Abstract
HIV type-1 (HIV-1) accounts for more than 25 million deaths and nearly 40 million people are infected worldwide. A significant obstacle in clearing virus from infected individuals is latently infected viral reservoirs. Latent HIV-1 can emerge with recrudescence as a productive infection later in disease progression and could provide a source for the emergence of resistant HIV-1. It is widely recognized that macrophages represent a latently infected viral reservoir and are a significant and critical HIV-1 target cell in vivo. Macrophages can be divided into multiple subsets of macrophage-like cells, all of which are susceptible to HIV-1 infection, including dendritic cells, Langerhans cells, alveolar macrophages, mucosal macrophages and microglial cells. Current antiretroviral therapy (ART) often displays differential antiviral activity in macrophages relative to CD4+ T-lymphocytes. Significant work has been performed to establish antiviral activity of many clinically approved ART in macrophages; however, a direct link between antiviral activity and specific mechanisms responsible for these antiviral effects are incompletely understood. This review identifies many understudied areas of research, along with topics for further research in the field of HIV therapy and eradication. Discussion focuses upon the known cellular pharmacology and antiviral activity of antiretroviral agents in macrophages and its relationship to latency, chronic HIV-1 infection and therapeutic strategies to eradicate systemic HIV-1 infection.
Introduction
Macrophages are a major and crucial target of HIV type-1 (HIV-1) infection and, as potential long-term HIV reservoirs, infected cells must be selectively destroyed to achieve HIV-1 eradication [1]. As macrophages function as potent antigen-presenting cells and mediators of both innate and acquired immunity, HIV-1-mediated macrophage deficiency is catastrophic to the global immune response. A significant obstacle in clearing virus from infected individuals is multiple latently infected viral sanctuaries (Figure 1). Latent HIV-1 can emerge with recrudescence as a productive infection later in disease progression and might also represent a source for the emergence of resistant HIV-1 [2-4]. Many regimens eventually fail, primarily because of lack of adherence to strict regimens, delayed toxicities and/or the emergence of drug-resistant HIV strains [5]. Several studies have begun to elucidate the relationship between antiretroviral therapy (ART) and CD4+ T-lymphocytes relative to toxicity, resistance, latency and chronic infection (reviewed in [6]). Recently, Richman et al. [6] presented a comprehensive review commenting on strategies to reduce or eliminate latent HIV-1 infection within the CD4+ T-lymphocyte compartment. This review presented a model wherein latent CD4+ T-lymphocytes could be reduced or eliminated by combination therapy designed to purge remaining latent CD4+ T-lymphocytes in tandem with novel therapeutics targeting pathways implicated as modulators of HIV-1 latency, including histone deacetylase inhibitors and modulators of Jak/STAT-, PKC-, NF-κB- and IL-7-mediated signal transduction cascades [6]. The application of this hypothesis within macrophages is undefined, but presents an integral overlap to facilitate greater understanding of the relationship between macrophages and ART cellular pharmacology.
Figure 1.
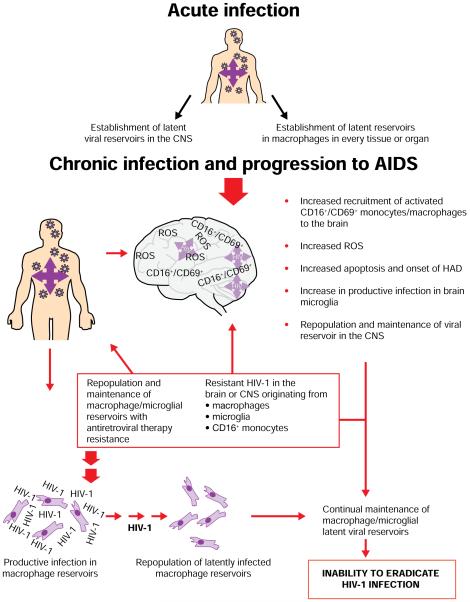
Mechanisms for establishment and maintenance of HIV-1 reservoirs in macrophages
Acute HIV type-1 (HIV-1) infection results in establishment of infection in macrophages in the central nervous system (CNS) and every organ or tissue. Chronic infection results in increased recruitment of activated CD16+/CD69+ monocytes/macrophages to the brain, increased reactive oxygen species (ROS) and apoptosis followed by onset of HIV-associated dementia (HAD). These mechanisms increase the productive infection in brain microglia and contribute to maintenance of an HIV-1 viral reservoir in the CNS. Resistant HIV-1 in the brain or CNS along with latently infected macrophages originating from other tissues and organs contributes to maintenance of macrophage viral reservoirs. Together, these factors contribute to the inability to eradicate systemic HIV-1 infection [2,4,7,13,16,24,27,28,31-33,75,77-79,84-90,110].
The function of macrophages in vivo presents a long-lived target for HIV-1 infection; the half-life (t1/2) of macrophages is significantly longer than that of an activated lymphocyte (weeks/years versus hours/days) [7,8]. More specifically, the life span of activated HIV-1-infected lymphocytes is relatively attenuated, with a t1/2 of approximately 0.8–1.1 days [9], whereas productively infected macrophages maintain viability and virus production for at least 30 days [10]. The latter study represents an in vitro assay and, although studies observing the t1/2 of HIV-1 infected macrophages in vivo are lacking, existing studies define a distinct advantage for macrophages when observing their life span and virus production over time. Viral dynamics in vivo indicate that CCR5 (R5)-using viruses predominate early during infection [11]. As macrophages display high CCR5 expression levels, they represent an early target for the establishment of both chronic and latent infection [2,4]. Macrophages interact with lymphocytes during antigen presentation, conferring direct infection to new CD4+ T-lymphocytic targets [12]. Macrophages have also been implicated as the causative agent in central nervous system (CNS) infection of HIV-1, which often manifests itself as HIV-associated dementia (HAD) during the later stages in the progression to AIDS [13]. For these reasons, understanding dynamics of ART pharmacology in macrophages, and subsequently eliminating productive infection in these cells, is critical to eliminating systemic HIV-1 infection.
Viral dynamics in macrophages is unique because these cells can be found in every organ system [2,3]. This represents multiple microenvironments from which HIV-1 can establish latent infection and in which ART is often present with significantly different antiviral activity profiles relative to circulating CD4+ T-lymphocytes [14,15]. Studies are conflicting as to the proposed mechanism(s) responsible for observed antiviral activity of ART in macrophages. Suggested mechanisms differ relative to the class of ART, activation state of the target cell, drug concentration and time after initial infection that cells are exposed to drug [16-18].
The efficacy of ART cellular pharmacology in macrophages has significant implications in disease progression. The interplay between ART cellular pharmacology in macrophages directly affects viral loads, selection of resistance mutations both within and between subsets of HIV-1 target cells, eradication of systemic virus and long-term patient survival [2,10,13,16,19-30] (Figure 1). Eradication of systemic HIV-1 infection is not possible without clearance of latently infected cells [4,13,29-36]. As macrophages are a sentinel target for HIV-1 infection and latency, understanding the cellular pharmacology of current antiretroviral therapy in macrophages is essential.
This manuscript provides state-of-the-art knowledge on HIV-1 replication in macrophages, especially relating to antiretroviral agents, and also defines understudied areas of research. It should be noted that the general term ‘macrophage’ is used here to indicate cells that are obtained from the circulating peripheral blood. However, in vivo, macrophages can be broadly categorized by mechanism of activation into classically activated (M1) and alternatively activated (M2) macrophages [37-40]. M1 classification is associated with prototypical secretion of proinflammatory cytokines interleukin (IL)-1β, IL-15, IL-18, IL-12 and tumour necrosis factor (TNF)-α, which modulate enhanced endocytic function coupled with increased capacity to eliminate intracellular pathogens [37-40]. M2 macrophages can be further classified into M2a, M2b, and M2c subsets, each with distinct functionalities and cytokine profiles [37-43]. In this review, the potency and efficacy of ART for each subset of macrophages are not defined nor are the dynamics of HIV-1 infection within M1 or M2 macrophages.
Entry inhibitors
Currently, only two anti-HIV drugs are approved by the US Federal Drug Administration (FDA) that inhibit entry of HIV-1 into host cells (Table 1). Enfuvirtide (Fuzeon®; ENF, T-20) is a peptide derived from a repeat sequence of the transmembrane portion of HIV-1 envelope, gp41, and inhibits the hairpin formation necessary for virus–host cell fusion to occur [44]. Maraviroc (Selzentry®/Celsentri®; UK-427, 857) is a small molecule that inhibits viral entry by binding to the CCR5 coreceptor and inhibiting the receptor–coreceptor viral envelope interaction required for HIV-1 entry into the host cell [45,46].
Table 1.
Potency of currently approved antiretroviral therapy in macrophages versus PBMCs
| Compound | Acute infection in macrophages EC50, nM |
Chronic infection in macrophages EC50, nM |
Acute infection in PBMCs EC50, nM |
|---|---|---|---|
| Entry inhibitors | |||
| Maraviroc | 0.5 | NA | 0.2–2.9 |
| Enfuvirtide | 20 | NA | NA |
| NNRTI | |||
| Delavirdine | 10 | NE | 6 |
| Efavirenz | 10 | NE | 10 |
| Etravirine | NA | NA | NA |
| Nevirapine | 50 | NE. | 40 |
| NRTI | |||
| Abacavir sulfate | 300 | NE | NA |
| Didanosine | 50 | NE | 500 |
| Lamivudine | 20 | NE | 40 |
| Stavudine | 240 | NE | 800 |
| Tenofovir disoproxil fumarate | 20 | NE | 370 |
| Zalcitabine | 3 | NE | 40 |
| Zidovudine | 20 | NE | 200 |
| Protease inhibitors | |||
| Amprenavir | 10 | 720 | NA |
| Atazanavir | NA | NA | NA |
| Darunavir | NA | NA | NA |
| Fosamprenavir | NA | NA | NA |
| Indinavir | 60 | 400 | 50 |
| Nelfinavir | 80 | 1.4×103 | 40 |
| Ritonavir | 120 | 3.3×103 | 20 |
| Saquinavir | 50 | 500 | 10 |
| Tipranavir | NA | NA | NA |
The 50% effective concentration (EC50) of US Federal Drug Administration-approved HIV type-1 antiretroviral therapy in acute versus chronically infected macrophages [14-17,22,29,35,45-47,52,119].
NA, not available; NE, not effective; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PBMCs, peripheral blood mononuclear cells.
Maraviroc has undergone extensive plasma pharmacokinetics and testing against multiple HIV-1 variants and clades [46]; however, studies assessing antiviral activity, toxicity or cellular pharmacology in macrophages are largely undefined. The median effective concentration (EC50) for maraviroc in primary macrophages is reported to be 0.5 nM for a subtype B M-R5 virus [29] versus 0.2–2.9 nM in activated lymphocytes for R5-using viruses across multiple subtypes [46] (Table 1). Follow-up studies across multiple donors and within macrophages at different stages of activation are not fully elucidated; therefore, one should always compare potency of antiviral agents in the same assay using the same experimental procedures.
Extensive studies assessing antiviral activity of either maraviroc or ENF in primary macrophages are lacking, although Yi et al. [47] recently offered data comparing the sensitivity of ENF upon CCR5- versus CXCR4-mediated HIV-1 entry into primary macrophages. ENF sensitivity was largely independent of the coreceptor usage, and overall ENF antiviral activity in macrophages correlated more closely with HIV-1 entry by CCR5 rather than with entry through CXCR4 [47] (Table 1). These data demonstrate the downstream effects of ENF relative to CXCR4- versus CCR5-mediated signalling and provide a foundation for understanding the effect of entry inhibitors on multiple cellular events in macrophages.
Reverse transcriptase inhibitors
Two classes of reverse transcriptase (RT) inhibitors exist for treatment of HIV-1 infection: nucleoside RT inhibitors (NRTIs; nucleoside analogues) and non-nucleoside RT inhibitors (NNRTIs). NRTIs have a well established regulatory pathway, with eight FDA-approved drugs for the treatment of HIV-1 infection and multiple drugs in various stages of clinical development [48,49]. NNRTIs also have a long history of FDA approval, with four drugs currently approved.
Nucleoside reverse transcriptase inhibitors
NRTIs present distinct clinical advantages: low plasma protein binding, sustained antiviral response when a dose is missed (because of their long intracellular half-life) and relative ease of chemical manufacture [48]. By 2008, the eight approved HIV-1 NRTI were zidovudine (Retrovir®; ZDV, AZT), didanosine (Videx®; ddI), zalcitabine (Hivid®; ddC), stavudine (Zerit®; d4T), abacavir sulfate (Ziagen®; ABC), lamivudine (Epivir®; 3TC), emtricitabine (Emtriva®; FTC) and tenofovir disoproxil fumarate (Viread®; TDF).
The target of NRTIs in HIV-1 infection is the action of virally encoded RT. This enzyme is active early in the viral replication cycle and converts the genetic information of the virus, which is stored as RNA, into DNA by reverse transcription, a process necessary for continued viral replication [48,49].
NRTIs are chiral small molecules that mimic natural nucleotides and require intracellular phosphorylation to become functionally active against HIV-1 RT. In the triphosphate form, NRTIs compete with one of the four naturally occurring dNTP, namely, dCTP, dTTP, dATP or dGTP, for binding and DNA chain elongation near the active site of HIV-1 RT [48]. As most NRTIs lack a 3′-hydroxyl terminus, incorporation of the analogue into the growing DNA strand results in termination of the DNA strand and the next phosphodiester bond is not formed. Because of these factors, both the concentration of cellular triphosphorylated drug and the levels of cellular dNTP pools play a key role in the efficacy of the NRTIs [49,50].
Antiviral activity of nucleoside reverse transcriptase inhibitors in macrophages
The antiviral activity of NRTIs in macrophages is significantly different for acute versus chronically infected cells. Mechanisms responsible for these observations are currently undefined, although multiple hypotheses exist to explain these findings. Natural dNTP levels have been historically demonstrated as a surrogate marker for replication and activation of mammalian cells [51]. Macrophages primarily remain in a resting, G1 state and undergo limited DNA synthesis [7,51,52]. Therefore, it follows that cellular dNTP levels are significantly lower in macrophages compared with an activated and dividing cell [15]. For acute infection, NRTI EC50 in macrophages demonstrates ranges from 3 to 300 nM depending on the NRTI tested (Table 1). By contrast, data for chronically infected macrophages demonstrate an EC50>25 μM or state that NRTIs are ‘not effective’ in chronically infected macrophages [16]. These differences indicate that chronic HIV-1 infection alters the cellular milieu in a manner that modulates the ability of NRTI to successfully become functionally active and/or subsequently inhibit viral reverse transcription. The significant difference in EC50 might be explained in part by differences in activation state, which could, in turn, affect dNTP levels. As NRTIs compete with endogenous nucleotides for incorporation into the growing viral DNA, differences in dNTP levels, as a function of activation state, could significantly alter NRTI-triphosphate levels.
Alternatively, the difference in number of infected cells in an acute versus chronic infection in vitro might affect the potency of the NRTIs. A chronically infected culture possesses a greater number of infected cells, which, in turn, can induce a cytokine milieu that promotes infection [53,54]. This milieu might modulate a globally activated system, which either reduces intracellular bioavailability of NRTIs or increases its catabolism. Interestingly, Aquaro et al. [14] reported that the constant exposure of macrophages to monocyte colony-stimulating factor (m-CSF) results in a significant increase in EC50 (Table 1), which might provide evidence of activation state affecting potency of NRTI.
As chronically infected cells contain an integrated proviral genome, which is downstream of the NRTI target, it follows that NRTIs will not be as effective in a population of cells containing integrated HIV-1. Despite this fact, a chronic infection contains a mixture of cells at various stages of infection. Newly established infection continues to occur, thus providing a functional target for NRTIs in some cells. Although integrated proviral genome is a characteristic of chronic infection, it is important to note that not all cells in vitro or in vivo are infected even as chronic infection is established. Thus, the mechanism of action of NRTIs along with its inability to inhibit viral replication in cells with integrated HIV-1 DNA is not applicable to all cells and to new infection established in cells neighbouring chronically infected cells. Whether the activation state (and thus the endogenous cellular dNTP pools) in neighbouring cells is altered as a function of paracrine stimulation from chronically infected neighbour cells is not defined. Together, the knowledge to date suggests a multimechanism system wherein NRTIs might not be as effective in chronically infected cells because of multiple factors.
The EC50 of NRTIs for acute infection in macrophages relative to CD4+ T-lymphocytes is significantly lower. Although the mechanisms are unknown, it follows that differences between cell types might be responsible for these data. These findings have led to the hypothesis that lower cellular dNTP pools in resting macrophages (compared with that of lymphocytes) results in decreased competition for cellular kinases. Thus, the ability of NRTI to undergo phosphorylation is increased, resulting in greater antiviral activity. A tandem hypothesis is that during chronic HIV-1 infection continuous, exposure to m-CSF might activate the macrophage resulting in increased dNTP levels. This, in turn, would increase competition for cellular kinases and decrease the ability of the NRTIs to become functionally active. Together, these mechanisms might be responsible for markedly different EC50 values for analogous drugs across macrophages and lymphocytes.
Viral resistance to nucleoside reverse transcriptase inhibitors in macrophages
Macrophages represent a long-lived reservoir for HIV-1 infection and survive for weeks or years within an infected individual [3,7,22]. Understanding the dynamics of ongoing or latent infection during the emergence of resistant HIV-1 is essential for managing chronic infection [55], drug regimens and the design of novel therapeutics that might confer less resistance for extended periods of time.
Studies assessing the effect of 3TC on the emergence of drug-resistant HIV-1 in macrophages over time surprisingly demonstrated a lack of emergence of resistance mutations [23]. When 3TC treated acutely infected macrophages exposed to supernatants that were transferred from infected macrophages (assay conducted for 105 days), no resistance mutations were observed, despite emergence of mutations that confer resistance to NRTI in lymphocytes [23]. These data suggest that the dynamics of 3TC in lymphocytes versus macrophages is different, and that the mechanisms responsible for the emergence of resistance in lymphocytes are not uniform in macrophages. The relationship between fitness and cell-specific emergence of resistant mutations was not defined, although potential differences in replicative kinetics across cell types might contribute to these findings. Other experiments demonstrate a resistance-associated loss of replicative capacity in acutely infected macrophages relative to activated CD4+ T-lymphocytes [56]. These data define the relationship between acute infection, NRTI activity and emergence of resistance. They also suggest a role for resistance-associated loss of fitness in macrophages.
Although it is favourable to correlate the lack of resistance mutations over time with NRTI treatment, the effect of NRTI on chronically infected macrophages is difficult to elucidate. Chronic infection directly relates to latency because latent infection often emerges later in disease progression. Although specific mechanisms responsible for reactivation in vivo are not fully understood, it is accepted that reactivation of previously latently infected macrophages can result in repopulation of the systemic system with virus, which contributes to disease progression (reviewed in [1,2,6,57]). It is well established that NRTI EC50 in activated and/or chronically infected macrophages is very high or cannot be established in vitro (Table 1), which provides a difficult environment from which to assess studies more easily defined in resting macrophages or CD4+ T-lymphocytes.
Non-nucleoside reverse trancriptase inhibitors
By 2009, four NNRTIs were approved by the FDA: etravirine (Intelence®; TMC-125), delavirdine (Rescriptor®, DLV), efavirenz (Sustiva®, Stocrin; EFV) and nevirapine (Viramune®; NVP). NNRTIs are chemically distinct from NRTI and inhibit HIV-1 RT by a different mechanism. NNRTIs interact with binding pockets of HIV-1 RT, inhibiting its enzymatic activity by causing conformational changes at or near the active site [58].
Antiviral activity of NNRTI in macrophages
Although the cellular dNTP pool does not directly affect the mechanism of action of NNRTIs, the EC50 differs significantly for NNRTI against acute versus chronic infection in macrophages (reviewed in Aquaro et al. [16]). The EC50 for inhibition of acute HIV-1 infection in macrophages ranges from 10 to 50 nM depending on the NNRTI; however, NNRTIs are not effective at inhibiting HIV-1 replication in chronically infected macrophages (Table 1). To date, the mechanism(s) responsible for ineffective inhibition of viral replication in macrophages by NNRTI (and NRTI) are incompletely understood. Similar principles as discussed with NRTI might be partially responsible for these data (establishment of integrated proviral genome downstream of NNRTI target). If this mechanism was solely responsible for differential activity of NNRTI in chronically activated macrophages, significant antiviral activity would probably still be observed because establishment of new infection and subsequent p24 production would be terminated. As observed in human lymphocytes, NRTI and NNRTI do not efficiently inhibit HIV-1 infection in chronically infected macrophages and data defining the mechanisms are lacking. NRTIs and NNRTIs cannot affect virus replication once the virus is integrated into the host genome. Additional studies elucidating the relationship between NRTIs, NNRTIs and HIV-1 infection in macrophages, could address current gaps in knowledge and allow for design of drugs with greater potency in populations of chronically infected macrophages, such as protease inhibitors (PIs), which are known to be effective in chronically infected cells (see below). Although many data correlate a significantly higher EC50 for activated macrophages versus lymphocytes, data observing intracellular accumulation of NNRTI are lacking. Intracellular accumulation of NNRTI within macrophages might present with a significantly diminished intracellular bioavailability profile relative to lymphocytes, which could present a direct link between differential activities of NNRTI in macrophages versus lymphocytes. Further experiments should be conducted to define the links between latent infection in macrophages, chronic HIV-1 infection and antiviral activity of both NRTIs and NNRTIs in macrophages at all stages of activation and infection.
Integrase inhibitors
HIV-1 integrase (IN) presents a novel and highly selective target for anti-HIV therapeutics. Raltegravir (Isentress®; MK-0518, RAL) received FDA approval in October 2007 and acts to inhibit integration of HIV-1 proviral genome into host cell DNA [59]. To date, studies are lacking in human macrophages. Future studies with RAL in macrophages might define any long-term relationship between IN inhibitors and toxicity, therapy outcome, chronic infection, establishment and emergence of resistant mutations, and latency.
Protease inhibitors
HIV-1 protease inhibitors (PIs) represent a class of ART with long-standing FDA approval. To date, 10 PIs have received FDA approval: amprenavir (Agenearase®; APV), tipranavir (Aptivus®; TPV), indinavir (Crixivan®; IDV), saquinavir (Invirase®; SQV), lopinavir/ritonavir (Kaletra®, Aluvia; LPV/r), fosamprenavir (Lexiva®, Telzir; FPV), ritonavir (Norvir®; RTV), darunavir (Prezista®; DRV), atazanavir (Reyataz®; ATV) and nelfinavir (Viracept®; NFV). PR cleaves HIV-1 Gag and Gag-Pol, resulting in a mature, infectious virion. PIs compete for binding in the active site with the natural substrate. Once bound, PIs cannot usually be cleaved, resulting in inactivation of the enzyme [60].
The hallmark of a PI is its ability to inhibit HIV-1 replication in macrophages (and lymphocytes) chronically infected with HIV-1. The EC50 of multiple PIs have been observed against both acute and chronic HIV-1 infection in macrophages (reviewed in [15,16]; Table 1). Unlike NRTIs and NNRTIs, PIs display potent activity in both acutely and chronically infected macrophages. Of the PIs tested, the EC50 in acutely infected macrophages ranged from 10 to 120 nM, whereas the EC50 in chronically infected macrophages ranged from 400 to 3.3×103 nM (Table 1). Some reports correlate higher rates of viral RNA metabolism in macrophages (relative to CD4+ T-lymphocytes) as a mechanism responsible for higher EC50 in macrophages [61]. As viral RNA production is mechanistically distinct from the action of PIs, it follows that this might contribute to higher EC50 of PIs in macrophages.
Understanding potential mechanisms responsible for antiviral activity of currently available ART is crucial. Correlating in vitro data with in vivo viral–host cell dynamics presents a unique and challenging obstacle. Plasma pharmacokinetics in vivo demonstrate inhibitory quotient values (minimum concentration [Cmin]/EC50 value, which adjusts for plasma protein binding) that are similar to the EC50 of PI in vitro for inhibition of chronic HIV-1 infection in macrophages [62]. Macrophages are unique because they can be found in every tissue compartment and organ systemically. Therefore, it is reasonable to suggest that many macrophages in vivo might be exposed to significantly lower levels of drug than those observed at inhibitory quotients or Cmin in plasma in the circulating periphery.
Data observed for chronically infected macrophages in vitro must be carefully compared with known pharmacology of drugs in vivo. As is the case with PIs, it is clear that antiviral activity is demonstrated, which might correlate with specific subsets of macrophages either in the circulating periphery or in specific tissue compartments displaying high bioavailability of drug. Levels of PI below the inhibitory quotient or Cmin in macrophages might represent a viral reservoir for the emergence of resistant viruses. Understanding specific interactions and mechanisms responsible for antiviral activity of PIs in macrophages and the relationship between differential activity of PI in macrophages at different levels of activation or exposure to HIV-1 infection is essential in abolishing establishment of latent HIV-1 infection. This area of study represents a rich foundation for continued studies to understand how antiviral activity and pharmacology of ART in macrophages contribute to establishment and maintenance of latent infection.
Toxicity of ART in macrophages
It is well established that administration of any ART is not always without detrimental consequence. Studies have extensively demonstrated a link between inhibition of mitochondrial DNA (mtDNA) polymerase-γ, mitochondrial toxicity and systemic side effects including cardiomyopathy [63]. PI use has been correlated with inhibition of apoptosis via multiple mechanisms in lymphocytes [64]; however, the effect of PI in macrophages is largely undefined. The toxicology profile of ART in macrophages remains a greatly understudied area of research.
Azzam et al. [65] demonstrated that treatment of macrophages with either ddI/d4T or 3TC/AZT results in a decrease in mtDNA content in both HIV-1-infected and -uninfected macrophages. The effect was more pronounced in infected macrophages. These observations were correlated with a decrease in complement-mediated phagocytosis, suggesting impairment in immune function. These findings were only observed with coadministration of multiple NRTI, demonstrating a potential synergistic toxicological effect. As the concentrations at which mtDNA content was impaired were ≥10 μM, this presents a debate as to the physiological relevance of these data relative to in vivo pharmacokinetics of NRTI. Nonetheless, these data demonstrate a system where, in concert, NRTI might result in mtDNA depletion in macrophages.
Relationship between ART and dendritic cells
Dendritic cells are macrophage-like cells that can be divided into two distinct subpopulations termed myeloid dendritic cells and plasmacytoid dendritic cells, both of which are susceptible to HIV-1 infection and express varying levels of both CD4 and chemokine coreceptors CXCR4 or CCR5 [66]. Dendritic cells are considered an early target for establishment and maintenance of infection, and represent a distinct viral reservoir [24]. These cells are potent antigen-presenting cells and have been implicated not only as a viral reservoir, but also as a mechanism of viral transmission to CD4+ T-lymphocytes during antigen presentation [24,66]. Dendritic cells are migratory, representing a mobile viral reservoir capable of infecting lymphocytes, as well as other macrophage-like cells, across multiple tissue compartments.
These cells are also primary modulators of both innate and acquired immunity, primarily by immune responses mediated by IL-12 and interferon-α, and stimulate clonal expansion of naive T-lymphocytes [24,66,67]. Chronic HIV-1 infection depletes levels of circulating dendritic cells, which directly correlates with increases in viral loads, decrease in CD4+ T-lymphocytes counts, significant immunological impairment hallmarked by opportunistic infection and progression to AIDS [24,66]. Dendritic cells represent a subpopulation of macrophages that are important modulators of functional immunity, while simultaneously acting as a viral reservoir and mechanism of transfer of HIV-1. Despite the sentinel role of dendritic cells in HIV-1 infection, and the obstacle that they represent in eradication, the pharmacological profile of FDA-approved ART in dendritic cells remains largely undefined.
Although EC50 values for many FDA-approved ART are defined for acute and chronic HIV-1 infection in macrophages [3,14-16,22,29,52], these data are lacking within dendritic cells. It follows that the cellular pharmacology of all FDA-approved ART are undefined within dendritic cells. Despite a lack of studies observing antiviral activity and cellular pharmacology of current ART, emerging studies have begun to exploit the antigen-presenting capacity of dendritic cells with therapies designed to modulate the immune response to HIV-1 [67-70].
DermaVir is a novel therapeutic in development by Genetic Immunity [70], which contains non-infectious viral DNA packaged with a 100 nm nanoparticle. The complex is designed to mimic the antigenic peptides present on dendritic cells, conferring enhanced immunity upon exposure of CD4+ T-lymphocytes to the antigenic peptides. Activation of HIV-1-specific lymphocytes from a previously naive pool would, in principle, expand effector and memory cell populations, resulting in a decrease in viraemia. Long-term data correlating the efficacy and safety of this therapeutic approach and its ability to affect dendritic cells across multiple tissue compartments and in distinct localizations, remains an ongoing area of research.
Relationship between ART and alveolar macrophages
Alveolar macrophages are localized to the pulmonary alveolus and primarily provide innate immunity by phagocytosis of extracellular micro-organisms and pathogens encountered during respiration. Alveolar macrophages express CD4, CXCR4 and CCR5, and are permissive to both CXCR4− and CCR5− using HIV-1 [71]. Although alveolar macrophages might represent a subset of cells functioning as viral reservoir for persistence and maintenance of HIV-1 [71,72], and are therefore a potential obstacle in the eradication of systemic HIV-1, studies elucidating the antiviral activity and cellular pharmacology of ART are lacking. Early work established that maintenance of AZT in alveolar macrophage cultures inhibits productive transfer of infection to lymphocytes [73,74]. However, further work with other ART, either alone or in combination, and complementing pharmacological data, are undefined.
The role of cytokines in modulating HIV-1 infection in alveolar macrophages is partially understood, as TNF-α, a proinflammatory cytokine associated with progression to AIDS [53,54,75,76], induces down-regulation of CCR5 surface expression coupled with paracrine and autocrine stimulation of MIP1-α, MIP1-β and RANTES, which are natural ligands for CCR5 [76]. Together, these cytokines in combination with decreased CCR5 expression might serve as complementing cytoprotective mechanisms in the presence of CCR5-using HIV-1 [76]. Together, these data establish a fundamental relationship between cytokines, CCR5 expression and HIV-1 pathogenesis, and provide an excellent foundation for studies elucidating the effect of FDA-approved CCR5 inhibitors or novel immune-based therapies on HIV-1 replication in alveolar macrophages. Novel strategies implementing small molecules designed to modulate cytokine expression, in particular TNF-α, within alveolar macrophages could provide a unique foundation for reduction and elimination of virus within distinct tissue reservoirs.
Relationship between mucosal macrophages and HIV-1 infection
Mucosal surfaces are densely populated with both macrophages and lymphocytes (reviewed in [77]). As HIV-1 infection is acquired in most cases through direct interaction with mucosal surfaces [77], understanding the dynamics for establishment of productive and latent infection, and its relationship to potency of ART within mucosal macrophages, represent critical questions that must be addressed before eradication can occur. Although studies defining the potency of ART across subsets of mucosal macrophages are lacking, a recent study by Shen et al. [77] provided insights on HIV-1 permissiveness of multiple subsets of mucosal macrophages.
These data define the receptor expression profile of vaginal and intestinal macrophages, and correlate surface expression of CD4, CXCR4, CCR5, CD14, CD89, CD16, CD32 and CD64 in vaginal mucosal macrophages with permissiveness to an R5-using HIV-1. In contrast, intestinal macrophages displayed low or absent receptor expression of each of the reported innate immune response receptors expressed in vaginal macrophages, and low or absent HIV-1 receptor and coreceptor expression. These data correlated with the inability to observe productive HIV-1 infection within intestinal macrophages. Together, these findings demonstrate the role of vaginal macrophages in establishment of acute infection and implicate a potential role for vaginal macrophages as a viral reservoir. Although the potency of ART across vaginal mucosal macrophages is currently unknown, defining the potency of current and novel ART within a subset of cells representing a front-line innate immune response to HIV-1 infection could serve as a valuable tool for design of novel therapeutics specifically targeting these cells. The data reported in Shen et al. [77] provide a foundation for extensive studies designed to elucidate the antiviral activity of ART against CCR5, CXCR4 or dual-tropic HIV-1 in vaginal macrophages.
Relationship between CD14lo/CD16hi monocytes and HIV-1 infection
A unique subset of non-terminally differentiated macrophages is the CD14lo/CD16hi monocytes. This subset of monocytes has been demonstrated to represent the approximately 10–15% of monocytes that are permissive to HIV-1 infection [78,79] and possess many macrophage and dendritic cell-like properties including high CCR5 and MHCII expression, and the ability to act as a migratory surveyor of multiple tissues [78,79]. As both tissue macrophages and dendritic cells perform similar functions, it follows that CD14lo/CD16hi monocytes might represent a significantly understudied viral reservoir (Figure 1). To this end, Jaworoski et al. [79] recently reported that the predominant site of HIV-1 infection within monocytes in vivo is the CD16hi subset. Although antiviral activity and cellular pharmacology of ART in CD14lo/CD16hi monocytes represent an unstudied area of research, novel drug design targeting monocyte subsets based on receptor expression profiles might present an area for future drug design.
Relationship between ART, microglial cells and the central nervous system
Microglial cells represent a significant target for HIV-1 infection within the CNS [25,32,80,81] and predominantly express CD4 and CCR5; however, subsets of microglial cells express CXCR4 and are permissive to CXCR4-using or dual-tropic (R5X4) viruses [26,82]. The CNS provides a distinct microenvironment because infection is established during acute infection, but remains largely latent until later in disease progression [83,84] (Figure 1). Factors including diminished immune function and CD4+ T-lymphocyte counts coupled with increased plasma viral loads, contribute to a systemic increase in infected, activated CD14+/CD16+- and CD14+/CD69+-expressing monocytes [85-87]. This, in turn, triggers increased trafficking of infected cells across the blood-brain barrier (BBB), which, in combination with the activation state of the cells, stimulates reactivation of latent infection within microglial cells in the CNS [86,87].
Clearance of both latent and productive infection in the CNS is essential in eradication of HIV-1 [32] and presents an immediate concern for current patients because HAD occurs in approximately 20% of HIV-infected individuals and is responsible for significant neurocognitive impairment [28]. Disease progression and increased viral loads in the CNS and brain are also associated with increased levels of activated monocytes and microglial cells [85,88], which correlate with progression to AIDS and an increased occurrence of either minor cognitive motor disorder (MCMD) or the more severe HAD [89,90]. Although the distinct mechanisms responsible for MCMD and HAD in HIV-infected persons are not fully elucidated, a link between activated monocytes, microglial cells and macrophages in the brain, and manifestation of clinical symptoms has been drawn [85,88-90]. Increased levels of activated cells of the monocytic lineage in the brain results in increased production of proinflammatory cytokines TNF-α, IL-1 and interferon-α [88-90], which function in a paracrine fashion to confer neuronal death and ultimately results in MCMD or HAD. Studies are conflicting when determining the relationship between administration of ART and the ability to eliminate or reduce MCMD and HAD [91,92], although Carvalhal et al. [93] correlates ZDV 3TC plus EFV treatment with decreased cognitive motor impairment and HAD. These data correlated with a statistically significant decrease in CNS viral loads, demonstrating that the effect of ART on MCMD and HAD might be an indirect mechanism facilitated through a decrease in overall viraemia. Although the interplay between ART and macrophages in MCMD and HAD is not fully elucidated, it is plausible that reduction of viraemia in the brain/CNS by some ART could correlate with decreased levels of activated monocytes/macrophages; therefore, decreasing cytokine-mediated neuronal death [85,87,89,90,94] (Figure 1). These data demonstrate the correlation between ART administration and decreased cognitive motor impairments, and highlight the need for novel therapeutics with greater BBB penetration.
Penetration of current ART across the BBB and in the CNS is poor, conferring a microenvironment wherein suboptimal levels of drug allow for emergence of mutations that confer resistance to ART, and the establishment and maintenance of viral reservoirs [57,95,96]. Understanding the cellular pharmacology of current ART in the CNS, and within subsets of primary HIV-1 targets within the CNS, remains a significant obstacle in the treatment of HIV-1-infected patients, and the design of novel therapeutics with increased CNS penetration and bioavailability is essential. Fortunately, new animal models for HIV-1 encephalitis in mice have been developed, which will facilitate the development of improved drug targeting within the brain and CNS [97,98].
As current ART display low penetration in the CNS, a focus on alternative mechanisms for reduction and elimination of HIV-1 and HAD in microglial cells is essential. Agrawal et al. [99] hypothesized that HIV-1 gp120-induced secretion of cytotoxic reactive oxygen species (ROS) could be reduced or eliminated and therefore examined the effect of vector-mediated delivery of antioxidant enzymes Cu/Zn-superoxide dismutase (SOD1) and/or glutathione peroxidase (GPx1) to primary neuronal cultures. A significant reduction in gp-120-induced apoptosis was observed, demonstrating the importance of non-traditional targets as mediators of HIV-1 infection in the CNS. Whether a vector-mediated delivery of cytoprotective agents can provide sustenance in HIV-1-infected persons, and whether this delivery system provides practical application, are ongoing areas of research. Nonetheless, this study highlights the necessity for reduction in both total viral loads in the CNS and elimination in HIV-1-mediated apoptosis and cytotoxicity. Novel targets with greater CNS penetration and potency represent an understudied area of research and remain a significant obstacle in systemic eradication of HIV-1.
Metabolism of antiretroviral therapy in macrophages
Studies on cellular drug interactions with antiretroviral agents prior to clinical trials are necessary to detect possible drug interactions. Dynamics of additive or synergistic drug effects in vitro provides a foundation for understanding how drugs might interact in vivo. Multiple studies have observed the effect of coadministration of ART in vitro in cell lines and primary heterogeneous lymphocyte populations [100-102]. Few studies have observed dynamics of drug interactions in macrophages. Pilot studies using nanoparticle-indinavir (NP-IDV) provide fundamental data about potential metabolism of NP-IDV in macrophages. Dou et al. [103] subjected primary macrophages to a single 50 μM dose of NP-IDV and demonstrated limited cytotoxicity for 6 days, and correlated these data with constant intracellular drug levels and minimal metabolism. As NP-IDV has not been tested in vivo, its systemic pharmacokinetic profile was not defined, presenting difficulty in assessing how these data might affect HIV-1 infection in vivo. Independent of current gaps in knowledge, this study presents a backbone for understanding metabolism of novel therapeutics in macrophages.
Novel therapeutics and potential targets in macrophages
Discovering novel targets to inhibit HIV-1 replication presents an important area of study. Combination ART remains a backbone of current treatment approaches [20] and the elimination of virus cannot occur by a single mechanism. Many new targets are currently being explored, including those targeting HIV-1 maturation, inhibition of HIV-1 accessory proteins, inhibition of cellular factors involved in viral replication or immune-based treatments [45,47,104-106] (Table 2). To date, most studies focus upon inhibition of viral replication in CD4+ T-lymphocytes, although recent studies have begun to define antiviral activity of novel therapeutics in macrophages. The potential novel therapeutics described in this review focus upon macrophage-related drug targets.
Table 2.
Potency of novel antiretroviral therapies in acute versus chronic macrophages
| Potential antiretroviral therapy |
Acute infection in macrophages EC50, nM |
Chronic infection in macrophages EC50, nM |
|---|---|---|
| BIT225 | 1,100 | U |
| Carbohydrate-binding agents | 80 | U |
| PI3K/Akt Inhibitors | 200a | U |
| siRNAs | NA | NA |
The 50% effective concentration (EC50) of HIV type-1 antiretroviral therapy in various stages of development for acute chronically infected macrophages.
EC50 observed in the presence of inhibitors of nitric oxide.
NA, not applicable; siRNA, small interfering RNA; U, undefined.
Small interfering RNAs (siRNAs) could be useful for the induction of potent gene silencing by degradation of cognate RNA. The use of siRNA for HIV-1 infection presents a unique challenge because systemic or directed silencing of CXCR4 coreceptor would result in mortality, and silencing of CCR5 coreceptor could represent a selective pressure for emergence of highly pathogenic CXCR4-using viruses. Independent of HIV-1 infection, sustained silencing in dividing cells is maintained for attenuated time frames (3–7 days); however, sustained silencing in non-dividing, terminally differentiated macrophages was examined in vitro as a model with potential therapeutic implications [107]. Replication kinetics of acute HIV-1 infection in primary macrophages stably transfected with CCR5-targeted siRNA alone or in combination with siRNA targeted against HIV-1 structural protein p24 were examined. HIV-1 infection was effectively eliminated when observed over 15 days in macrophages coexpressing CCR5 and p24 siRNAs [107]. Although these results provide a promising foundation, significant complexities of HIV-1 infection in macrophages persist and cannot be rectified with siRNA-mediated inhibition of HIV-1. Toxicity of siRNA in the context of HIV-1 infection is largely undefined, although Anderson and Akkina [108] provided a study presenting various methods of siRNA-mediated knockdown of CCR5 and their corresponding toxicities in transgenic macrophages. Whether in vivo introduction of siRNA would result in significant toxicity is undefined and, in combination with selective pressure for emergence of pathogenic CXCR4-using viruses, continues to present a significant obstacle in the use of siRNA as a therapeutic tool for treatment of HIV-1 infection.
Carbohydrate-binding agents (CBAs) have been described as potential inhibitors of HIV-1 infection [105]. CBAs target the heavily glycosylated HIV-1 envelope, impairing the ability of macrophages or dendritic cells to recognize and perform antigen presentation to CD4+ T-lymphocytes, subsequently impairing transfer infection [12,105]. Pilot studies in acutely infected primary macrophages demonstrate that CBA molecules demonstrate antiviral activity at concentrations as low as 80 nM (Table 2). The toxicological profile or the ability of CBAs to inhibit chronic HIV-1 infection is not currently defined. As the mechanism of action of CBAs is predominantly extracellular, and could effectively inhibit transfer of infection, these drugs might prove efficacious when coadministered with other classes of ART.
PI3K/Akt pathway inhibitors present a novel anti-HIV-1 target that has undergone recent study. The PI3K/Akt pathway is a cell survival pathway that is activated upon apoptotic stress and functions to activate downstream modulators of cell survival [109]. Recent reports have hypothesized that inhibition of the PI3K/Akt pathway might result in super-sensitivity and ultimately cell death in HIV-1-infected macrophages because they are exposed to normal cellular stressors [104]. Chugh et al. [104] demonstrated that PI3K/Akt inhibitors could inhibit HIV-1 replication in acutely infected primary macrophages. Interestingly, antiviral activity was only observed when drugs were coadministered with a compound that positively modulates nitric oxide-induced cytotoxicity in HIV-1 infection [110,111]. In addition, concentration of PI3K/Akt inhibitors conferring inhibition of viral replication was 200 nM (Table 2). The effect of these inhibitors on chronically infected macrophages, or macrophages at different stages of activation, is not known. This study provided an interesting novel drug target and demonstrated efficacy at high concentrations. Although this concentration appears high, and unlikely to be physiologically relevant, these data provide a foundation for studies that exploit cellular signalling pathways that are activated in HIV-1 infected, but not uninfected cells.
Vehicle-based delivery of currently approved ART to macrophages remains an ongoing area of research. Peptide fragments designed to bind specifically to macrophages, thus conferring phagocytosis, provide an attractive mechanism to target macrophages and macrophage-like cells. Dutta and Jain [69] recently evaluated the ability of EFV-loaded tuftsin to inhibit HIV-1 replication in primary macrophages. A significant reduction in supernatant p24 levels were observed relative to controls; however, studies defining the relationship between this drug and replicative kinetics for CXCR4, CCR5 or dual-tropic viruses is not fully elucidated. Whether efavirenz-loaded peptides provide a realistic approach for treating infection in humans and the ability of a drug that specifically targets phagocytic cells to indirectly or directly affect lymphocytes are important questions that must be answered before these studies can be regarded as therapeutic strategies.
Immunotoxins might provide a mechanism for targeted elimination of HIV-1-infected cells, and both novel design of immunotoxin-based antiviral agents and their effect on macrophages remains an ongoing area of research. Berger et al. [112] recently reported the use of Pseudomonas aeruginosa Exotoxin A linked to a specific protein that binds HIV-1 Env (3B3Mab against a highly conserved region of the CD4+ binding site). Inhibition of spreading infection was observed in both primary lymphocytes and macrophages, and addition of an RT inhibitor eliminated infectious virus in lymphocyte cultures. Whether this agent can demonstrate antiviral activity in primary macrophages at different stages of activation and infection are not defined; however, these studies provide a foundation for further studies. One major limitation of this experimental protein is its inability to cross the BBB; thus, precluding its use as a monotherapy and instead limiting it to combination administration with other agents demonstrating greater CNS penetration.
Inhibitors targeting HIV-1 accessory proteins provide a targeted approach for elimination of HIV-1 within infected cells. Vpu provides an attractive target because interference with Vpu acts post-integration to confer abnormal packaging of newly formed virions. Khoury et al. [113] demonstrated that BIT225, an inhibitor of Vpu displays antiviral activity against an R5-using HIV-1 in primary macrophages during acute and chronic infection (EC50 1,000 nM; Table 2). In addition, transfer of infection from primary macrophages to more permissive CD4+ T-lymphocytes was also significantly reduced. These data provide the framework for a novel class of therapeutics targeting components of the virus that are essential for productive replication.
Cellular factors affecting pharmacology of ART in macrophages
Bioavailability of drugs is in the treatment of HIV-1 infection [56,106,114]. Many ARTs are highly plasma protein bound, metabolized by cytochromes in the intestinal epithelium or lack the lipophilicity to traverse the lipid bilayer of cells efficiently [106,114-116]. Some drugs are substrates for efflux transporters, whose intrinsic function is to prevent cellular toxicity by performing rapid efflux of intracellular substrates. The link between efflux transporter expression and activity, and modulation of these transporters by ART has been extensively studied both in vitro and in vivo. To date, studies correlating higher efflux transporter expression and activity with treatment failure and poor clinical prognosis are conflicting [114,117,118].
P-glycoprotein (P-gp/MDR1) and multidrug resistance transporters 1, 4 and 5 (MRP1, MRP4 and MRP5) are expressed in macrophages [119-121]. As efflux transporters can limit the amount of bioavailable intracellular drug, understanding the link between these efflux transporters and bioavailability could provide a foundation for design of novel therapeutics that might not bind with high affinity to these transporters.
Relative to macrophages, efflux transporter expression and antiviral activity of AZT and IDV has been correlated in a study by Jorajuria et al. [119]. Inhibition of P-gp resulted in increased antiviral activity and increased intracellular IDV levels, whereas inhibition of P-gp did not correlate with increased anti-HIV activity of AZT. These data suggest that AZT is a substrate for MRP4 and MRP5, but not P-gp, whereas IDV is a substrate for P-gp.
Efflux transporters are a natural defence mechanism to prevent cellular toxicity and might decrease intracellular bioavailability of ART in HIV-1 target cells. Decreased bioavailability can result in suboptimal levels of drug, resulting in an increased likelihood for emergence of resistant HIV-1 [122]. As macrophages express P-gp, MRP4 and MRP5, and multiple ARTs have been demonstrated to be substrates for these transporters [119-121], it follows that this link might reduce the intracellular concentration of ART in macrophages. Long-term administration of ART could expedite emergence of resistant HIV-1 in macrophages, facilitating a viral reservoir for resistant HIV-1 within these long-lived cells. In addition, long-lived macrophages with suboptimal levels of drug might also remain latently infected, and emerge later in disease progression, transferring productive infection of resistant viruses [123]. In this way, the relationship between cellular factors affecting intracellular drug concentration directly correlates with latency, viral reservoirs and chronic HIV-1 infection. Understanding how current ARTs interact with efflux transporters and the effect of this relationship on therapy outcome and long-term patient survival is essential when designing novel therapeutics.
Conclusions
Macrophages represent a crucial target for establishment and maintenance of chronic and latent HIV-1 infection [2,3,21,31,33]. Various macrophage-like cells, including dendritic cells, alveolar macrophages, monocyte precursors and microglial cells, contribute to the complex interplay between systemic infection and administration of ART [2,3,16,21,22,25,26,31,33,73,84,87,124]. Macrophages are found in every organ system and tissue, and, because of high CCR5 expression, represent a target for early establishment and maintenance of latent viral reservoirs [2,4,7,71].
HIV-1 infection in alveolar macrophages can contribute to cytokine-mediated immune dysfunction by promoting a proinflammatory milieu that favours disease progression, whereas, in some cases, increasing levels of cytoprotective chaemokines including MIP1-α, MIP1-β and RANTES [76,125]. Infection in CD16hi HIV-1 permissive monocytes can contribute to increased trafficking of HIV-1-infected monocytes and macrophages across the BBB [86,87]. Increased trafficking across the BBB, in turn, positively modulates a proinflammatory cytokine milieu coupled with release of ROS in the brain, eventually culminating in an increased risk of HAD and MCMD [88,89,94].
Although the cellular pharmacology of ART remains an understudied area of research, the significantly higher EC50 in macrophages versus lymphocytes suggests that ART are not as potent across macrophages [126]. In addition, penetration of ART across the BBB is usually poor [57,95,96] and the ability to deliver effective concentrations of drug to macrophages across all organs and tissue compartments remains an important goal towards viral eradication. The cellular pharmacology of ART in macrophages directly affects viral loads, emergence of mutations that confer resistance both within and between subsets of HIV-1 target cells, eradication of systemic virus and long-term patient survival (Figure 1). Eradication of systemic HIV-1 infection is not possible without clearance of latently infected cells. For these reasons, understanding dynamics of ART pharmacology in macrophages and subsequently eliminating productive infection in these cells, is critical to eliminating systemic HIV-1 infection.
Acknowledgements
This work is supported in part by NIH grants 1R01-RR0-25996, 2P30-AI-50409 (CFAR), 5R37-AI-041980, R01-RR-25996, 5R01-AI-071846 and 5R37-AI-025899, and by the Department of Veterans Affairs.
Footnotes
Disclosure statement
RFS is a founder and major shareholder of Idenix Pharmaceuticals, Pharmasset Inc. and RFS Pharma. CG declares no competing interests.
References
- 1.Stevenson M. Can HIV be cured? Sci Am. 2008;299:78–83. doi: 10.1038/scientificamerican1108-78. [DOI] [PubMed] [Google Scholar]
- 2.Aquaro S, Balestra E, Cenci A, Francesconi M, Caliò R, Perno CF. HIV infection in macrophage: role of long-lived cells and related therapeutical strategies. J Biol Regul Homeost Agents. 1997;11:69–73. [PubMed] [Google Scholar]
- 3.Aquaro S, Calio R, Balzarini J, Bellocchi MC, Garaci E, Perno CF. Macrophages and HIV infection: therapeutical approaches toward this strategic virus reservoir. Antiviral Res. 2002;55:209–225. doi: 10.1016/s0166-3542(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 4.Kedzierska K, Crowe SM. The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr Med Chem. 2002;9:1893–1903. doi: 10.2174/0929867023368935. [DOI] [PubMed] [Google Scholar]
- 5.Pereira CF, Paridaen JT. Anti-HIV drug development-an overview. Curr Pharm Des. 2004;10:4005–4037. doi: 10.2174/1381612043382459. [DOI] [PubMed] [Google Scholar]
- 6.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 7.Montaner LJ, Crowe SM, Aquaro S, Perno CF, Stevenson M, Collman RG. Advances in macrophage and dendritic cell biology in HIV-1 infection stress key understudied areas in infection, pathogenesis, and analysis of viral reservoirs. J Leukoc Biol. 2006;80:961–964. doi: 10.1189/jlb.0806488. [DOI] [PubMed] [Google Scholar]
- 8.Ryan GB, Spector WG. Natural selection of long-lived macrophages in experimental granulomata. J Pathol. 1969;99:139–151. doi: 10.1002/path.1710990208. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz M, Louie M, Hurley A, et al. A novel antiviral intervention results in more accurate assessment of human immunodeficiency virus type 1 replication dynamics and T-cell decay in vivo. J Virol. 2003;77:5037–5038. doi: 10.1128/JVI.77.8.5037-5038.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly J, Beddall MH, Yu D, Iyer SR, Marsh JW, Wu Y. Human macrophages support persistent transcription from unintegrated HIV-1 DNA. Virology. 2008;372:300–312. doi: 10.1016/j.virol.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters PJ, Sullivan WM, Duenas-Decamp MJ, et al. Non-macrophage-tropic human immunodeficiency virus type 1 R5 envelopes predominate in blood, lymph nodes, and semen: implications for transmission and pathogenesis. J Virol. 2006;80:6324–6332. doi: 10.1128/JVI.02328-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groot F, Welsch S, Sattentau QJ. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood. 2008;111:4660–4663. doi: 10.1182/blood-2007-12-130070. [DOI] [PubMed] [Google Scholar]
- 13.Verani A, Gras G, Pancino G. Macrophages and HIV-1: dangerous liaisons. Mol Immunol. 2005;42:195–212. doi: 10.1016/j.molimm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Aquaro S, Perno CF, Balestra E, et al. Inhibition of replication of HIV in primary monocyte/macrophages by different antiviral drugs and comparative efficacy in lymphocytes. J Leukoc Biol. 1997;62:138–143. doi: 10.1002/jlb.62.1.138. [DOI] [PubMed] [Google Scholar]
- 15.Aquaro S, Perno CF. Assessing the relative efficacy of antiretroviral activity of different drugs on macrophages. Methods Mol Biol. 2005;304:445–453. doi: 10.1385/1-59259-907-9:445. [DOI] [PubMed] [Google Scholar]
- 16.Aquaro S, Svicher V, Schols D, et al. Mechanisms underlying activity of antiretroviral drugs in HIV-1-infected macrophages: new therapeutic strategies. J Leukoc Biol. 2006;80:1103–1110. doi: 10.1189/jlb.0606376. [DOI] [PubMed] [Google Scholar]
- 17.Perno CF, Aquaro S, Rosenwirth B, et al. In vitro activity of inhibitors of late stages of the replication of HIV in chronically infected macrophages. J Leukoc Biol. 1994;56:381–386. doi: 10.1002/jlb.56.3.381. [DOI] [PubMed] [Google Scholar]
- 18.Smith AJ, Scott WA. The influence of natural substrates and inhibitors on the nucleotide-dependent excision activity of HIV-1 reverse transcriptase in the infected cell. Curr Pharm Des. 2006;12:1827–1841. doi: 10.2174/138161206776873572. [DOI] [PubMed] [Google Scholar]
- 19.Almond LM, Hoggard PG, Edirisinghe D, Khoo SH, Back DJ. Intracellular and plasma pharmacokinetics of efavirenz in HIV-infected individuals. J Antimicrob Chemother. 2005;56:738–744. doi: 10.1093/jac/dki308. [DOI] [PubMed] [Google Scholar]
- 20.Anderson AM, Lennox JL. Antiretroviral therapy: when to start and which drugs to use. Curr Infect Dis Rep. 2008;10:332–339. doi: 10.1007/s11908-008-0053-4. [DOI] [PubMed] [Google Scholar]
- 21.Aquaro S, Bagnarelli P, Guenci T, et al. Long-term survival and virus production in human primary macrophages infected by human immunodeficiency virus. J Med Virol. 2002;68:479–488. doi: 10.1002/jmv.10245. [DOI] [PubMed] [Google Scholar]
- 22.Aquaro S, Guenci T, Di Santo F, Francesconi M, Caliò R, Perno CF. Potent antiviral activity of amprenavir in primary macrophages infected by human immunodeficiency virus. Antiviral Res. 2004;61:133–137. doi: 10.1016/j.antiviral.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Aquaro S, Svicher V, Ceccherini-Silberstein F, et al. Limited development and progression of resistance of HIV-1 to the nucleoside analogue reverse transcriptase inhibitor lamivudine in human primary macrophages. J Antimicrob Chemother. 2005;55:872–878. doi: 10.1093/jac/dki104. [DOI] [PubMed] [Google Scholar]
- 24.Crowe S, Zhu T, Muller WA. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol. 2003;74:635–641. doi: 10.1189/jlb.0503204. [DOI] [PubMed] [Google Scholar]
- 25.Gabuzda DH, Sobel RA. HIV antigen in brains of patients with AIDS. Ann Neurol. 1987;22:668. doi: 10.1002/ana.410220526. [DOI] [PubMed] [Google Scholar]
- 26.Gorry PR, Bristol G, Zack JA, et al. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J Virol. 2001;75:10073–10089. doi: 10.1128/JVI.75.21.10073-10089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorry PR, Churchill M, Crowe SM, Cunningham AL, Gabuzda D. Pathogenesis of macrophage tropic HIV-1. Curr HIV Res. 2005;3:53–60. doi: 10.2174/1570162052772951. [DOI] [PubMed] [Google Scholar]
- 28.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 29.Perno CF, Svicher V, Schols D, Pollicita M, Balzarini J, Aquaro S. Therapeutic strategies towards HIV-1 infection in macrophages. Antiviral Res. 2006;71:293–300. doi: 10.1016/j.antiviral.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Wahl SM, Greenwell-Wild T, Vazquez N. HIV accomplices and adversaries in macrophage infection. J Leukoc Biol. 2006;80:973–983. doi: 10.1189/jlb.0306130. [DOI] [PubMed] [Google Scholar]
- 31.Aquaro S, Calio R, Balestra E, et al. Clinical implications of HIV dynamics and drug resistance in macrophages. J Biol Regul Homeost Agents. 1998;12:23–27. [PubMed] [Google Scholar]
- 32.Bagasra O, Lavi E, Bobroski L, et al. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Balestra E, Perno CF, Aquaro S, et al. Macrophages: a crucial reservoir for human immunodeficiency virus in the body. J Biol Regul Homeost Agents. 2001;15:272–276. [PubMed] [Google Scholar]
- 34.Kazmierczak K, Potash MJ. Host and virus strain dependence in activation of human macrophages by human immunodeficiency virus type 1. J Neurovirol. 2007;13:452–461. doi: 10.1080/13550280701510104. [DOI] [PubMed] [Google Scholar]
- 35.Perno CF, Balestra E, Francesconi M, et al. Antiviral profile of HIV inhibitors in macrophages: implications for therapy. Curr Top Med Chem. 2004;4:1009–1015. doi: 10.2174/1568026043388565. [DOI] [PubMed] [Google Scholar]
- 36.Smith PD, Meng G, Salazar-Gonzalez JF, Shaw GM. Macrophage HIV-1 infection and the gastrointestinal tract reservoir. J Leukoc Biol. 2003;74:642–649. doi: 10.1189/jlb.0503219. [DOI] [PubMed] [Google Scholar]
- 37.Classen A, Lloberas J, Celada A. Macrophage activation: classical versus alternative. Methods Mol Biol. 2009;531:29–43. doi: 10.1007/978-1-59745-396-7_3. [DOI] [PubMed] [Google Scholar]
- 38.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 39.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 40.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 41.Bogdan C, Thüring H, Dlaska M, Röllinghoff M, Weiss G. Mechanism of suppression of macrophage nitric oxide release by IL-13: influence of the macrophage population. J Immunol. 1997;159:4506–4513. [PubMed] [Google Scholar]
- 42.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cassol E, Cassetta L, Rizzi C, Alfano M, Poli G. M1 and M2a polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. J Immunol. 2009;182:6237–6246. doi: 10.4049/jimmunol.0803447. [DOI] [PubMed] [Google Scholar]
- 44.Matthews T, Salgo M, Greenberg M, Chung J, DeMasi R, Bolognesi D. Enfuvirtide: the first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat Rev Drug Discov. 2004;3:215–225. doi: 10.1038/nrd1331. [DOI] [PubMed] [Google Scholar]
- 45.MacArthur RD, Novak RM. Reviews of anti-infective agents: maraviroc: the first of a new class of antiretroviral agents. Clin Infect Dis. 2008;47:236–241. doi: 10.1086/589289. [DOI] [PubMed] [Google Scholar]
- 46.Dorr P, Westby M, Dobbs S, et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yi Y, Loftin L, Wang L, Ratcliffe SJ, Isaacman-Beck J, Collman RG. Entry coreceptor use and fusion inhibitor T20 sensitivity of dual-tropic R5X4 HIV-1 in primary macrophage infection. J Acquir Immune Defic Syndr. 2008;47:285–292. doi: 10.1097/QAI.0b013e31816520f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schinazi RF, Hernandez-Santiago BI, Hurwitz SJ. Pharmacology of current and promising nucleosides for the treatment of human immunodeficiency viruses. Antiviral Res. 2006;71:322–334. doi: 10.1016/j.antiviral.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Painter GR, Almond MR, Mao S, Liotta DC. Biochemical and mechanistic basis for the activity of nucleoside analogue inhibitors of HIV reverse transcriptase. Curr Top Med Chem. 2004;4:1035–1044. doi: 10.2174/1568026043388358. [DOI] [PubMed] [Google Scholar]
- 50.Diamond TL, Roshal M, Jamburuthugoda VK, et al. Macrophage tropism of HIV-1 depends on efficient cellular dNTP utilization by reverse transcriptase. J Biol Chem. 2004;279:51545–51553. doi: 10.1074/jbc.M408573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hakansson P, Hofer A, Thelander L. Regulation of mammalian ribonucleotide reduction and dNTP pools after DNA damage and in resting cells. J Biol Chem. 2006;281:7834–7841. doi: 10.1074/jbc.M512894200. [DOI] [PubMed] [Google Scholar]
- 52.Perno CF, Newcomb FM, Davis DA, et al. Relative potency of protease inhibitors in monocytes/macrophages acutely and chronically infected with human immunodeficiency virus. J Infect Dis. 1998;178:413–422. doi: 10.1086/515642. [DOI] [PubMed] [Google Scholar]
- 53.Barcellini W, Rizzardi GP, Borghi MO, Fain C, Lazzarin A, Meroni PL. TH1 and TH2 cytokine production by peripheral blood mononuclear cells from HIV-infected patients. AIDS. 1994;8:757–762. doi: 10.1097/00002030-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Becker Y. The changes in the T helper 1 (Th1) and T helper 2 (Th2) cytokine balance during HIV-1 infection are indicative of an allergic response to viral proteins that may be reversed by Th2 cytokine inhibitors and immune response modifiers - a review and hypothesis. Virus Genes. 2004;28:5–18. doi: 10.1023/B:VIRU.0000012260.32578.72. [DOI] [PubMed] [Google Scholar]
- 55.Siliciano RF. Scientific rationale for antiretroviral therapy in 2005: viral reservoirs and resistance evolution. Top HIV Med. 2005;13:96–100. [PubMed] [Google Scholar]
- 56.Perez-Bercoff D, Wurtzer S, Compain S, Benech H, Clavel F. Human immunodeficiency virus type 1: resistance to nucleoside analogues and replicative capacity in primary human macrophages. J Virol. 2007;81:4540–4550. doi: 10.1128/JVI.01620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGee B, Smith N, Aweeka F. HIV pharmacology: barriers to the eradication of HIV from the CNS. HIV Clin Trials. 2006;7:142–153. doi: 10.1310/AW2H-TP5C-NP43-K6BY. [DOI] [PubMed] [Google Scholar]
- 58.Souza TM, Cirne-Santos CC, Rodrigues DQ, et al. The compound 6-chloro-1,4-dihydro-4-oxo-1-(beta-D-ribofuranosyl) quinoline-3-carboxylic acid inhibits HIV-1 replication by targeting the enzyme reverse transcriptase. Curr HIV Res. 2008;6:209–217. doi: 10.2174/157016208784324930. [DOI] [PubMed] [Google Scholar]
- 59.Anker M, Corales RB. Raltegravir (MK-0518): a novel integrase inhibitor for the treatment of HIV infection. Expert Opin Investig Drugs. 2008;17:97–103. doi: 10.1517/13543784.17.1.97. [DOI] [PubMed] [Google Scholar]
- 60.Imamichi T. Action of anti-HIV drugs and resistance: reverse transcriptase inhibitors and protease inhibitors. Curr Pharm Des. 2004;10:4039–4053. doi: 10.2174/1381612043382440. [DOI] [PubMed] [Google Scholar]
- 61.Ortiz GM, Wellons M, Brancato J, et al. Structured antiretroviral treatment interruptions in chronically HIV-1-infected subjects. Proc Natl Acad Sci U S A. 2001;98:13288–13293. doi: 10.1073/pnas.221452198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murphy RL. Reviving protease inhibitors: new data and more options. J Acquir Immune Defic Syndr. 2003;33(Suppl 1):S43–52. [PubMed] [Google Scholar]
- 63.Scruggs ER, Dirks Naylor AJ. Mechanisms of zidovudine-induced mitochondrial toxicity and myopathy. Pharmacology. 2008;82:83–88. doi: 10.1159/000134943. [DOI] [PubMed] [Google Scholar]
- 64.Chavan S, Kodoth S, Pahwa R, Pahwa S. The HIV protease inhibitor Indinavir inhibits cell-cycle progression in vitro in lymphocytes of HIV-infected and uninfected individuals. Blood. 2001;98:383–389. doi: 10.1182/blood.v98.2.383. [DOI] [PubMed] [Google Scholar]
- 65.Azzam R, Lal L, Goh SL, et al. Adverse effects of antiretroviral drugs on HIV-1 infected and uninfected human monocyte-derived macrophages. J Acquir Immune Defic Syndr. 2006;42(1):19–28. doi: 10.1097/01.qai.0000214809.83218.88. [DOI] [PubMed] [Google Scholar]
- 66.Donaghy H, Stebbing J, Patterson S. Antigen presentation and the role of dendritic cells in HIV. Curr Opin Infect Dis. 2004;17:1–6. doi: 10.1097/00001432-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Frank I, Stossel H, Gettie A, et al. A fusion inhibitor prevents spread of immunodeficiency viruses, but not activation of virus-specific T cells, by dendritic cells. J Virol. 2008;82:5329–5339. doi: 10.1128/JVI.01987-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dutta T, Garg M, Jain NK. Targeting of efavirenz loaded tuftsin conjugated poly(propyleneimine) dendrimers to HIV infected macrophages in vitro. Eur J Pharm Sci. 2008;34:181–189. doi: 10.1016/j.ejps.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Dutta T, Jain NK. Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochim Biophys Acta. 2007;1770:681–686. doi: 10.1016/j.bbagen.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 70.Lori F, Calarota SA, Lisziewicz J. Nanochemistry-based immunotherapy for HIV-1. Curr Med Chem. 2007;14:1911–1919. doi: 10.2174/092986707781368513. [DOI] [PubMed] [Google Scholar]
- 71.Worgall S, Connor R, Kaner RJ, et al. Expression and use of human immunodeficiency virus type 1 coreceptors by human alveolar macrophages. J Virol. 1999;73:5865–5874. doi: 10.1128/jvi.73.7.5865-5874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rich EA, Orenstein JM, Jeang KT. A macrophage-tropic HIV-1 that expresses green fluorescent protein and infects alveolar and blood monocyte-derived macrophages. J Biomed Sci. 2002;9:721–726. doi: 10.1159/000067293. [DOI] [PubMed] [Google Scholar]
- 73.Hammer SM, Gillis JM, Pinkston P, Rose RM. Effect of zidovudine and granulocyte-macrophage colony-stimulating factor on human immunodeficiency virus replication in alveolar macrophages. Blood. 1990;75:1215–1219. [PubMed] [Google Scholar]
- 74.Park IW, Koziel H, Hatch W, Li X, Du B, Groopman JE. CD4 receptor-dependent entry of human immunodeficiency virus type-1 env-pseudotypes into CCR5−, CCR3−, and CXCR4-expressing human alveolar macrophages is preferentially mediated by the CCR5 coreceptor. Am J Respir Cell Mol Biol. 1999;20:864–871. doi: 10.1165/ajrcmb.20.5.3547. [DOI] [PubMed] [Google Scholar]
- 75.Pemberton LA, Stone E, Price P, van Bockxmeer F, Brew BJ. The relationship between ApoE, TNFA, IL1a, IL1b and IL12b genes and HIV-1-associated dementia. HIV Med. 2008;9:677–680. doi: 10.1111/j.1468-1293.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 76.Lane BR, Markovitz DM, Woodford NL, Rochford R, Strieter RM, Coffey MJ. TNF-alpha inhibits HIV-1 replication in peripheral blood monocytes and alveolar macrophages by inducing the production of RANTES and decreasing C-C chemokine receptor 5 (CCR5) expression. J Immunol. 1999;163:3653–3661. [PubMed] [Google Scholar]
- 77.Shen R, Richter HE, Clements RH, et al. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J Virol. 2009;83:3258–3267. doi: 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ellery PJ, Tippett E, Chiu YL, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 79.Jaworowski A, Kamwendo DD, Ellery P, et al. CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with Plasmodium falciparum malaria and HIV-1 infection. J Infect Dis. 2007;196:38–42. doi: 10.1086/518443. [DOI] [PubMed] [Google Scholar]
- 80.Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 81.Vazeux R. AIDS encephalopathy and tropism of HIV for brain monocytes/macrophages and microglial cells. Pathobiology. 1991;59:214–218. doi: 10.1159/000163648. [DOI] [PubMed] [Google Scholar]
- 82.Albright AV, Shieh JT, Itoh T, et al. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol. 1999;73:205–213. doi: 10.1128/jvi.73.1.205-213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.An SF, Groves M, Gray F, Scaravilli F. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J Neuropathol Exp Neurol. 1999;58:1156–1162. doi: 10.1097/00005072-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 84.Davis LE, Hjelle BL, Miller VE, et al. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 85.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 86.Ancuta P, Kunstman KJ, Autissier P, et al. CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology. 2006;344:267–276. doi: 10.1016/j.virol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 87.Dunfee R, Thomas ER, Gorry PR, Wang J, Ancuta P, Gabuzda D. Mechanisms of HIV-1 neurotropism. Curr HIV Res. 2006;4:267–278. doi: 10.2174/157016206777709500. [DOI] [PubMed] [Google Scholar]
- 88.Rumbaugh JA, Nath A. Developments in HIV neuropathogenesis. Curr Pharm Des. 2006;12:1023–1044. doi: 10.2174/138161206776055877. [DOI] [PubMed] [Google Scholar]
- 89.Sippy BD, Hofman FM, Wallach D, Hinton DR. Increased expression of tumor necrosis factor-alpha receptors in the brains of patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:511–521. [PubMed] [Google Scholar]
- 90.Tyor WR, Glass JD, Griffin JW, et al. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- 91.Goodkin K, Wilkie FL, Concha M, et al. Subtle neuropsychological impairment and minor cognitive-motor disorder in HIV-1 infection. Neuroradiological, neurophysiological, neuroimmunological, and virological correlates. Neuroimaging Clin N Am. 1997;7:561–579. [PubMed] [Google Scholar]
- 92.Jevtovic Dj, Vanovac V, Veselinovic M, Salemovic D, Ranin J, Stefanova E. The incidence of and risk factors for HIV-associated cognitive-motor complex among patients on HAART. Biomed Pharmacother. 2008;63:561–565. doi: 10.1016/j.biopha.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 93.Carvalhal AS, Rourke SB, Belmonte-Abreu P, Correa J, Goldani LZ. Evaluation of neuropsychological performance of HIV-infected patients with minor motor cognitive dysfunction treated with highly active antiretroviral therapy. Infection. 2006;34:357–360. doi: 10.1007/s15010-006-6610-6. [DOI] [PubMed] [Google Scholar]
- 94.Roy A, Jana A, Yatish K, et al. Reactive oxygen species up-regulate CD11b in microglia via nitric oxide: Implications for neurodegenerative diseases. Free Radic Biol Med. 2008;45:686–699. doi: 10.1016/j.freeradbiomed.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eggers C, Hertogs K, Stürenburg HJ, van Lunzen J, Stellbrink HJ. Delayed central nervous system virus suppression during highly active antiretroviral therapy is associated with HIV encephalopathy, but not with viral drug resistance or poor central nervous system drug penetration. AIDS. 2003;17:1897–1906. doi: 10.1097/00002030-200309050-00008. [DOI] [PubMed] [Google Scholar]
- 96.Smit TK, Brew BJ, Tourtellotte W, Morgello S, Gelman BB, Saksena NK. Independent evolution of human immunodeficiency virus (HIV) drug resistance mutations in diverse areas of the brain in HIV-infected patients, with and without dementia, on antiretroviral treatment. J Virol. 2004;78:10133–10148. doi: 10.1128/JVI.78.18.10133-10148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Persidsky Y, Gendelman HE. Murine models for human immunodeficiency virus type 1-associated dementia: the development of new treatment testing paradigms. J Neurovirol. 2002;8(Suppl 2):49–52. doi: 10.1080/13550280290167993. [DOI] [PubMed] [Google Scholar]
- 98.Potula R, Poluektova L, Knipe B, et al. Inhibition of indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in an animal model of HIV-1 encephalitis. Blood. 2005;106:2382–2390. doi: 10.1182/blood-2005-04-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agrawal L, Louboutin JP, Reyes BA, Van Bockstaele EJ, Strayer DS. Antioxidant enzyme gene delivery to protect from HIV-1 gp120-induced neuronal apoptosis. Gene Ther. 2006;13:1645–1656. doi: 10.1038/sj.gt.3302821. [DOI] [PubMed] [Google Scholar]
- 100.Hernandez-Santiago BI, Mathew JS, Rapp KL, Grier JP, Schinazi RF. Antiviral and cellular metabolism interactions between Dexelvucitabine and lamivudine. Antimicrob Agents Chemother. 2007;51:2130–2135. doi: 10.1128/AAC.01543-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Balzarini J, Naesens L, Aquaro S, et al. Intracellular metabolism of cyclosaligenyl 3′-azido-2′,3′-dideoxythymidine monophosphate, a prodrug of 3′-azido-2′,3′-dideoxythymidine (zidovudine) Mol Pharmacol. 1999;56:1354–1361. doi: 10.1124/mol.56.6.1354. [DOI] [PubMed] [Google Scholar]
- 102.Borroto-Esoda K, Vela JE, Myrick F, Ray AS, Miller MD. In vitro evaluation of the anti-HIV activity and metabolic interactions of tenofovir and emtricitabine. Antivir Ther. 2006;11:377–384. [PubMed] [Google Scholar]
- 103.Dou H, Morehead J, Destache CJ, et al. Laboratory investigations for the morphologic, pharmacokinetic, and anti-retroviral properties of indinavir nanoparticles in human monocyte-derived macrophages. Virology. 2007;358:148–158. doi: 10.1016/j.virol.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 104.Chugh P, Bradel-Tretheway B, Monteiro-Filho CM, et al. Akt inhibitors as an HIV-1 infected macrophage-specific anti-viral therapy. Retrovirology. 2008;5:11. doi: 10.1186/1742-4690-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pollicita M, Schols D, Aquaro S, et al. Carbohydrate-binding agents (CBAs) inhibit HIV-1 infection in human primary monocyte-derived macrophages (MDMs) and efficiently prevent MDM-directed viral capture and subsequent transmission to CD4+ T lymphocytes. Virology. 2008;370:382–391. doi: 10.1016/j.virol.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 106.Warnke D, Barreto J, Temesgen Z. Antiretroviral drugs. J Clin Pharmacol. 2007;47:1570–1579. doi: 10.1177/0091270007308034. [DOI] [PubMed] [Google Scholar]
- 107.Song E, Lee SK, Dykxhoorn DM, et al. Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J Virol. 2003;77:7174–7181. doi: 10.1128/JVI.77.13.7174-7181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Anderson J, Akkina R. Complete knockdown of CCR5 by lentiviral vector-expressed siRNAs and protection of transgenic macrophages against HIV-1 infection. Gene Ther. 2007;14:1287–1297. doi: 10.1038/sj.gt.3302958. [DOI] [PubMed] [Google Scholar]
- 109.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 110.Zhao ML, Kim MO, Morgello S, Lee SC. Expression of inducible nitric oxide synthase, interleukin-1 and caspase-1 in HIV-1 encephalitis. J Neuroimmunol. 2001;115:182–191. doi: 10.1016/s0165-5728(00)00463-x. [DOI] [PubMed] [Google Scholar]
- 111.Polazzi E, Levi G, Minghetti L. Human immunodeficiency virus type 1 Tat protein stimulates inducible nitric oxide synthase expression and nitric oxide production in microglial cultures. J Neuropathol Exp Neurol. 1999;58:825–831. doi: 10.1097/00005072-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 112.Berger EA, Kennedy PE, Bera TK, Gallo M, Pastan I. Immunotoxins to selectively deplete reservoirs of HIV-infected cells that persist in the face of highly suppresive antiretroviral therapy. Global Antiviral Journal. 2008;4(Suppl 1):13. [Google Scholar]
- 113.Khoury G, Ewart G, Luscombe C, Miler M, Wilkinson J. Inhibiting Vpu function with the novel compound BIT225 results in inhibition of HIV-1 release from human macrophage reservoirs. Global Antiviral Journal. 2008;4(Suppl 1):15. [Google Scholar]
- 114.Kiser JJ, Aquilante CL, Anderson PL, King TM, Carten ML, Fletcher CV. Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47:298–303. doi: 10.1097/qai.0b013e31815e7478. [DOI] [PubMed] [Google Scholar]
- 115.Owen A, Pirmohamed M, Khoo SH, Back DJ. Pharmacogenetics of HIV therapy. Pharmacogenet Genomics. 2006;16:693–703. doi: 10.1097/01.fpc.0000236338.41799.57. [DOI] [PubMed] [Google Scholar]
- 116.Granfors MT, Wang JS, Kajosaari LI, Laitila J, Neuvonen PJ, Backman JT. Differential inhibition of cytochrome P450 3A4, 3A5 and 3A7 by five human immunodeficiency virus (HIV) protease inhibitors in vitro. Basic Clin Pharmacol Toxicol. 2006;98:79–85. doi: 10.1111/j.1742-7843.2006.pto_249.x. [DOI] [PubMed] [Google Scholar]
- 117.Janneh O, Jones E, Chandler B, Owen A, Khoo SH. Inhibition of P-glycoprotein and multidrug resistance-associated proteins modulates the intracellular concentration of lopinavir in cultured CD4 T cells and primary human lymphocytes. J Antimicrob Chemother. 2007;60:987–993. doi: 10.1093/jac/dkm353. [DOI] [PubMed] [Google Scholar]
- 118.Sankatsing SU, Cornelissen M, Kloosterboer N, et al. Antiviral activity of HIV type 1 protease inhibitors nelfinavir and indinavir in vivo is not influenced by P-glycoprotein activity on CD4+ T cells. AIDS Res Hum Retroviruses. 2007;23:19–27. doi: 10.1089/aid.2006.0027. [DOI] [PubMed] [Google Scholar]
- 119.Jorajuria S, Dereuddre-Bosquet N, Becher F, et al. ATP binding cassette multidrug transporters limit the anti-HIV activity of zidovudine and indinavir in infected human macrophages. Antivir Ther. 2004;9:519–528. [PubMed] [Google Scholar]
- 120.Dallas S, Schlichter L, Bendayan R. Multidrug resistance protein (MRP) 4- and MRP 5-mediated efflux of 9-(2-phosphonylmethoxyethyl)adenine by microglia. J Pharmacol Exp Ther. 2004;309:1221–1229. doi: 10.1124/jpet.103.063966. [DOI] [PubMed] [Google Scholar]
- 121.Dallas S, Zhu X, Baruchel S, Schlichter L, Bendayan R. Functional expression of the multidrug resistance protein 1 in microglia. J Pharmacol Exp Ther. 2003;307:282–290. doi: 10.1124/jpet.103.054304. [DOI] [PubMed] [Google Scholar]
- 122.Bangsberg DR. Preventing HIV antiretroviral resistance through better monitoring of treatment adherence. J Infect Dis. 2008;197(Suppl 3):S272–S278. doi: 10.1086/533415. [DOI] [PubMed] [Google Scholar]
- 123.Gillim-Ross L, Cara A, Klotman ME. HIV-1 extrachromosomal 2-LTR circular DNA is long-lived in human macrophages. Viral Immunol. 2005;18:190–196. doi: 10.1089/vim.2005.18.190. [DOI] [PubMed] [Google Scholar]
- 124.Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 125.Coffey MJ, Woffendin C, Phare SM, Strieter RM, Markovitz DM. RANTES inhibits HIV-1 replication in human peripheral blood monocytes and alveolar macrophages. Am J Physiol. 1997;272:L1025–L1029. doi: 10.1152/ajplung.1997.272.5.L1025. [DOI] [PubMed] [Google Scholar]
- 126.Gavegnano C, Fromentin E, Schinazi RF. Lower levels of nucleoside analog triphosphates in primary human macrophages compared to human lymphocytes could impair potency of antiretroviral drugs in human viral reservoirs. Global Antiviral Journal. 2008;4(Suppl 1):70. [Google Scholar]