Abstract
During the 10-year period from 1997 through 2006, the reported mean annual incidence rate of leptospirosis in the state of Hawaii was 3.3/100,000 with a range of 22–60 infections reported each year. Because the clinical presentation is highly variable, however, leptospirosis illness is challenging to recognize and may be underdiagnosed. To assess whether the incidence may be substantially higher than reported figures indicate, we retrospectively studied the prevalence of anti-Leptospira IgM antibodies among specimens obtained over a 12-month period (May 2001 to April 2002) from patients presenting with febrile illness during a dengue fever outbreak in Hawaii. Of 1206 patients testing negative or indeterminate for dengue, 54 (4.5%; 95% confidence interval: 3.3%–5.7%) were positive for anti-Leptospira IgM antibodies using a commercially available dipstick enzyme-linked immunosorbent assay (ELISA). The most common clinical symptoms reported by laboratory-positive leptospirosis patients were fever (92%), headache (88%), and myalgia (83%). Three clinical symptoms were significantly less common among persons laboratory positive for leptospirosis when compared with the 122 patients who had been diagnosed with dengue fever during the outbreak: rash (p < 0.0001), chills (p = 0.05), and petechiae (p = 0.0005). Laboratory-positive leptospirosis infections were identified in persons exposed on each of the 5 most populous islands and illness onsets spanned a 10-month period, reflecting an endemic pattern of disease. If added to the figures obtained via routine passive surveillance, the number of leptospirosis infections identified through this study would more than double the annual incidence rate for Hawaii during 2001. These findings indicate that many leptospiral infections in Hawaii go undiagnosed. Physicians should maintain a high index of suspicion for leptospirosis when assessing patients presenting with acute febrile illness among residents and visitors to Hawaii.
Key Words: Leptospirosis, Dengue fever, Hawaii, Annual incidence, ELISA, Underrecognition
Introduction
Leptospirosis is a bacterial zoonosis caused by a pathogenic spirochete from the genus Leptospira. The disease is maintained in nature through the chronic renal infection of host animals and the bacterium is shed in their urine. Human infection results from either direct contact with the urine of an infected animal or indirectly through contact with contaminated water or soil.
Leptospires can affect many different human tissues, producing a wide array of clinical manifestations, ranging from a mild undifferentiated febrile illness to severe multiorgan failure and death (Bharti et al. 2003). As a consequence, leptospirosis infection may be clinically indistinguishable from many febrile illnesses including typhus, influenza, viral encephalitis, and dengue fever (Flannery et al. 2001). Furthermore, laboratory confirmation of leptospirosis by the gold standard microscopic agglutination test (MAT) is technically challenging because of the need to maintain panels of live leptospires in culture for long periods, and time consuming as paired acute and convalescent serum samples need to be tested together.
Leptospirosis has a global distribution but is most common in tropical regions where warm, wet conditions promote the survival of the leptospires (Levett 2001, McBride et al. 2005). Within the United States, the annual incidence rates of leptospirosis in Hawaii are consistently much higher than those reported from the U.S. mainland (Effler et al. 2002). Still the challenges associated with recognizing and confirming infection suggest leptospirosis may be underdiagnosed among patients presenting with clinically compatible febrile illness in Hawaii. To assess this possibility, we used a commercially available leptospirosis IgM enzyme-linked immunosorbent assay (ELISA) to retrospectively test serum samples originally collected from patients being evaluated for dengue fever during an outbreak of dengue in 2001–2002.
Materials and Methods
In September 2001, the Hawaii State Department of Health (HDOH) contacted all licensed physicians in Hawaii requesting that they report any patients presenting with a dengue-like illness (DLI). DLI was defined as fever or chills and 2 or more of the following symptoms: myalgia, headache, arthralgia, retro-orbital pain, rash, or any hemorrhagic symptom (Effler et al. 2005). In addition, active surveillance for DLI was implemented in all acute care hospitals and major clinics throughout the state between September 12, 2001 and April 30, 2002. Clinical and travel histories for each patient with DLI were reviewed by HDOH staff, and whenever possible, serologic specimens were obtained and forwarded to the HDOH State Laboratories Division (SLD) for anti-dengue IgM and/or IgG antibodies, as previously described (Effler et al. 2005). Of the 1644 persons tested during the outbreak, 122 patients had confirmed dengue infection and the remainder (1522) lacked serologic evidence of recent dengue infection. Following the outbreak, aliquots of sera were linked via a unique number to limited patient demographic and clinical information and stored at −20°C.
Resources did not permit testing all of the 1522 patients who lacked serologic evidence of acute dengue infection for anti-Leptospira IgM antibodies; instead a sample of 1206 (79%) patients was compiled by selecting stored sera without prior knowledge of patient characteristics. The number of specimens originally collected from each patient during the 2001–2002 dengue outbreak varied; thus, the number of serum samples from each patient tested for leptospirosis also varied, ranging from 1 to 4. The serum set tested for leptospirosis ultimately consisted of 1681 serum specimens from 1206 patients. This study was approved by the Institutional Review Board of the University of Hawaii.
All serum samples were tested using the PAN-BIO IgM DIP-S-TICKS leptospirosis test (PAN-BIO, Inc., Columbia, MD) according to the manufacturer's protocol. Anti-Leptospira IgM antibodies were detected qualitatively and each assay strip contained positive and negative controls. The assay strip was scored on a scale of 1 to 4 dots. Strips with more than 2 dots were recorded as positive tests, strips with 2 dots were recorded as indeterminate, and those with less than 2 dots were scored as a negative. Samples that initially tested positive for anti-Leptospira IgM antibodies were retested using the same procedure. If the results of the second test were also positive, then the individual that submitted the specimen was considered laboratory positive for leptospirosis.
Statistical analysis
Demographic and clinical characteristics for patients who tested positive for leptospirosis were compared with: (1) those with confirmed dengue infection as described elsewhere (Effler et al. 2005), and (2) those without laboratory evidence of dengue or leptospirosis using Fisher's exact test in StatXact, version 4.0.1 (Cytel Software Corporation, Cambridge, MA). All tests were 2-tailed and p ≤ 0.05 was considered statistically significant. Confidence intervals (95% CI) for proportions were calculated using exact binomial equation.
Results
Fifty-four (4.5%) of the 1206 patients tested had duplicate positive test results for anti-Leptospira IgM antibodies in 1 or more specimens and hence were classified as having laboratory-positive leptospirosis infections; 1141 (94.6%) patients were negative and 11 (0.9%) were indeterminate for leptospirosis on all specimens tested. Thirty-one of the laboratory-positive leptospirosis patients had more than 1 serum specimen available for testing; of these, 20 (65%) seroconverted between acute and convalescent sera.
Of the 53 laboratory-positive leptospirosis patients with known gender, 33 (62%) were males and 20 (38%) were females (Table 1). The median age of laboratory-positive leptospirosis patients was 37 years (range, 10–67 years). While the greatest number of laboratory-positive patients (n = 13) were in the 30–39 year age group, those in the 10–19 year age group had the highest rate of infection (7%) (Table 1).
Table 1.
Demographic Characteristics of Patients Laboratory Positive for Leptospirosis and Those Negative for Leptospirosis and Dengue Infection, Hawaii, 2001–2002
| |
Leptospirosis positive (n = 54) |
Negative for leptospirosis and dengue (n = 1141) |
||
|---|---|---|---|---|
| n | % | n | % | |
| Gender | ||||
| Male | 33 | 61 | 548 | 48 |
| Female | 20 | 37 | 577 | 51 |
| Unknown | 1 | 2 | 16 | 1 |
| Island/county | ||||
| Hawaii | 13 | 24 | 96 | 8 |
| Kauai | 9 | 17 | 104 | 9 |
| Maui | 20 | 37 | 386 | 34 |
| Oahu | 12 | 22 | 554 | 49 |
| Unknown | 0 | 0 | 1 | 0 |
| Age group (years) | ||||
| 0–9 | 0 | 0 | 3 | 0 |
| 10–19 | 9 | 17 | 117 | 10 |
| 20–29 | 5 | 9 | 156 | 14 |
| 30–39 | 13 | 24 | 246 | 22 |
| 40–49 | 10 | 19 | 224 | 20 |
| 50–59 | 7 | 13 | 143 | 13 |
| 60–69 | 4 | 7 | 46 | 4 |
| ≥70 | 0 | 0 | 29 | 3 |
| Unknown | 6 | 11 | 177 | 16 |
Detailed data on dengue-positive patients are available in Effler et al. (2005).
Positive patients came from 5 islands in 4 counties, with Maui county having the greatest number of infections (n = 20) and Hawaii and Kauai counties having the highest patient positivity rates (12% and 8%, respectively) (Table 1).
Leptospirosis infection was detected in persons experiencing onset of illness during a 10-month period from July 2001 through April 2002, with the highest monthly total occurring in October. The number of positive patients was, however, strongly correlated with the total number of patients tested each month (r = 0.98). As shown in Figure 1, the proportion of all patients that tested positive for anti-Leptospira IgM antibodies remained fairly constant (range, 2.3%–5.9%) during the 8-month period from September 2001 through April 2002, a time when 30 or more persons were tested each month.
FIG. 1.
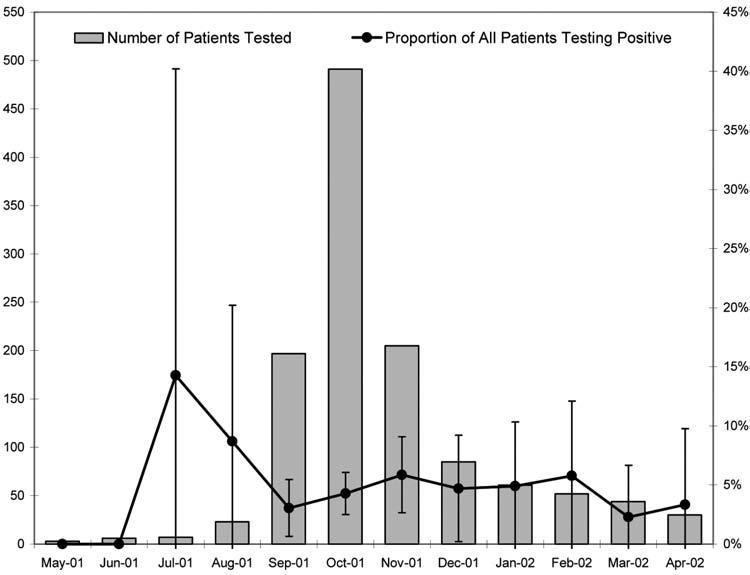
Number of patients tested for leptospirosis and proportion of all patients testing positive, by month, Hawaii, May 2001 to April 2002. Excludes 2 leptospirosis-negative patients without documentation for date of illness onset. Confidence intervals (95%) for proportions were calculated using exact binomial equation.
Table 2 compares the symptoms reported by leptospirosis-positive patients with those who tested negative for both dengue and leptospirosis, and those with laboratory-confirmed dengue infection. Fever, headache, chills, myalgia, and arthralgia were the most commonly reported symptoms, each reported by the majority of patients in all 3 patient groups. Statistically significant differences were observed between patients with leptospirosis and those with dengue fever for several signs/symptoms: chills (p = 0.05), rash (p = 0.0001), and a history of petechiae (p = 0.0005) were each more likely to be reported by patients with dengue.
Table 2.
Clinical Presentation of Patients with Leptospirosis or Dengue Infection Compared with Those Lacking Laboratory Evidence for Either Infection, Hawaii, 2001–2002
| |
|
|
|
P |
|
|---|---|---|---|---|---|
| |
No. with specific sign or symptom/no. with data available (%) |
|
|||
| Clinial sign or symptom | Leptospirosis positive | Negative for dengue and leptospirosis | Dengue positive | Leptospirosis positive compared with negative for dengue and leptospirosis | Leptospirosis positive compared with dengue positive |
| Fever | 49/53 (92) | 1008/1106 (91) | 114/120 (95) | 1.0 | 0.50 |
| Headache | 44/50 (88) | 902/1047 (86) | 104/116 (90) | 0.84 | 0.79 |
| Myalgia | 43/52 (83) | 838/1055 (79) | 106/115 (92) | 0.72 | 0.10 |
| Chills | 35/49 (71) | 739/1032 (72) | 100/117 (85) | 1.0 | 0.05 |
| Arthralgia | 33/48 (69) | 599/987 (61) | 85/112 (76) | 0.29 | 0.43 |
| Nausea/vomiting | 23/50 (46) | 533/1032 (52) | 59/119 (50) | 0.47 | 0.74 |
| Eye/retro-orbital pain | 21/48 (44) | 494/958 (52) | 68/114 (60) | 0.30 | 0.08 |
| Diarrhea | 18/48 (38) | 335/1010 (33) | 39/118 (33) | 0.53 | 0.59 |
| Cough | 14/47 (30) | 433/1012 (43) | 25/118 (21) | 0.10 | 0.31 |
| Sore throat | 14/49 (29) | 346/990 (35) | 27/117 (22) | 0.44 | 0.55 |
| Rash | 13/48 (27) | 357/1038 (34) | 79/117 (68) | 0.35 | <0.0001 |
| Nasal congestion | 12/49 (24) | 338/996 (34) | 26/119 (22) | 0.21 | 0.69 |
| Purpura | 2/44 (5) | 39/970 (4) | 1/116 (1) | 0.70 | 0.18 |
| Epistaxis | 2/46 (4) | 35/979 (4) | 9/113 (8) | 0.68 | 0.51 |
| Petechiae | 1/46 (2) | 68/968 (7) | 28/118 (24) | 0.36 | 0.0005 |
| Hematuria | 1/46 (2) | 22/965 (2) | 1/111 (1) | 1.0 | 0.50 |
| Jaundice | 0/46 (0) | 25/930 (3) | 5/108 (5) | 0.63 | 0.32 |
Detailed data on dengue-positive patients are available in Effler et al. (2005).
Discussion
The outbreak of dengue that occurred in Hawaii in 2001–2002 provided a unique opportunity to assess the incidence of leptospirosis in a population of febrile patients that may not, under nonoutbreak circumstances, have presented for clinical evaluation or have had specimens collected for diagnostic serology. Nearly 1 in 20 individuals in this large sample of febrile patients had laboratory evidence of recent leptospirosis infection. In addition, leptospirosis infection was identified in specimens collected throughout most of the year and from patients residing on all major Hawaiian islands. If added to the figures obtained via routine passive surveillance, the number of leptospirosis infections identified through this study would increase the annual incidence rate for Hawaii during 2001 from 2.8 to 6.5/100,000. It is also noteworthy that more than a third of all leptospirosis infections identified in this study were from Maui, an island which accounted for only 1.4% of all leptospirosis cases reported during the 25-year period from 1974 through 1998 (Katz et al. 2002). These data indicate that leptospirosis is endemic throughout Hawaii and is substantially underdiagnosed.
Our findings complement those recently reported for dengue outbreak investigations in Puerto Rico (Sanders et al. 1999) and Bangladesh (LaRocque et al. 2005), where a significant proportion of leptospirosis cases were diagnosed after retesting sera from persons presenting with dengue-like illness but who were laboratory negative for dengue. Also consistent with our findings, the presence of a skin rash or petechiae helped differentiate dengue patients from those found to have leptospirosis (Bruce et al. 2005, LaRocque et al. 2005, Levett 2003).
Several factors likely contribute to the underdiagnosis of leptospirosis in our setting. First, as reconfirmed in this study, the clinical manifestations of leptospirosis are known to be diverse and not readily distinguishable from those accompanying other febrile illnesses. Therefore, physicians may have attributed febrile illnesses to other causes, omitted leptospirosis from the differential diagnosis, and not obtained appropriate laboratory tests.
Second, even if leptospirosis is suspected, the laboratory confirmation of leptospirosis remains a major challenge. Isolation and culture require specialized laboratory techniques and may take several weeks. The definitive diagnostic serologic assay for leptospirosis, the microscopic agglutination test (MAT), is methodologically complex and established in few laboratories (Levett 2003). In addition, MAT confirmation requires demonstration of a 4-fold titer change between paired acute and convalescent serum samples. Our experience in Hawaii suggests that approximately 30% of patients initially evaluated for leptospirosis do not have a convalescent serum sample obtained, excluding the possibility of adequately assessing MAT confirmation (Katz et al. 2002).
Third, though IgM-ELISA tests that can be interpreted on single specimens are now widely available for leptospirosis, they exhibit suboptimal sensitivities early in the course of illness (25%–50%) (Effler et al. 2002). The low sensitivity on acute-phase specimens limits the utility of IgM-ELISA tests for guiding clinical management decisions, reducing the incentive for physicians to order the test. Low sensitivity on acute specimens also likely results in some patients being “ruled-out” for leptospirosis prematurely (smits et al. 1999, Cumberland et al. 1999, Gussenhoven et al. 1997).
Our study has limitations. Anti-Leptospira IgM antibodies can persist for years after initial exposure (McBride et al. 2005) and IgM antibodies detected by the DIP-S-TICKS assay may remain detectable for at least as long as 3 months after symptom onset (WHO 2003). It is therefore difficult to conclusively differentiate between current and past infections. This calls into question whether the acute symptoms reported by patients were caused by an acute leptospirosis infection or by a more recent infection of different etiology. However, in this study, the majority (65%) of the 31 laboratory-positive leptospirosis patients with 2 or more specimens available demonstrated seroconversion, indicating that the leptospirosis infections were recent, and likely the source of the clinical illness.
A second limitation is the aforementioned suboptimal sensitivity of the DIP-S-TICKS IgM test when applied to acute-phase specimens. In this study, 312 (41%) of the individuals with only 1 specimen available for testing submitted that specimen during the first week after symptom onset, a time when IgM assays have demonstrated low sensitivity (<50%) among patients in Hawaii (Effler et al. 2002). It is therefore likely that the rates of leptospiral infection in our study population are higher than reported.
A third potential limitation is that the specificity of the DIP-S-TICKS IgM is imperfect; therefore, it is possible some patients may have been misclassified as having leptospirosis when they did not. However, a previous evaluation of this assay in Hawaii comparing it with the MAT found the specificity to be quite acceptable in our patient population, i.e., 95% (Effler et al. 2002), and this finding has been corroborated in more recent assessments conducted elsewhere (Bajani et al. 2003, Berlioz-Arthaud 2007). The high specificity of the leptospirosis DIP-S-TICKS IgM assay would support that the majority of the laboratory-positive patients we identified likely represent true infections.
In summary, our findings suggest that leptospirosis is underdiagnosed in persons presenting with febrile illness in Hawaii. These data underscore the need for physicians to maintain a high level of clinical suspicion for the disease when presented with patients exhibiting an undifferentiated febrile illness. This is particularly important as early diagnosis and initiation of antibiotic therapy early in the course of infection may reduce the severity and duration of illness.
Acknowledgments
This work was supported by grants U50/CCU912395-06 and U50/CCU912395-07 from the U.S. Public Health Service, and P20RR018727 from the National Center for Research Resources of the National Institutes of Health.
References
- Berlioz-Arthaud A. Evaluation of reagents for the serological diagnosis of leptospirosis. www.spc.int/phs/PPHSN/Services/LabNet/kits-Lepto-eval-VE.pdf. [Dec 4;2007 ]. www.spc.int/phs/PPHSN/Services/LabNet/kits-Lepto-eval-VE.pdf
- Bajani MD. Ashford DA. Bragg SL. Woods CW, et al. Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis. J Clin Microbiol. 2003;41:803–809. doi: 10.1128/JCM.41.2.803-809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti AR. Nally JE. Ricaldi JN. Matthias MA, et al. Leptospirosis: a zoonotic disease of global importance. Lancet. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- Bruce MG. Sanders EJ. Leake JA. Zaidel O, et al. Leptospirosis among patients presenting with dengue-like illness in Puerto Rico. Acta Trop. 2005;96:36–46. doi: 10.1016/j.actatropica.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Cumberland P. Everard CO. Levett PN. Assessment of the efficacy of an IgM-ELISA and microscopic agglutination test (MAT) in the diagnosis of acute leptospirosis. Am J Trop Med Hyg. 1999;61:731–734. doi: 10.4269/ajtmh.1999.61.731. [DOI] [PubMed] [Google Scholar]
- Effler PV. Bogard AK. Domen HY. Katz AR, et al. Evaluation of eight rapid screening tests for acute leptospirosis in Hawaii. J Clin Microbiol. 2002;40:1464–1469. doi: 10.1128/JCM.40.4.1464-1469.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effler PV. Pang L. Kitsutani P. Vorndam V, et al. Dengue fever, Hawaii, 2001–2002. Emerg Infect Dis. 2005;11:742–749. doi: 10.3201/eid1105.041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery B. Pereira MM. De Freitas Velloso L. De Castro Carvalho C, et al. Referral pattern of leptospirosis cases during a large urban epidemic of dengue. Am J Trop Med Hyg. 2001;65:657–663. doi: 10.4269/ajtmh.2001.65.657. [DOI] [PubMed] [Google Scholar]
- Gussenhoven GC. van der Hoorn MA. Goris MG. Terpstra WJ, et al. LEPTO dipstick, a dipstick assay for detection of Leptospira-specific immunoglobulin M antibodies in human sera. J Clin Microbiol. 1997;35:92–97. doi: 10.1128/jcm.35.1.92-97.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz AR. Ansdell VE. Effler PV. Middleton CR, et al. Leptospirosis in Hawaii, 1974–1998: epidemiologic analysis of 353 laboratory confirmed cases. Am J Trop Med Hyg. 2002;66:61–70. doi: 10.4269/ajtmh.2002.66.61. [DOI] [PubMed] [Google Scholar]
- LaRocque RC. Breiman RF. Ari MD. Morey RE, et al. Leptospirosis during dengue outbreak, Bangladesh. Emerg Infect Dis. 2005;11:766–769. doi: 10.3201/eid1105.041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levett PN. Murray PR. Baron EJ. Pfaller MA. Jorgensen JH, et al. Manual of Clinical Microbiology. 8th. Washington, DC: ASM Press; 2003. Leptospirosis and leptonema; pp. 929–936. [Google Scholar]
- Levett PN. Branch SL. Edwards CN. Detection of dengue infection in patients investigated for leptospirosis in Barbados. Am J Trop Med Hyg. 2000;62:112–114. doi: 10.4269/ajtmh.2000.62.112. [DOI] [PubMed] [Google Scholar]
- McBride AJA. Athanazio DA. Reis MG. Ko Al. Leptospirosis. Curr Opin Infect Dis. 2005;18:376–386. doi: 10.1097/01.qco.0000178824.05715.2c. [DOI] [PubMed] [Google Scholar]
- Sanders EJ. Rigau-Pérez JG. Smits HL. Deseda CC, et al. Increase of leptospirosis in dengue-negative patients after a hurricane in Puerto Rico in 1996. Am J Trop Med Hyg. 1999;61:399–404. doi: 10.4269/ajtmh.1999.61.399. [DOI] [PubMed] [Google Scholar]
- Smits HL. Ananyina YV. Chereshsky A. Dancel L, et al. International multicenter evaluation of the clinical utility of a dipstick assay for detection of Leptospira-specific immunoglobulin M antibodies in human serum specimens. J Clin Microbiol. 1999;37:2904–2909. doi: 10.1128/jcm.37.9.2904-2909.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control. Geneva: WHO; 2003. [Google Scholar]


