesia combined with the tranquilizer, phencyclidine hydrochloride. Dogs that died of technical complications or intussusception were excluded. Serum bilirubin, alkaline phosphatase, serum glutamic oxalacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), and complete blood counts were obtained frequently in all animals. The patency of vascular anastomoses was established at autopsy.
Group 1
The liver flow was studied in 10 unmodified recipients. In 8 of these, serial postoperative measurements were done daily or every other day until the death of the animal; in the other 2, measurements were done only on the first posttransplant day. In 9 of these experiments the liver blood flow was also measured in the donor animal on the day before transplantation.
Group 2
Five recipients were administered antilymphocyte globulin (ALG) and azathioprine. ALG was given daily for 5 days pretransplant and 30 days after operation; subsequent injections were twice a week. The preparation and the dosage of ALG was the same as in previous reports from this institution.4,19 Azathioprine was given daily from the day of transplantation. The dose varied between 1 and 8 mg. per kilogram of body weight per day, depending on the white blood cell count of the animal. Blood flow measurements were done for as long as 19 days, usually every third day.
Group 3
Five untreated recipients were used for pathologic studies. The donor liver was biopsied before transplantation. Postoperatively, biopsies were obtained every second or third day until death. Each tissue sample was divided into 3 pieces. The first piece was immediately diced up into tiny fragments, fixed in osmium tetroxide, processed, and embedded in Araldite. Sections 0.5μ thick were cut, stained with Azur II, and examined by light microscopy. Later, very thin sections were examined in a Siemens Elmiskop 1A electron microscope. The second piece was snap-frozen at −70° C, and sections cut on a cryostat were examined in ultraviolet light after treatment with fluorescein isothiocyanate-conjugated antisera to canine IgG and complement. The third piece was fixed in 10 percent formalin, processed, and embedded in paraffin wax. Sections were examined by ordinary light microscopy after they had been stained with hematoxylin and eosin, van Gieson’s method for elastic and methyl green pyronin.
Flow studies
The liver blood flow was studied in the unanesthetized state by measuring the washout of the inert, radioactive gas, xenon-133. The procedure was essentially the same as that used by Hollenberg and Dougherty.3 Approximately 500 μc of Xe133 dissolved in 1 to 3 ml. of isotonic saline was rapidly injected through indwelling catheters in the portal and the hepatic arterial systems (Fig. 1), giving a peak radioactivity over the liver of 30,000 to 100,000 counts per minute. To obtain return to background radioactivity between the injections, 10 to 30 minutes were required. The washout of the isotope was monitored externally with a collimated, 1 inch sodium iodide crystal scintillation detector mounted above the liver approximately 1 cm. from the dog. A linear rate meter and a recorder were used.
Fig. 1.
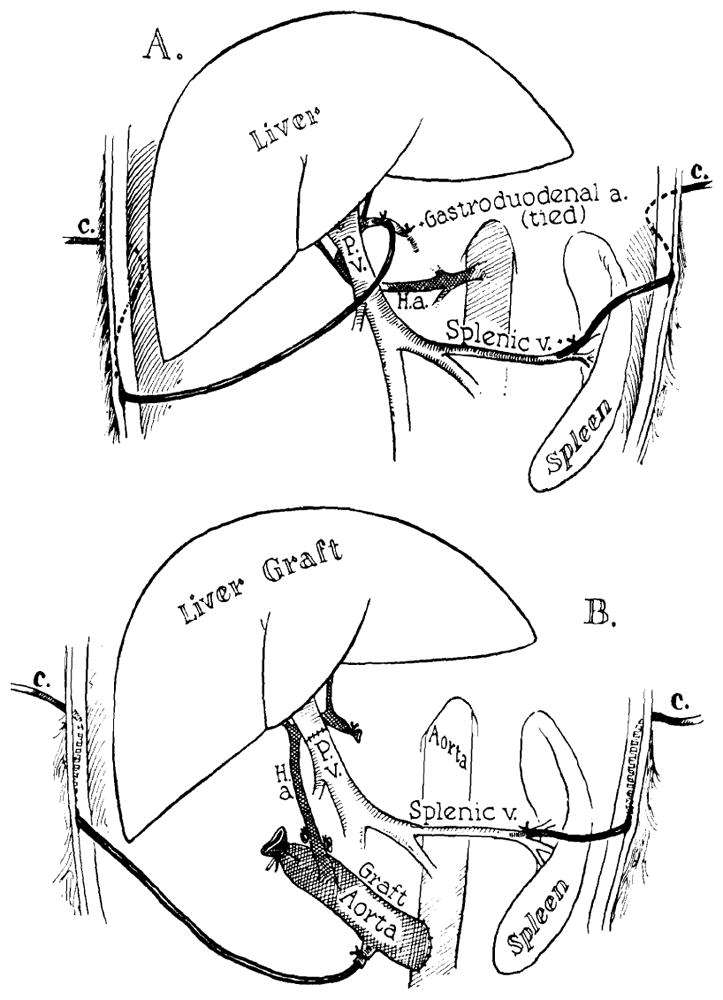
The placement of indwelling catheters in the portal and hepatic arterial systems in the donor (A) and the recipient animal (B). Note that the gastroduodenal artery in the donor is tied to ensure delivery of the isotope solely to the liver.
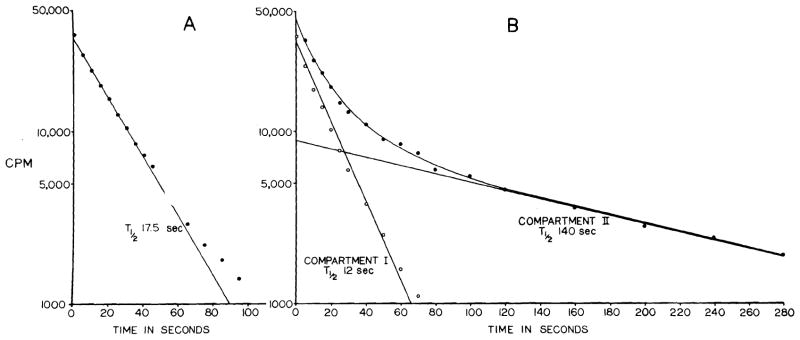
To obtain the disappearance rate constant, K, the curves were replotted in a semilogarithmic system where , T½ being the half time of disappearance. In most instances the first 9/10 of the semilogarithmic plot was an apparently straight line, only the last part being curved (Fig. 2, A). In some others, the plot was a curved line throughout (Fig. 2, B). The first type was regarded as an essentially one-compartment system, and T½ was assessed directly from the straight part of the plot. In the other type a second compartment was subtracted graphically, and T½ for this and the resulting first compartment were assessed. The flow in the second compartment was always less than 20 percent of that in the first, and in the results only the flow values for the first compartment are given. Knowing K, the blood flow was calculated as follows23:
Fig. 2.
Semilogarithmic plots of typical disappearance curves following injection of Xe133. Usually most of the plot was a straight line (A). In some instances, however, it was a curve that could be resolved into 2 compartments (B).
Where L is the partition coefficient between tissue and blood (this was calculated for the prevailing hematocrit according to Veall and Mallett25 with the relative solubility values of Xe133 in plasma, erythrocyte, and liver given by Conn.1), and P is the specific gravity which for the liver is 1.02.13
If the solubility of Xe133 in liver tissue were to change during rejection, a systematic error would be introduced in the flow calculations. The relative solubility of the isotope in normal and rejected liver tissue was, therefore, compared in two experiments, in vitro. After the livers had been perfused with lactated Ringer’s solution until they became clear of blood, equal aliquots of homogenized tissue were mixed with equal amounts of Xe133 in gas sampling tubes at 37° C. for 30 minutes. The average ratio between the activity in homogenates of normal liver and rejected liver homografts were 0.91 and 1.02, respectively. It was concluded that the same solubility value could be used throughout without inducing an error exceeding 10 percent.
Concomitant with the blood flow studies, cardiac output was measured by an indicator dilution method with Xe133 as the tracer substance.17 An isotonic saline solution of the isotope was infused at a known constant rate (R) into the systemic venous circulation, and samples were taken in the pulmonary artery through a cardiac catheter. After counting the activity per milliliter in the infused solution (I) and in the mixed venous blood (B), the cardiac output was calculated according to the formula:
RESULTS
Liver blood flow in normal dogs
The mean FPI and FAI in 19 normal dogs studied with the present technique were 221 ± 52 (SD) milliliters per 100 Gm. tissue per minute and 178 ± 50 (SD) ml., respectively.
Liver blood flow in untreated recipients
In the 9 experiments in which studies were obtained before and one day after transplantation, the hepatic blood flow was usually slightly lower in the recipient than it had previously been in the donor. The declines in FPI and FAI were of the same magnitude. Part of the difference in flow could be accounted for by lower cardiac outputs in the recipient animals. Mean values are given in Fig. 3.
Fig. 3.
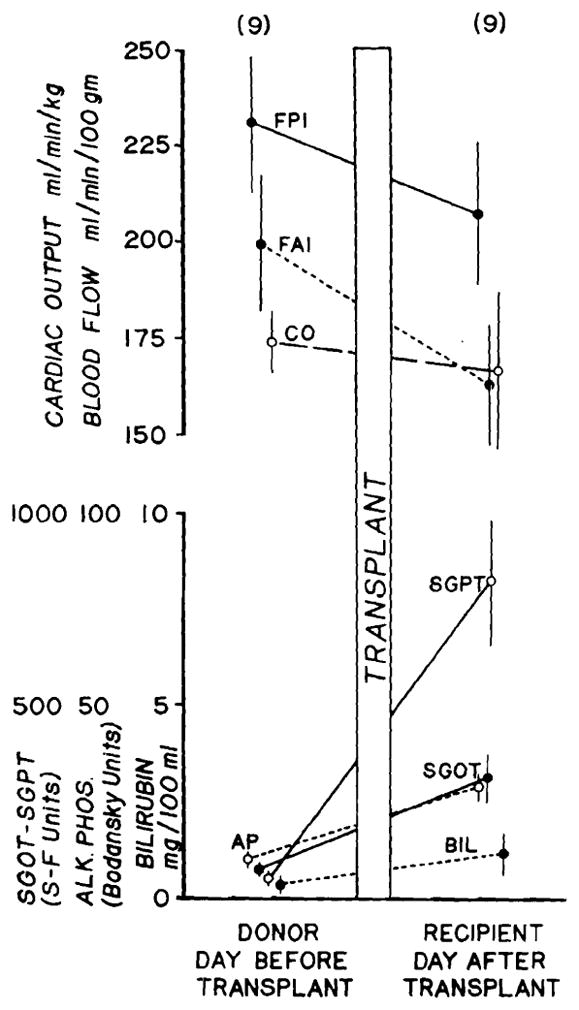
Liver blood flow as measured by portal injection (FPI) and hepatic arterial injection (FAI) in the donor and recipient on the days before and after transplantation. Cardiac output and liver function are also shown. Mean values ± SE in 9 experiments are depicted.
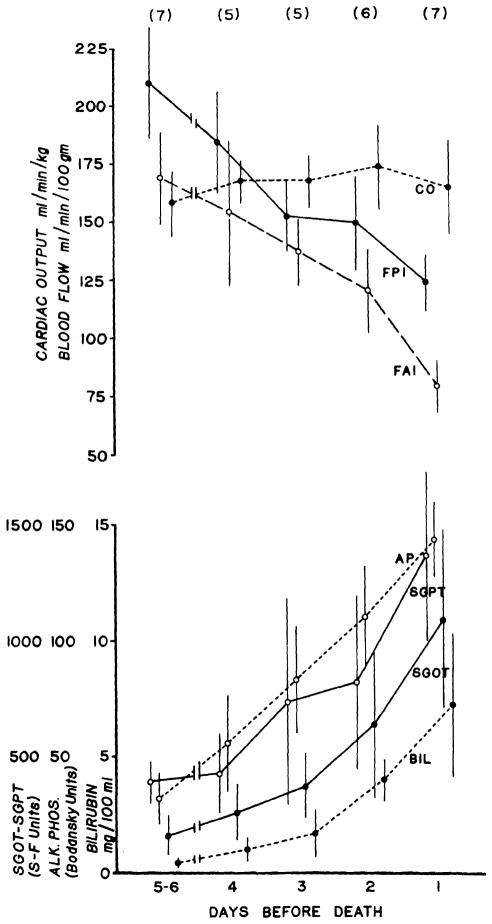
Of the 8 dogs that were studied serially after transplantation, 7 died of rejection after 6 to 10 days, with a mean survival of 8 days. In these animals, there was a progressive deterioration in liver function. Postmortem microscopic examination of the livers showed typical features of rejection.20 A decrease in liver blood flow occurred concomitantly with the deterioration in hepatic function (Fig. 4). The decreases in FPI and FAI were ultimately 41 and 54 percent, respectively. The mean changes in the cardiac output were small (Fig. 4) and could not account for the changes in flow.
Fig. 4.
Liver blood flow, cardiac output, and liver function in 7 unmodified recipients that died of rejection. The mean values ± SE are shown, as well as the number of observations (in parentheses) for each day.
One of the untreated recipients lived for 22 days and ultimately died with pneumonia and wasting. Until death the dog’s liver chemistries were normal, except for an elevated alkaline phosphatase. There was no histologic evidence of rejection. During the first 3 postoperative days, this animal had a subnormal liver blood flow as well as a reduced cardiac output. Subsequently, both values became supernormal for a few days and then settled within normal limits until the last day of study.
Liver blood flow in recipients given immunosuppression
One of the 5 dogs treated with ALG and azathioprine died with typical features of rejection 13 days postoperatively. None of the remaining animals had a clinically diagnosed rejection during the first 20 days. One died after 14 days of an anaphylactic reaction during a blood transfusion One died after 4 months and 4 days due to an intestinal volvulus; histologically, the homograft was normal except for a few mononuclear cells in the portal tracts. The other 2 are still alive after 7 months.
The changes in blood flow in the dog that died of early rejection were similar during the first 9 days to those in unmodified recipients; the flow decreased concomitantly with a deterioration in liver function. However, on the eleventh posttransplantation day, the flow improved at the same time as the liver chemistries had begun to return toward normal. The cardiac output was unchanged during these events. On the thirteenth postoperative day, the day of death, there was again a drop in blood flow along with a deterioration in liver function. At this time, cardiac output was also markedly decreased (Fig. 5).
Fig. 5.
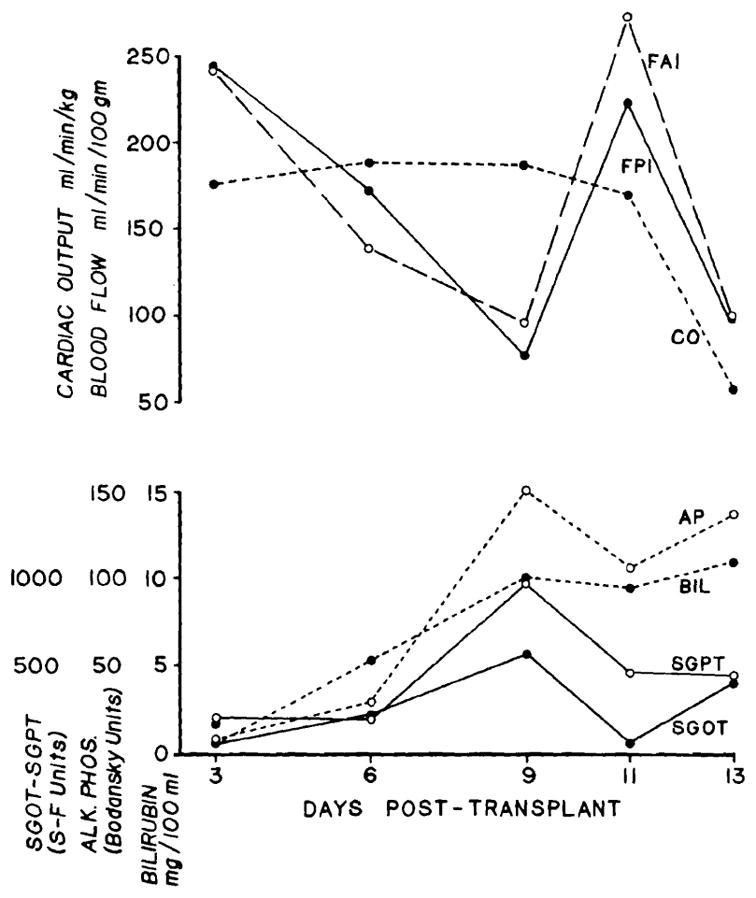
Liver blood flow, cardiac output, and liver function in a recipient receiving immunosuppression. The animal died of rejection 13 days after transplant. Note the concomitant improvement of flow and liver function on Day 11, indicating reversibility of the flow changes.
The remaining 4 animals, which had little or no evidence of rejection as judged by liver function tests, did not have any significant changes in blood flow or cardiac output during the study period of 13 to 19 days (Fig. 6).
Fig. 6.
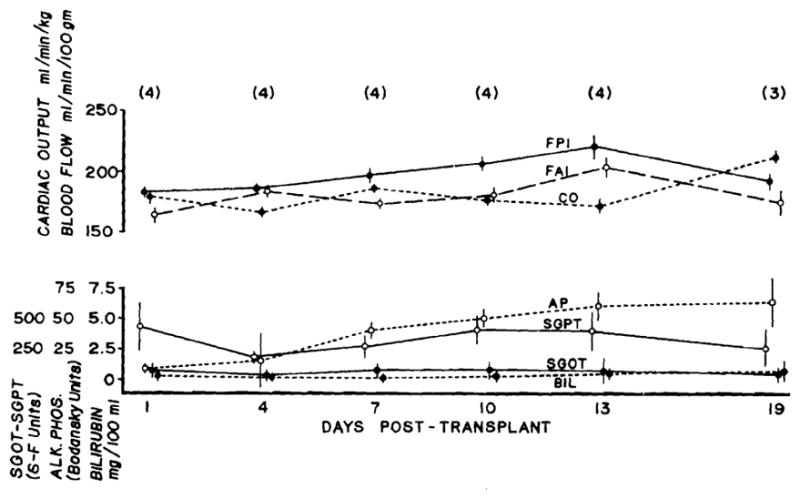
Liver blood flow, cardiac output, and liver function in 4 recipients which received immunosuppression and had little or no evidence of rejection. Mean values ± SE are given, as well as the numbers of observation (in parentheses).
Serial pathologic changes in rejecting liver homografts
The 5 untreated dogs had evidence of a typical rejection, as judged by liver chemistries. All died in 4 to 9 days. The biopsies obtained on Day 2 showed slight dilatation and congestion of the centrilobular sinusoids and damage to a variable number of the hepatocytes in the central zones of the lobules. In the injured cells there was dilatation and destruction of the rough endoplasmic reticulum, clumping of swollen mitochondria, and shedding of cytoplasm. Macrophages containing ingested fragments of cytoplasm and organelles were present in the tissue spaces. These cells, together with neutrophil polymorphonuclear leukocytes and occasional lymphocytes, were common around the portal and central veins. The lymphocytes were ordinary, small lymphocytes, with few organelles in their cytoplasm. No immunoglobulins were detected at this stage.
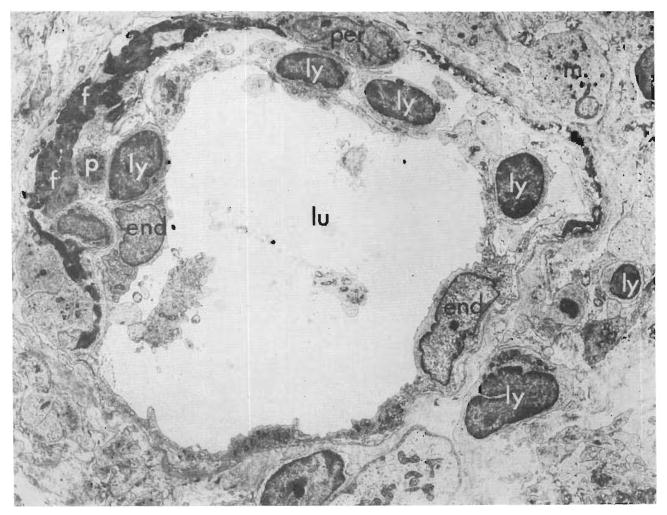
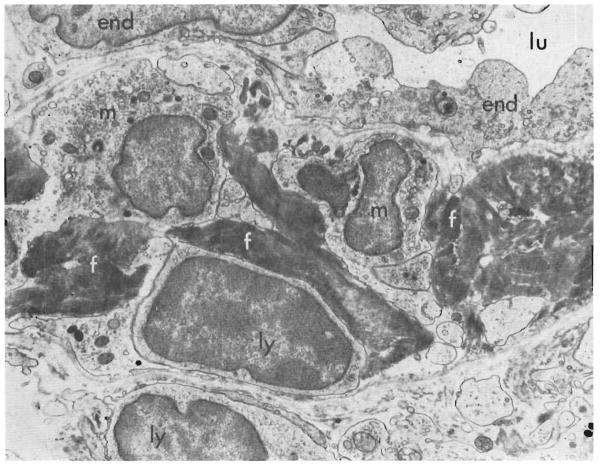
By the fourth day cellular infiltration was marked around the portal and central veins. Large lymphoid cells with pyroninophilic cytoplasm that was full of polyribosomes, but lacking in rough endoplasmic reticulum, were common and could be seen beneath the endothelium of the veins (Figs. 7 and 9) and in the space of Disse. Some were touching the endothelial cells. The tenuous walls of many of the centrilobular sinusoids appeared disrupted (Fig. 8), and fibrin lay in the venous subendothelial spaces (Figs. 7 and 9). A few plasma cells with abundant rough endoplasmic reticulum were present, particularly in the portal tracts. The centrizonal hepatocytes were now necrotic, and in one hepatic homograft the centrilobular bile canaliculi lacked microvilli and contained bile plugs. Immunofluorescence showed no deposits of immunoglobulin G and complement in the vessel walls, but the cytoplasm of several of the infiltrating cells “stained” positively for IgG.
Fig. 7.
Biopsy, 4 days after transplantation, of an hepatic homograft in an untreated dog. Electron micrograph showing a central hepatic vein. Lymphocytes (ly), platelets (p), and fibrin (f) lie beneath the endothelial lining (end) of the vessel. Fluid and cells lie in the perivascular space (per = pericyte; m = macrophage; lu = lumen of vein). (Lead stain. ×2,250.)
Fig. 9.
Biopsy of untreated canine hepatic homograft, 4 days after transplantation. Electron micrograph showing part of wall of a vein in a small portal tract. The wall is infiltrated by lymphocytes (ly), macrophages (m), and fibrin (f) (lu = lumen of vein; end = endothelial lining cells.) (Lead stain, ×6,000.)
Fig. 8.
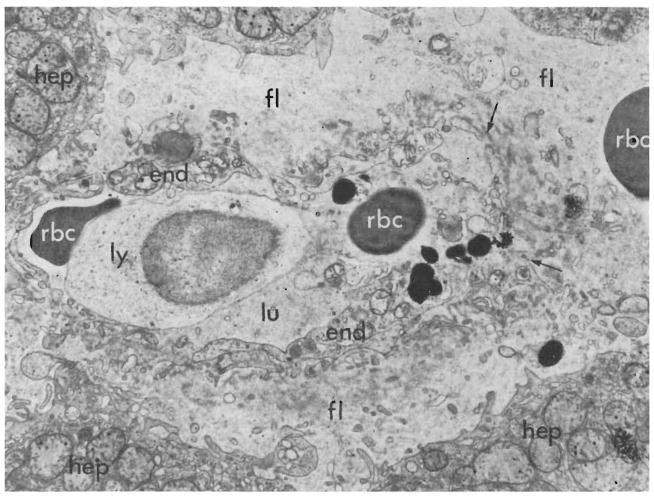
Biopsy of canine hepatic homograft, 4 days after transplantation. Electron micrograph showing a centrilobular sinusoid. The endothelial lining (end) is ruptured at the point marked with arrow. The space of Disse is wider than normal and contains a red cell (rbc) and fluid (fl). A lymphoid cell (ly) and two erythrocytes (rbc) are present in the lumen (lu) of the sinusoid. The adjacent hepatocytes (hep) are injured, as shown by swelling and clumping of their mitochondria and loss of their rough endoplasmic reticulum. (Lead stain. ×6,000.)
The later biopsies were less instructive. Necrosis of hepatocytes was widespread, and large numbers of lymphoid cells, many macrophages, some polymorphs, and plasma cells were present in the portal and central zones and lying between the necrotic hepatocytes. The lumina of several of the central veins were completely blocked by collections of infiltrating mononuclear cells. Cholestasis was pronounced in the better-preserved peripheral areas of the lobules. In one hepatic homograft, 8 days after transplanation there was deposition of IgG and complement in the walls of several small arteries, and, ultra-structurally, a homogeneous, finely granular deposit was present between the endothelium and the internal elastic lamina. By light microscopy a few of these affected arteries showed “fibrinoid necrosis” of their walls. No deposits of immunoglobulin or complement were found in the other homografts.
DISCUSSION
Techniques for liver blood flow determination during rejection must be independent of liver function. The measurement of the clearance of inert radioactive gases fulfills this requirement and has the further advantage of permitting daily studies with a minimum of manipulation of the animal. The method does not differentiate clearly between the fractional contribution to total liver blood from the hepatic arterial and portal venous sources since there is variable presinusoidal or intrasinusoidal communication of the 2 vascular systems.12 Consequently, the washout of the isotope is not due solely to tissue perfusion by the vascular system which receives the injection. Nevertheless, the fact that the flow values actually differ with the route of isotope administration indicates that the intrahepatic mixing is incomplete.3, 13
The results obtained with this method after homotransplantation of the liver show that total liver blood flow decreases significantly at the time of rejection. Conversely, the decline was not seen in one untreated animal which did not have a rejection, and it was prevented altogether in other dogs which received adequate immunosuppression. On one occasion, flow fell during rejection in a treated animal and was later restored when liver chemistries began to improve.
Some indirect evaluation of the importance of relative changes in portal and hepatic arterial circulation can be deduced from observations of Hollenberg and Dougherty.3 They found that total occlusion of the hepatic artery decreases both the FPI and FHI to about ⅔, while occlusion of the portal vein decreases FHI to approximately ⅓ of control values. The recorded changes in our animals would thus seem to be best explained by a decrease in both portal and arterial flow. The decrease might well, however, be more marked in one of these systems than in the other.
In those animals which had clinically evident rejection, the reduction in liver blood flow did not precede the characteristic abnormalities of liver function. The temporal relation between flow alterations and the biochemical changes was almost absolute. Thus, the order of events was not completely comparable to that reported in rejecting canine kidney homografts in which renal blood flow has been noted prior to any deterioration in function.6, 14
Nevertheless, the findings confirm earlier suspicions that there is an important component of ischemia in the rejection of liver homografts. On the grounds of histologic findings, it was initially suggested that blood flow was choked off at a sinusoidal level.8, 18 Subsequently, Moore and his associates9 proposed, on the basis of angiographic evidence, that lesions of larger vessels within the portal tracts were responsible for devascularization of discrete, rather large areas of hepatic parenchyma. The hypothesis was weakened, although not refuted, by the rarity of structural abnormalities of these larger vessels in the liver homografts of either treated or untreated recipients.20 In the latter study, the possibility was raised again, on the basis of preliminary electronmicroscopic observations, that injury to the sinusoidal bed was responsible. In some areas, mononuclear cells were found to be fused to, and presumably damaging, the centrisinusoidal endothelium. The possible analogy was pointed out between these findings and the peritubular capillary lesions described by Kountz7 and Porter11 and their associates in rejecting renal homografts.
The fact that blood flow is rapidly restored upon reversal of rejection could be explained by an initial mechanism of heightened vasomotor reactivity, although an attempt by Moore and his associates9 to affect the course of rejection by intra-arterial administration of a vasodilating agent was not successful. Consequently, the principal effort in the present study was to find an anatomic explanation for the hemodynamic changes. The findings with electronmicroscopy suggest that the reduced blood flow is mainly due to damage to the veins and sinusoidal bed of the homograft. The sinusoids appear to have actually disrupted, while the central veins became narrowed by masses of cells in their lumina and by cells and fibrin lifting the endothelium from the vessel wall. The portal veins showed similar changes in their walls, but their lumina were less frequently filled with infiltrating cells. Platelet aggregates were not seen in these vessels.
These findings are compatible with the hypothesis that acute rejection in the untreated hepatic homograft is predominantly cell mediated. We have no evidence that circulating antibody plays a significant role at this time. Deposition of IgG and complement on and in vessel walls was not apparent until 8 days after transplantation, at a time when the rejection process was far advanced. Paronetto and associates,10 in their studies on auxiliary hepatic homografts without a portal blood supply, also found that gammaglobulin did not appear in the walls of the hepatic arteries until the second week after transplantation. There is still no proof that the lymphoid cells damage the vascular endothelium of the hepatic graft, but it is probable that this occurs. No evidence was obtained in these orthotopic liver grafts of the periportal “piecemeal” necrosis described by Paronetto and co-workers.10
SUMMARY
The blood flow in orthotopic canine liver homografts was investigated in awake animals with a Xe133 washout technique. Cardiac output was also studied. Ten dogs which received no immunosuppressive treatment lived for as long as 22 days (mean 8.5 days). With the onset of rejection, as diagnosed by elevations in bilirubin, SGOT, and SGPT, there was a significant decrease in both components of liver blood flow which could not be accounted for by changes in cardiac output.
These findings were correlated with the immunofluorescent and ultrastructural findings in 5 additional experiments in which biopsies of homografts were obtained before and every second day for 4 to 8 days after orthotopic liver homotransplantation to untreated recipients. It was found that lymphoid cell infiltration of the grafts commenced at about 4 days after transplantation and resulted in damage to the walls of the portal and central veins and to the centrilobular sinusoids. Localization of immunoglobulin G and complement in the homograft was a rare and late phenomenon.
Another 5 recipients were given immunosuppression with horse antilymphocyte globulin and azathioprine. When there was no biochemical indication of rejection, the liver blood flow was essentially unchanged in animals studied for as long as 19 days after transplant. The findings in these 3 experimental groups indicate that decreased blood flow and consequent ischemia is an important factor in the rejection of liver homografts, that such changes can be prevented by effective immunosuppression, and that an anatomic basis for the flow alterations could be the disruption of the centrilobular sinusoidal walls and the intraluminal and subendothelial accumulation of host lymphoid cells in the central and portal veins.
Acknowledgments
This work was supported by United States Public Health Service Grants F05-TW-1154, AM 06283, AM 06344, HE 07735, AM 07772, AI 04152, FR 00051, and FR 00069, and by grants from the British Heart Foundation. Dr. Clark Memorial Fund, Medical Research Council, St. Mary’s Hospital Research Fund, and the Wellcome Trust, Received for publication Aug. 14, 1967.
Footnotes
It should be emphasized that flow values obtained on portal and arterial injection (designed FPI and FAI, respectively) do not represent an absolute measure of fractional flow from either source, as will be discussed later.
References
- 1.Conn HL., Jr Equilibrium distribution of radioxenon in tissue: Xenon-hemoglobin association curve. J Appl Physiol. 1961;16:1065. doi: 10.1152/jappl.1961.16.6.1065. [DOI] [PubMed] [Google Scholar]
- 2.Dempster WJ. The effects of cortisone on the homotransplanted kidney. Arch Internat Pharmacodyn. 1953;95:253. [PubMed] [Google Scholar]
- 3.Hollenberg M, Dougherty J. Liver blood flow measured by portal venous and hepatic arterial routes with Kr85, Am. J Physiol. 1966;210:926. doi: 10.1152/ajplegacy.1966.210.5.926. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki Y, Porter KA, Amend JR, Marchioro TL, Zühlke V, Starzl TE. The preparation and testing of horse anti-dog and antihuman antilymphoid plasma or serum and its protein fractions. Surg Gynec & Obst. 1967;124:1. [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson BT, Mannick JA. Serial blood flow in first set renal homotransplants undergoing rejection. Surg Gynec & Obst. 1964;119:1265. [PubMed] [Google Scholar]
- 6.Kountz SL, Laub DR, Cohn R. Detecting and treating early renal homotransplant rejection. J A M A. 1965;191:997. doi: 10.1001/jama.1965.03080120031008. [DOI] [PubMed] [Google Scholar]
- 7.Kountz SL, Williams MA, Williams PL, Kapros C, Dempster WJ. Mechanism of rejection of homotransplanted kidneys. Nature (London) 1963;199:257. doi: 10.1038/199257a0. [DOI] [PubMed] [Google Scholar]
- 8.McBride RA, Wheeler HB, Smith LL, Moore FD, Dammin GJ. Homotransplantation of the canine liver as an orthotopic vascularized graft. Am J Path. 1962;41:501. [PMC free article] [PubMed] [Google Scholar]
- 9.Moore FD, Birtch AG, Dagher F, Veith F, Krisher JA, Order SE, Shucart WA, Dammin GJ, Couch NP. Immunosuppression and vascular insufficiency in liver transplantation. Ann New York Acad Sc. 1964;120:729. doi: 10.1111/j.1749-6632.1964.tb34765.x. [DOI] [PubMed] [Google Scholar]
- 10.Paronetto F, Horowitz RE, Sicular A, Burrows L, Kark AE, Popper H. Immunologic observations on homografts. I The canine liver. Transplantation. 1965;3:303. doi: 10.1097/00007890-196505000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Porter KA, Peart WS, Kenyon JR, Joseph NH, Hoehn RJ, Calne RY. Rejection of kidney homotransplants. Ann New York Acad Sc. 1964;120:472. doi: 10.1111/j.1749-6632.1964.tb34746.x. [DOI] [PubMed] [Google Scholar]
- 12.Rabinovici N, Vardi J. The intrahepatic portal vein-hepatic artery relationship. Surg Gynec & Obst. 1965;120:38. [PubMed] [Google Scholar]
- 13.Rees JR, Redding VJ, Ashfield R. Hepatic blood flow measurement with xenon 133. Lancet. 1964;2:562. doi: 10.1016/s0140-6736(64)90623-3. [DOI] [PubMed] [Google Scholar]
- 14.Retik AB, Hollenberg NK, Rosen SM, Merrill JP, Murray JE. Cortical ischemia in renal allograft rejection. Surg Gynec & Obst. 1967;124:989. [PubMed] [Google Scholar]
- 15.Rolle GK, Taylor AC, Charipper HA. A study of vascular changes in skin grafts in mice and their relationship to homograft breakdown. J Cell Comp Physiol. 1959;53:215. doi: 10.1002/jcp.1030530206. [DOI] [PubMed] [Google Scholar]
- 16.Rosen SM, Retik AB, Hollenberg NK, Merrill JP, Murray JE. Effect of immunosuppressive therapy on the intrarenal distribution of blood flow in dog renal allograft rejection. S Forum. 1966;17:233. [PubMed] [Google Scholar]
- 17.Sanders RJ, Sullivan RG. Cardiac output determination with radioactive krypton,85. J Surg Res. doi: 10.1016/0022-4804(68)90002-4. In press. [DOI] [PubMed] [Google Scholar]
- 18.Starzl TE, Kaupp HA, Jr, Brock DR, Linman JW. Studies on the rejection of the transplanted homologous dog liver. Surg Gynec & Obst. 1961;112:135. [PMC free article] [PubMed] [Google Scholar]
- 19.Starzl TE, Marchioro TL, Porter KA, Iwasaki Y, Cerilli GJ. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation and in human renal homotransplantation. Surg Gynec & Obst. 1967;124:301. [PMC free article] [PubMed] [Google Scholar]
- 20.Starzl TE, Marchioro TL, Porter KA, Taylor PD, Faris TD, Herrmann TJ, Hlad CJ, Waddell WR. Factors determining short- and long-term survival after orthotopic liver homotransplantation in the dog. Surgery. 1965;58:131. [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE, Marchioro TL, Rifkind D, Holmes JH, Rowlands DT, Waddell WR. Factors in successful renal transplantation. Surgery. 1964;56:296. [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor AC, Lehrfeld JW. Determination of survival time of skin homografts in the rat by observation of vascular changes in the graft. Plast & Reconst Surg. 1953;12:423. doi: 10.1097/00006534-195312000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Thorburn GD, Kopald HH, Herd JA, Hollenberg M, O’Morchoe CCC, Barger A. C Intrarenal distribution of nutrient blood flow determined with krypton85 in the unanesthetized dog. Circulation Res. 1963;13:290. doi: 10.1161/01.res.13.4.290. [DOI] [PubMed] [Google Scholar]
- 24.Truniger B, Rosen SM, Kriek H, Merrill JP, Murray JE. Intrarenal distribution of blood flow in the rejecting homotransplanted dog kidney. S Forum. 1965;16:254. [PubMed] [Google Scholar]
- 25.Veall N, Mallett BL. The partition of trace amounts of xenon between human blood and brain tissues at 37° C. Physiol M Biol. 1965;10:375. [Google Scholar]
- 26.Williams PL, Williams MA, Kountz SL, Dempster WJ. Ultrastructural and haemodynamic studies in canine renal transplants. J Anat. 1964;98:545. [PMC free article] [PubMed] [Google Scholar]