Abstract
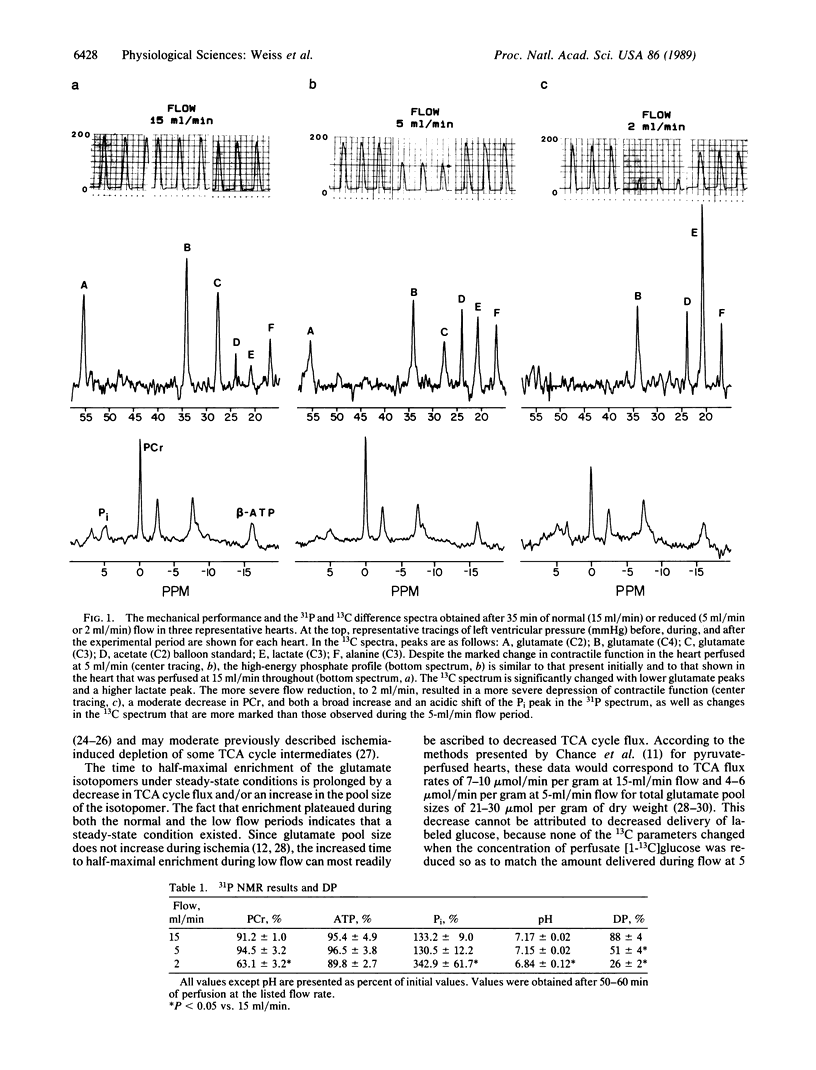
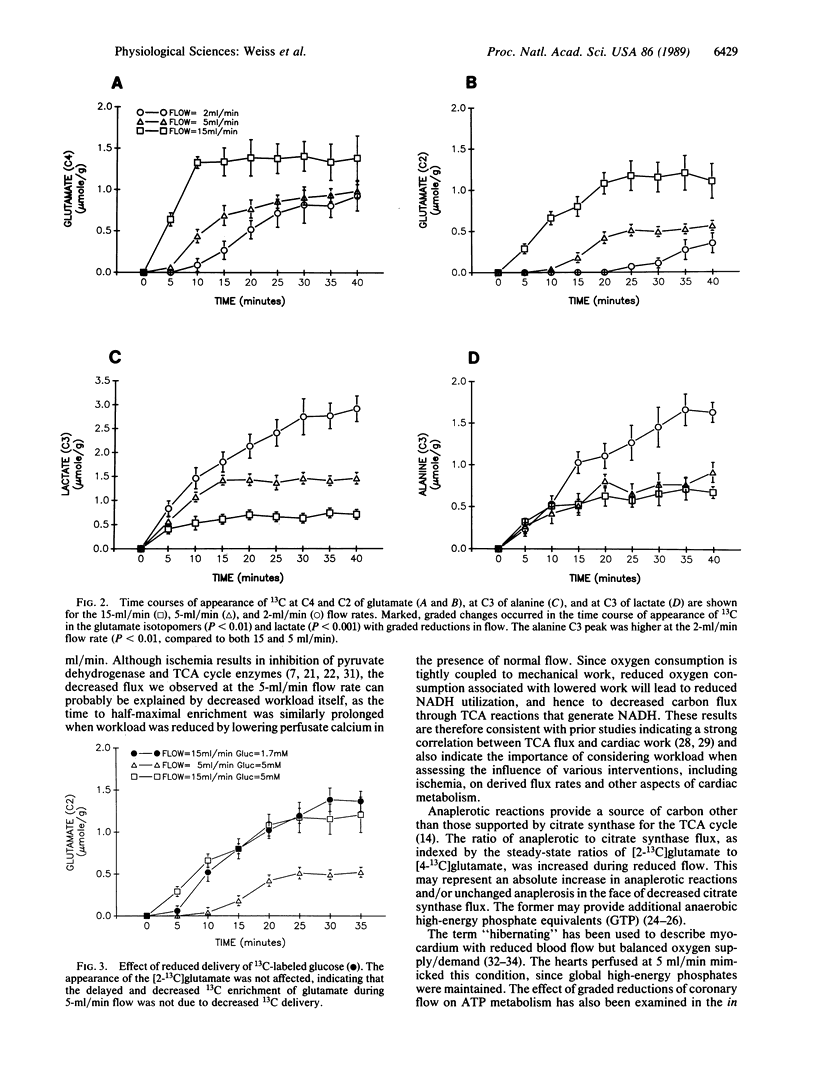
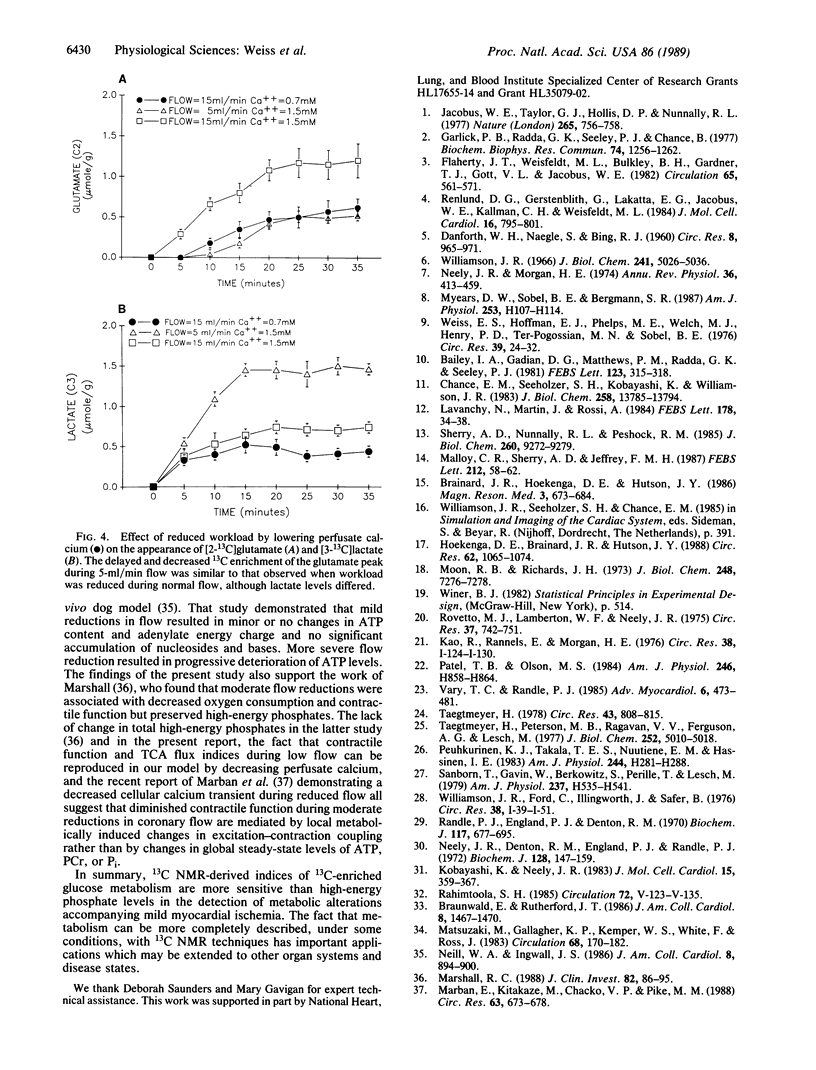
13C NMR spectroscopy may offer a unique ability to characterize the metabolic response to graded reduction in coronary flow since it allows repeated, nondestructive identification of products of intermediary metabolism in the same heart. The sensitivity of 13C parameters of glucose metabolism was compared with changes in levels of phosphocreatine, ATP, and pH as determined by 31P NMR in the intact, beating rat heart model during graded reductions in coronary flow. Experiments were performed during 60 min of perfusion with [1-13C]glucose (5 mM) at normal flow (15 ml/min) and at the reduced flow rates of 5 and 2 ml/min. During flow at 5 ml/min, isovolumic developed pressure fell to 51 +/- 4% of control. Although phosphocreatine, ATP, and pH were not changed, [3-13C]lactate was increased (1.46 +/- 0.12 mumol/g of wet weight vs. 0.63 +/- 0.08 during normal flow). In addition, the time to 50% maximum enrichment of [2-13C]glutamate was prolonged (17 +/- 1 min vs. 9 +/- 1 min during normal flow), indicating that glucose-supported flux through the tricarboxylic acid (TCA) cycle was decreased. The relative anaplerotic contribution to citrate synthase-supported TCA flux was increased from 6% to 35%. These 13C metabolic changes could not be reproduced by reduced [1-13C]glucose delivery in the absence of ischemia, although similar reduced TCA flux indices were reproduced in additional hearts when workload was reduced by low calcium (0.7 mM) perfusion. Therefore, the information provided by 13C NMR spectroscopy can be a more sensitive indicator of flow-induced alterations in cardiac metabolism than that provided by the much more commonly used 31P NMR technique.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey I. A., Gadian D. G., Matthews P. M., Radda G. K., Seeley P. J. Studies of metabolism in the isolated, perfused rat heart using 13C NMR. FEBS Lett. 1981 Jan 26;123(2):315–318. doi: 10.1016/0014-5793(81)80317-1. [DOI] [PubMed] [Google Scholar]
- Brainard J. R., Hoekenga D. E., Hutson J. Y. Metabolic consequences of anoxia in the isolated, perfused guinea pig heart: anaerobic metabolism of endogenous amino acids. Magn Reson Med. 1986 Oct;3(5):673–684. doi: 10.1002/mrm.1910030504. [DOI] [PubMed] [Google Scholar]
- Braunwald E., Rutherford J. D. Reversible ischemic left ventricular dysfunction: evidence for the "hibernating myocardium". J Am Coll Cardiol. 1986 Dec;8(6):1467–1470. doi: 10.1016/s0735-1097(86)80325-4. [DOI] [PubMed] [Google Scholar]
- Chance E. M., Seeholzer S. H., Kobayashi K., Williamson J. R. Mathematical analysis of isotope labeling in the citric acid cycle with applications to 13C NMR studies in perfused rat hearts. J Biol Chem. 1983 Nov 25;258(22):13785–13794. [PubMed] [Google Scholar]
- DANFORTH W. H., NAEGLE S., BING R. J. Effect of ischemia and reoxygenation on glycolytic reactions and adenosine-triphosphate in heart muscle. Circ Res. 1960 Sep;8:965–971. doi: 10.1161/01.res.8.5.965. [DOI] [PubMed] [Google Scholar]
- Flaherty J. T., Weisfeldt M. L., Bulkley B. H., Gardner T. J., Gott V. L., Jacobus W. E. Mechanisms of ischemic myocardial cell damage assessed by phosphorus-31 nuclear magnetic resonance. Circulation. 1982 Mar;65(3):561–570. doi: 10.1161/01.cir.65.3.561. [DOI] [PubMed] [Google Scholar]
- Garlick P. B., Radda G. K., Seeley P. J. Phosphorus NMR studies on perfused heart. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1256–1262. doi: 10.1016/0006-291x(77)91653-9. [DOI] [PubMed] [Google Scholar]
- Hoekenga D. E., Brainard J. R., Hutson J. Y. Rates of glycolysis and glycogenolysis during ischemia in glucose-insulin-potassium-treated perfused hearts: A 13C, 31P nuclear magnetic resonance study. Circ Res. 1988 Jun;62(6):1065–1074. doi: 10.1161/01.res.62.6.1065. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Taylor G. J., 4th, Hollis D. P., Nunnally R. L. Phosphorus nuclear magnetic resonance of perfused working rat hearts. Nature. 1977 Feb 24;265(5596):756–758. doi: 10.1038/265756a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Neely J. R. Effects of ischemia and reperfusion on pyruvate dehydrogenase activity in isolated rat hearts. J Mol Cell Cardiol. 1983 Jun;15(6):359–367. doi: 10.1016/0022-2828(83)90320-6. [DOI] [PubMed] [Google Scholar]
- Lavanchy N., Martin J., Rossi A. Glycogen metabolism: a 13C-NMR study on the isolated perfused rat heart. FEBS Lett. 1984 Dec 3;178(1):34–38. doi: 10.1016/0014-5793(84)81234-x. [DOI] [PubMed] [Google Scholar]
- Malloy C. R., Sherry A. D., Jeffrey F. M. Carbon flux through citric acid cycle pathways in perfused heart by 13C NMR spectroscopy. FEBS Lett. 1987 Feb 9;212(1):58–62. doi: 10.1016/0014-5793(87)81556-9. [DOI] [PubMed] [Google Scholar]
- Marban E., Kitakaze M., Chacko V. P., Pike M. M. Ca2+ transients in perfused hearts revealed by gated 19F NMR spectroscopy. Circ Res. 1988 Sep;63(3):673–678. doi: 10.1161/01.res.63.3.673. [DOI] [PubMed] [Google Scholar]
- Marshall R. C. Correlation of contractile dysfunction with oxidative energy production and tissue high energy phosphate stores during partial coronary flow disruption in rabbit heart. J Clin Invest. 1988 Jul;82(1):86–95. doi: 10.1172/JCI113606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M., Gallagher K. P., Kemper W. S., White F., Ross J., Jr Sustained regional dysfunction produced by prolonged coronary stenosis: gradual recovery after reperfusion. Circulation. 1983 Jul;68(1):170–182. doi: 10.1161/01.cir.68.1.170. [DOI] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973 Oct 25;248(20):7276–7278. [PubMed] [Google Scholar]
- Myears D. W., Sobel B. E., Bergmann S. R. Substrate use in ischemic and reperfused canine myocardium: quantitative considerations. Am J Physiol. 1987 Jul;253(1 Pt 2):H107–H114. doi: 10.1152/ajpheart.1987.253.1.H107. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Denton R. M., England P. J., Randle P. J. The effects of increased heart work on the tricarboxylate cycle and its interactions with glycolysis in the perfused rat heart. Biochem J. 1972 Jun;128(1):147–159. doi: 10.1042/bj1280147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill W. A., Ingwall J. S. Stabilization of a derangement in adenosine triphosphate metabolism during sustained, partial ischemia in the dog heart. J Am Coll Cardiol. 1986 Oct;8(4):894–900. doi: 10.1016/s0735-1097(86)80432-6. [DOI] [PubMed] [Google Scholar]
- Patel T. B., Olson M. S. Regulation of pyruvate dehydrogenase complex in ischemic rat heart. Am J Physiol. 1984 Jun;246(6 Pt 2):H858–H864. doi: 10.1152/ajpheart.1984.246.6.H858. [DOI] [PubMed] [Google Scholar]
- Peuhkurinen K. J., Takala T. E., Nuutinen E. M., Hassinen I. E. Tricarboxylic acid cycle metabolites during ischemia in isolated perfused rat heart. Am J Physiol. 1983 Feb;244(2):H281–H288. doi: 10.1152/ajpheart.1983.244.2.H281. [DOI] [PubMed] [Google Scholar]
- Randle P. J., England P. J., Denton R. M. Control of the tricarboxylate cycle and its interactions with glycolysis during acetate utilization in rat heart. Biochem J. 1970 May;117(4):677–695. doi: 10.1042/bj1170677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renlund D. G., Gerstenblith G., Lakatta E. G., Jacobus W. E., Kallman C. H., Weisfeldt M. L. Perfusate sodium during ischemia modifies post-ischemic functional and metabolic recovery in the rabbit heart. J Mol Cell Cardiol. 1984 Sep;16(9):795–801. doi: 10.1016/s0022-2828(84)80003-6. [DOI] [PubMed] [Google Scholar]
- Rovetto M. J., Lamberton W. F., Neely J. R. Mechanisms of glycolytic inhibition in ischemic rat hearts. Circ Res. 1975 Dec;37(6):742–751. doi: 10.1161/01.res.37.6.742. [DOI] [PubMed] [Google Scholar]
- Sanborn T., Gavin W., Berkowitz S., Perille T., Lesch M. Augmented conversion of aspartate and glutamate to succinate during anoxia in rabbit heart. Am J Physiol. 1979 Nov;237(5):H535–H541. doi: 10.1152/ajpheart.1979.237.5.H535. [DOI] [PubMed] [Google Scholar]
- Sherry A. D., Nunnally R. L., Peshock R. M. Metabolic studies of pyruvate- and lactate-perfused guinea pig hearts by 13C NMR. Determination of substrate preference by glutamate isotopomer distribution. J Biol Chem. 1985 Aug 5;260(16):9272–9279. [PubMed] [Google Scholar]
- Taegtmeyer H. Metabolic responses to cardiac hypoxia. Increased production of succinate by rabbit papillary muscles. Circ Res. 1978 Nov;43(5):808–815. doi: 10.1161/01.res.43.5.808. [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H., Peterson M. B., Ragavan V. V., Ferguson A. G., Lesch M. De novo alanine synthesis in isolated oxygen-deprived rabbit myocardium. J Biol Chem. 1977 Jul 25;252(14):5010–5018. [PubMed] [Google Scholar]
- Vary T. C., Randle P. J. Regulation of pyruvate dehydrogenase complex activity during myocardial ischemia. Adv Myocardiol. 1985;6:473–481. [PubMed] [Google Scholar]
- Weiss E. S., Hoffman E. J., Phelps M. E., Welch M. J., Henry P. D., Ter-Pogossian M. M., Sobel B. E. External detection and visualization of myocardial ischemia with 11C-substrates in vitro and in vivo. Circ Res. 1976 Jul;39(1):24–32. doi: 10.1161/01.res.39.1.24. [DOI] [PubMed] [Google Scholar]
- Williamson J. R. Glycolytic control mechanisms. II. Kinetics of intermediate changes during the aerobic-anoxic transition in perfused rat heart. J Biol Chem. 1966 Nov 10;241(21):5026–5036. [PubMed] [Google Scholar]


